Abstract
Bacterial cell growth necessitates synthesis of peptidoglycan. Assembly of this major constituent of the bacterial cell wall is a multistep process starting in the cytoplasm and ending in the exterior cell surface. The intracellular part of the pathway results in the production of the membrane-anchored cell wall precursor, Lipid II. After synthesis this lipid intermediate is translocated across the cell membrane. The translocation (flipping) step of Lipid II was demonstrated to require a specific protein (flippase). Here, we show that the integral membrane protein FtsW, an essential protein of the bacterial division machinery, is a transporter of the lipid-linked peptidoglycan precursors across the cytoplasmic membrane. Using Escherichia coli membrane vesicles we found that transport of Lipid II requires the presence of FtsW, and purified FtsW induced the transbilayer movement of Lipid II in model membranes. This study provides the first biochemical evidence for the involvement of an essential protein in the transport of lipid-linked cell wall precursors across biogenic membranes.
Keywords: antibiotic target, cell wall synthesis, flippase activity, FtsW, Lipid II
Introduction
The cell wall or peptidoglycan layer is unique and essential to bacteria. Most steps involved in its biosynthesis are therefore exploited as targets for well-known antibiotics or are explored for designing novel drugs. The central events in the building of peptidoglycan enclose the synthesis of two lipid intermediates on the cytoplasmic side of the membrane, Lipid I and Lipid II, and the subsequent transport of the latter across the bacterial membrane. In brief, the cytoplasmic step of peptidoglycan precursor synthesis culminates in the production of UDP-N-acetylmuramyl-pentapeptide (UDP-MurNAc-pentapeptide) from UDP-N-acetyl-glucosamine (UDP-GlcNAc). This compound is coupled to undecaprenyl phosphate to form Lipid I by MraY, an integral membrane protein. The subsequent addition of GlcNAc by MurG, an enzyme associated with the membrane, yields Lipid II (its structure is illustrated in Figure 1A). Thereafter, Lipid II is translocated to the exterior surface of the cell by an unknown mechanism and incorporated into the peptidoglycan through transglycosylation and transpeptidation reactions by penicillin-binding proteins (PBPs; Höltje, 1998; Cabeen and Jacobs-Wagner, 2007; Bouhss et al, 2008; den Blaauwen et al, 2008; Vollmer and Bertsche, 2008).
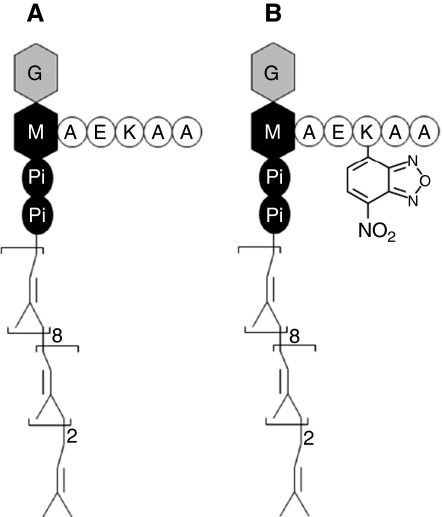
Figure 1.
Schematic representation of the chemical structure of Lipid II (A) and NBD-labelled Lipid II (B). This peptidoglycan precursor consists of an undecaprenyl chain, phosphate (Pi), MurNAc (M) and GlcNAc (G). The pentapeptide moiety of MurNAc is symbolized by circles. NBD fluorophore is attached at the lysine of the pentapeptide moiety residue.
The molecular and cellular details of the transport of peptidoglycan subunits across the cytoplasmic membrane are as yet unidentified. Recently, the translocation process was studied in Escherichia coli inner membrane vesicles using a fluorescent 7-nitro-2,1,3-benzoxadiazol-4-yl (NBD) analogue of Lipid II (van Dam et al, 2007; its structure is illustrated in Figure 1B). The transport machinery was shown not to be dependent on metabolic energy, but was demonstrated to be protein facilitated. To date, no specific proteins have been identified yet. Potential transporters (flippases) are predicted to be inner membrane proteins, essential and conserved in most eubacteria producing peptidoglycan cell wall. On the basis of these characteristics and its effects on peptidoglycan synthesis, FtsW has been suggested to act as a flippase decades ago (Matsuhashi, 1994; Höltje, 1998). FtsW is an essential division protein with 10 predicted transmembrane segments and belongs to the SEDS (shape, elongation, division and sporulation) family, which includes RodA and SpoVE proteins. At least one member of the SEDS family appears to be present in all bacteria that have a peptidoglycan cell wall. RodA, FtsW and Bacillus subtilis SpoVE are thought to participate in separate peptidoglycan synthesis complexes acting during elongation, division and sporulation, respectively. Within these complexes, each protein from the SEDS family is accompanied by its cognate PBP. For example, FtsW and PBP3 (FtsI) are important during division, whereas RodA and PBP2 operate specifically during the elongation step of rod-shaped bacteria. The gene encoding FtsW is located on the dcw (division and cell wall) cluster in close proximity to the gene encoding PBP3. FtsW was shown to be localized to the septum during division in E. coli and to interact with PBP3 (Boyle et al, 1997; Mercer and Weiss, 2002; Pastoret et al, 2004; Fraipont et al, 2011), thereby connecting the cell wall synthesis and the division machinery. More interestingly, the gene encoding FtsW is always located next to that encoding MurG, which suggests that they interact.
Altogether, these considerations support the old speculation that FtsW could act as Lipid II transporter, implying that RodA and SpoVE fulfil these roles during elongation and sporulation, respectively.
Results
A FRET-based assay to study Lipid II translocation
To address the role of FtsW in the flipping process, we developed a novel fluorescence resonance energy transfer (FRET)-based assay to study the translocation of the membrane-anchored cell wall precursor Lipid II in bacterial membrane vesicles. This assay makes use of the specific recognition of Lipid II by vancomycin (Breukink and de Kruijff, 2006) and the inability of this antibiotic to cross the membrane. Using NBD-labelled Lipid II as a donor and tetramethylrhodamine cadaverine (TMR)-labelled vancomycin as an acceptor of the fluorescence, a strong FRET signal (a fluorescence signal yielded by the energy transfer between NBD and TMR fluorophores when they are in close proximity) is detectable only when Lipid II is present (Figure 2). This FRET signal is absent when the fluorescence of NBD-Lipid II that is located in the outer leaflet of the vesicles has been quenched beforehand by the membrane impermeant reductant dithionite (Supplementary Figure S1).
Figure 2.
The interaction between fluorescently labelled vancomycin and Lipid II leads to FRET. In this assay, NBD-Lipid II (labelled at the amino group of the lysine at position 3 of the pentapeptide) and vancomycin-TMR (labelled at its C terminus with tetramethylrhodamine cadaverine) are used as a FRET pair. Fluorescence spectra of NBD-Lipid II (in LUVs prepared as described in Materials and methods) and vancomycin-TMR separately and together are presented (while being exited at the wavelength of the NBD group). The energy transfer between NBD and TMR fluorophores yields a fluorescence signal when they are in close proximity of each other. This is generally reflected by a decrease in fluorescence of NBD-Lipid II and increase in fluorescence of vancomycin-TMR. A.U.: arbitrary units.
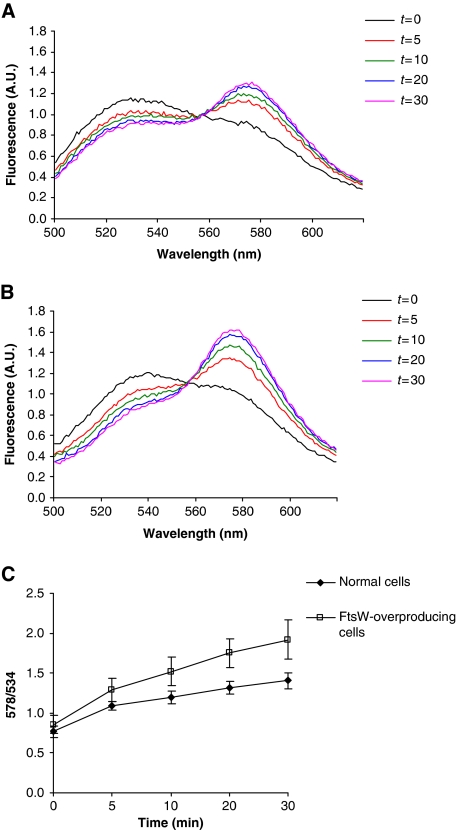
We then assessed if this FRET approach would be suitable to assay Lipid II transport in right-side-out (RSO) membrane vesicles prepared from E. coli cells. The assay is based on the synthesis of NBD-labelled Lipid II at the inner leaflet of RSOs, which will be translocated across the membrane to appear at the outer leaflet rendering it accessible to vancomycin (see the hypothetical plot in Supplementary Figure S2, right panel). The appearance of Lipid II at the outer leaflet and concomitant binding to TMR-labelled vancomycin will lead to a decrease in the NBD fluorescence, which will be accompanied by an increase of TMR fluorescence (Supplementary Figure S2, left panel). In the RSOs, synthesis of fluorescently labelled Lipid II was enabled by following a freeze–thaw procedure to introduce the precursors NBD-UDP-MurNAc-pentapeptide and UDP-GlcNAc into the lumen of the vesicles. When RSO vesicles derived from wild-type E. coli prepared in this way were incubated at 14°C (All FRET measurements were carried out at 14°C to prevent the decrease in the fluorescence of NBD-labelled Lipid II in time at elevated temperatures resulting from transglycosylase activity, most likely of PBPs as reported on earlier in van Dam et al (2007). This decrease leads to a reduction in the total fluorescence of the FRET signal when measurements are performed at temperatures around 25°C.) in the presence of vancomycin-TMR, an increase in the fluorescence of the latter accompanied by a decrease in the NBD fluorescence was detected (Figure 3A). This gradual increase in the FRET signal reflects the appearance of Lipid II on the outside of the vesicles, demonstrating that the assay is capable of measuring Lipid II transport in bacterial membranes. Upon overexpression of FtsW in the same E. coli strain, the translocation of Lipid II was considerably increased (Figure 3B). This indicates that FtsW is involved in the transit of Lipid II from the inner to the outer leaflet of the membrane. Enhanced translocation of Lipid II was also detectable when transport of NBD-Lipid II was monitored using membrane vesicles derived from cells overexpressing Streptococcus pneumoniae FtsW (Supplementary Figure S3), which signifies that the effect of FtsW is species independent. Overexpression of other (control) proteins did not result in an augmentation of Lipid II translocation (Supplementary Figure S4A–C).
Figure 3.
Expression of FtsW increases the translocation of NBD-Lipid II from the inner to the outer leaflet of the bacterial membrane. Generation of a FRET signal was monitored over time in RSO vesicles prepared from wild-type TOP10F’ strain (A), and TOP10F’ harbouring a plasmid encoding His-tagged FtsW, where the expression of the ftsW gene is under the control of an IPTG-inducible promoter (B). The time course of fluorescence was monitored during 30 min. Assaying the wild-type strain yielded a gradual increase in the FRET signal in time (A). Overexpression of the ftsW gene causes a significant increase in the FRET signal (B). Background signals obtained from RSO vesicles, where neither NBD-UDP-MurNAc-pentapeptide nor UDP-GlcNAc were incorporated and were subtracted from each measurement. Synthesis of NBD-Lipid II in the vesicles was monitored using TLC. All measurements are representative of at least three independent experiments. A.U.: arbitrary units. (C) The time course of the FRET signal in (A) and (B) displayed as a 578/534 ratio, where 578 nm is the emission maximum of vancomycin-TMR and 534 nm is the emission maximum of NBD-Lipid II. Error bars represent s.d. of the mean value of the ratios measured at 578±5 and 534±5 nm.
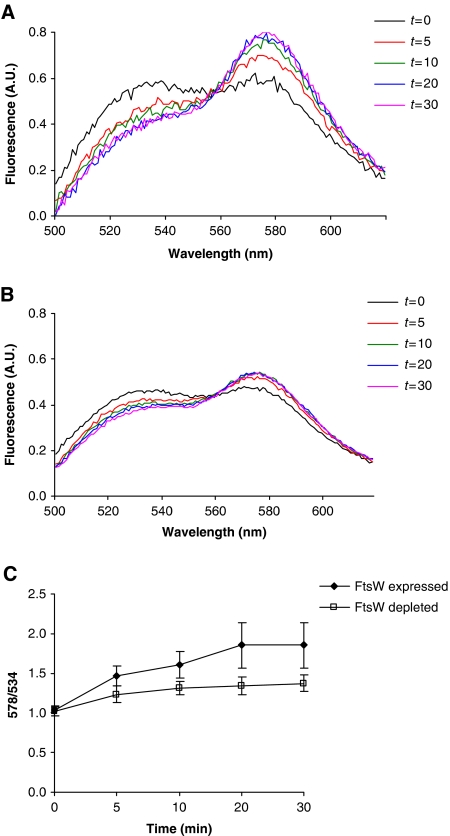
To further probe the role of FtsW in Lipid II translocation, we determined the transport of Lipid II in bacterial membranes depleted of FtsW. For this we isolated membranes from an FtsW depletion strain of E. coli (Boyle et al, 1997). While Lipid II transport in RSOs prepared from this strain (under conditions that allow expression of FtsW) was similar to that in RSOs derived from the wild-type strain (compare Figures 3A to 4A), depletion of FtsW resulted in reduced Lipid II transport (Figure 4B and C). This implies that the translocation of NBD-Lipid II requires the presence of FtsW. The remaining observed transport activity is likely due to the presence of the FtsW homologue, RodA.
Figure 4.
The translocation of NBD-Lipid II across the bacterial membrane is affected by the depletion of FtsW. The LMC1436 strain (FtsW depletion strain) was grown in the presence of arabinose to induce the expression of FtsW, and RSO vesicles were prepared as described before. These behaved similarly as the wild-type E. coli strains in yielding a moderate augmentation in fluorescence in time (A). When this strain was grown in the presence of glucose (inducing the depletion of FtsW), a reduced FRET signal (reflecting a reduced Lipid II translocation) is obtained (B) compared with that where the expression of FtsW was induced. The fluorescence spectra in (B) were normalized to the same scale as in (A). All measurements are representative of at least three independent experiments. A.U: arbitrary units. (C) The time course of the FRET signal in (A) and (B) displayed as a 578/534 ratio, where 578 nm is the emission maximum of vancomycin-TMR and 534 nm is the emission maximum of NBD-Lipid II. Error bars represent s.d. of the mean value of the ratios measured at 578±5 and 534±5 nm.
FtsW is directly involved in the transport of Lipid II
To demonstrate whether FtsW is directly involved in Lipid II transport, we purified E. coli FtsW (Figure 5A) and tested its effect on the topology of Lipid II in model membranes using a dithionite reduction assay (McIntyre and Sleight, 1991; Kol et al, 2001) adapted to determine the topology of Lipid II (van Dam et al, 2007). To this end, FtsW was reconstituted in large unilamellar vesicles (LUVs) with NBD-Lipid II. In these proteoliposomes (with a diameter of ∼150 nm) NBD-Lipid II is symmetrically distributed between the inner and outer leaflets. As illustrated in Figure 5B, LUVs reconstituted in the absence of protein demonstrate a reduction of ∼50% of the fluorescence signal of NBD-Lipid II upon addition of dithionite. This corresponds to the quenching of NBD-Lipid II pool residing in the outer leaflet of the LUVs. The remaining 50% is localized to the inner leaflet of the LUVs and is protected from quenching by the membrane impermeant reductant. When 0.1% Triton X-100 was added to permeabilize the vesicles, complete quenching of all fluorescence was achieved.
Figure 5.
FtsW induces transbilayer movement of NBD-Lipid II in proteoliposomes. (A) Coomassie-stained SDS–PAGE gel analysis of purified FtsW. The arrow at ∼37 kDa points to the FtsW band. (B) LUVs containing NBD-Lipid II symmetrically distributed between the inner and outer leaflets of the bilayer and solubilized with Triton X-100 were reconstituted with no protein, the control protein KcsA or FtsW following the procedure detailed in Materials and methods. After addition of dithionite (1), a reduction of almost 50% of the fluorescence signal is displayed by protein-free vesicles and proteoliposomes containing KscA. In contrast, ∼70% quenching is obtained when FtsW-containing proteoliposomes were employed. When 0.1% Triton X-100 was added (2) to permeabilize the vesicles, a complete quenching of all the fluorescence is achieved. All measurements were carried out at 20°C and are representative of at least three independent experiments. A.U.: arbitrary units.
We assayed several control proteins for their effect on Lipid II topology. Among these KcsA, MraY and SecYEG, KcsA is the potassium channel of the soil bacterium Streptomyces lividans (van Dalen et al, 2002). Under defined conditions, this well-characterized inner membrane protein was shown to induce translocation of the C6NBD-PG phospholipid analogue through its transmembrane α-helices, albeit at long incubation times (Kol et al, 2003). MraY is an integral membrane enzyme that assembles Lipid I. As a combination of synthesis and transport would likely be efficient for the cell, MraY may well be the transporter in addition to being a synthesis enzyme. By virtue of its importance as a preserved constituent of bacterial membranes and also in the translocation of secretory proteins, SecYEG would also be a candidate Lipid II transporter. After inclusion of the purified KcsA, MraY and SecYEG into LUVs containing NBD-Lipid II, no significant differences regarding the level of protected fluorescent pools were found between protein-free vesicles (Figure 5B, dotted black trace), vesicles with KcsA (Figure 5B, grey trace), vesicles with MraY (Supplementary Figure S5A, red trace), vesicles with SecYEG (Supplementary Figure S5B, red trace). This demonstrates that the topology of Lipid II is not affected by the presence of these proteins.
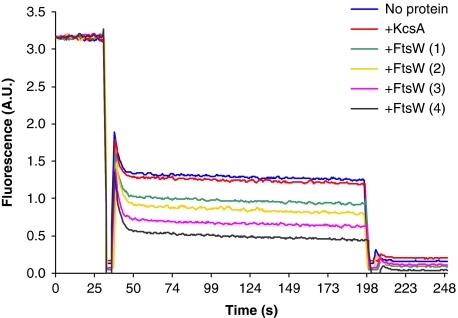
A completely different picture emerged when FtsW-containing proteoliposomes were assayed. With a protein to phospholipids molar ratio of ∼1:20 000, a reduction of ∼70% in fluorescence of NBD-Lipid II was visible, reflecting that only 30% of the NBD-labelled Lipid II remained protected from quenching by dithionite, suggesting that FtsW facilitated translocation of NBD-labelled Lipid II from the inner to the outer leaflet (Figure 5B, black trace). An intriguing observation is the persistence of a pool of protected NBD-labelled Lipid II, which can be explained as follows. The reconstitution procedure allows for the generation of a pool of NBD-Lipid II containing vesicles devoid of FtsW. Of these vesicles, only 50% of NBD-Lipid II is accessible to dithionite reduction. Therefore, the level of reduction by dithionite is expected to be dependent on the amount of transporters in the vesicle preparations, and the addition of more FtsW should result in fewer transporter-less LUVs. Indeed, the extent of quenching of fluorescence was shown to be FtsW concentration dependent, as measured from vesicles reconstituted with different amounts of FtsW (Figure 6). Yet, even at the highest amount of FtsW, a pool of NBD-labelled Lipid II remained protected from quenching by dithionite. This is best explained by aggregation of FtsW during the reconstitution procedure that is thereby not incorporated into the proteoliposomes and which would still allow for a significant amount of vesicles devoid of FtsW. These findings are consistent with results reported previously for translocation by other transporters of phospholipids and dolichol-linked oligosaccharides across the yeast endoplasmic reticulum (Vehring et al, 2007; Sanyal et al, 2008).
Figure 6.
The effect of FtsW in facilitating the transbilayer movement of Lipid II in model membranes is concentration dependent. Proteoliposomes were reconstituted in the presence of FtsW at the following protein/phospholipid molar ratio: 1:40 000 (1), 1:20 000 (2), 1:10 000 (3) and 1:5000 (4). The assay was performed as delineated under Figure 5. The percentage of quenching of fluorescence is dependent on the concentration of FtsW used in the reconstitution procedure. All measurements were carried out at 20°C and are representative of at least three independent experiments. A.U.: arbitrary units.
The effect of FtsW in the reconstituted system is specific
The effect obtained with proteoliposomes containing FtsW can be explained by two possibilities: (i) the presence of FtsW allows Lipid II translocation across the membrane or (ii) can be due to FtsW-dependent leakiness of the proteoliposomes to dithionite. To exclude the latter possibility, we carried out control experiments using NBD-labelled UDP-MurNAc-pentapeptide that was present in the lumen of the vesicles. In all combinations no significant difference between FtsW and KcsA-containing vesicles was detectable, revealing that the proteoliposomes remained sealed under all tested conditions (Supplementary Figure S6), and hence emphasizing the involvement of FtsW in the transport of Lipid II.
Our results using dithionite quenching were additionally confirmed using the FRET-based assay. We reconstituted vesicles in the absence of protein, with KcsA or FtsW. Addition of vancomycin-TMR yielded a higher FRET signal in the presence of FtsW than in the control situations; protein-free vesicles and vesicles reconstituted with KcsA (Figure 7). This result can be understood in the following way. Whereas in vesicles devoid of transporter (no protein or with KcsA), the vancomycin-TMR can bind to only half the pool of NBD-Lipid II, in vesicles harbouring FtsW, the vancomycin-TMR eventually binds to the most of the Lipid II pool that is flipped to the outer leaflet. Moreover, after subjecting these proteoliposomes to quenching by dithionite on ice, at a temperature that allows the reduction reaction but blocks the translocation, prior to the addition of vancomycin-TMR similar results were obtained (Supplementary Figure S7). Thus, a much larger Lipid II pool is accessible for vancomycin in the presence of FtsW, confirming its role as a Lipid II transporter.
Figure 7.
Increased accessibility of Lipid II to vancomycin in proteoliposomes containing FtsW. Vesicles without any protein or reconstituted with the control protein KcsA or with FtsW were prepared according to the procedure described before. FRET measurements were then carried out at 14°C after addition of vancomycin-TMR. FtsW-containing vesicles display a much higher FRET signal than the protein-free and KcsA-containing vesicles. All measurements are representative of at least three independent experiments. A.U.: arbitrary units.
Discussion
The enzymes involved in the assembly of cell wall peptidoglycan have been known for decades. However, the protein that transports the lipid-linked (peptidoglycan) precursors across the cytoplasmic membrane was the last key step in this fundamental process that remained to be identified. Attempts to unravel this route have been hampered by the unavailability of convenient assays, allowing for studying the flippase activity and the biochemical events of transport experimentally. On the basis of fluorescence studies to assay the transport of NBD-Lipid II across bacterial inner membrane vesicles and on biochemical evidence accumulated from the reconstituted system, this work reveals that FtsW is a Lipid II transporter. In view of the identification of FtsW as the transporter of peptidoglycan subunits, it is anticipated that other members of SEDS family such as the FtsW homologues, RodA and SpoVE, are also likely to participate in the translocation of Lipid II during cell elongation and spore peptidoglycan synthesis in B. subtilis, respectively. Moreover, our findings argue against the latest reports that proposed MviN (MurJ) as the putative Lipid II flippase (Inoue et al, 2008; Ruiz, 2008). In this respect, our results are consistent with the finding that was very recently reported, describing that MviN homologues in B. subtilis are not essential for growth and do not seem to have a role as the flippase of Lipid II (Fay and Dworkin, 2009). Furthermore, FtsW was encountered among the preserved proteins, previously revealed by the bioinformatics search for candidates for Lipid II flippase (Ruiz, 2008). In spite of this, a role of this protein as a Lipid II flippase was not considered, as FtsW was absent in the peptidoglycan-bearing Vesicomyosocious okutanii (that does possess RodA), and two of its homologues were present in the peptidoglycan-less Mollicute Eubacterium dolichum DSM 3991 (Ruiz, 2008). Yet, there are several examples of proteins specifically required for a defined process; such as, peptidoglycan biosynthesis, in bacteria possessing a cell wall that are also encountered in bacteria lacking this biosynthetic route. In this regard, it is worthwhile to note the recent elaboration of the existence of a functional and essential Lipid II biosynthesis pathway in the cell wall-less bacteria Chlamydia and Wolbachia (Henrichfreise et al, 2009).
Given the apparent requirement of MviN for peptidoglycan synthesis, it is however not excluded that this protein might also be involved in a different way, for example, by interacting with other (redundant) proteins required for this process. To confirm that MviN has no direct role in transport of Lipid II, we tested the effect of this protein in bacterial membrane vesicles as well as in model membranes (Supplementary Figures S8 and S9). In contrast to overexpression of FtsW (Figure 3B), overexpression of MviN did not result in enhanced transport of NBD-labelled Lipid II (Supplementary Figure S8). Similarly, co-reconstitution of purified MviN and Lipid II in vesicles did not lead to an increased accessibility of NBD-Lipid II for dithionite (Supplementary Figure S9), showing that this protein has no direct role in Lipid II translocation.
Although the mechanism underlying the flipping route remains to be elucidated, the present work identifies for the first time a specific protein as a transporter of complex sugars across biological membranes. This process was previously demonstrated to be independent of any metabolic energy. Therefore, it is conceivable that the transport process occurs through facilitated diffusion, allowing Lipid II to equilibrate between the two leaflets of the inner membrane. As the peptidoglycan synthesis was shown to occur very fast, the transmembrane movement of Lipid II should at least match this speed that was estimated to occur at a rate of ∼5000 molecules s−1 (Sanyal and Menon, 2009). As expected, in our assays the translocation process was shown to occur rapidly. The transbilayer movement of Lipid II was accomplished within few seconds to minutes after addition of dithionite or vancomycin, respectively. The time required for the reduction reaction by dithionite and or binding of vancomycin poses therefore a constraint for studying the kinetics of the transport process.
The obvious difference in rate of transport between our two assays points to the existence of a regulatory process in the biological membranes that is absent in the reconstituted system and that FtsW-mediated transmembrane transport is not the rate-limiting step.
In this regard, it is worthwhile to note that, in the bacterial membrane vesicles, not all of the synthesized NBD-labelled Lipid II was transported. This can be deduced by comparing the FRET signal or ratio of the acceptor/donor fluorescence (578/534 nm) in Figure 2 (3.9, here all NBD-labelled Lipid II molecules are bound to vancomycin-TMR) to the maximal ratio obtained in Figure 3C (1.9 for membranes from FtsW overproducing strain). This plateau level of translocation is most likely caused by an obstruction of the transporter channel by newly transported NBD-labelled Lipid II that is bound by vancomycin-TMR. Hence, the maximal FRET signal in the translocation assay reflects the actual amount of (active) transporters in the system, thus explaining the increased FRET signal for membranes with increased levels of FtsW and decreased FRET signal for membranes with depleted levels of FtsW.
In addition to its necessity as an essential protein in cell division, FtsW is present in virtually all eubacteria that synthesize a peptidoglycan cell wall and has no counterpart in humans. Therefore, revealing its specific function will not only further our understanding of the fundamental cellular processes involved in bacterial growth but may also prove valuable in the design of novel antibiotics.
Materials and methods
Phospholipids and bacterial cell wall precursors
All phospholipids were purchased from Avanti Polar Lipids (Alabaster, AL). Stock solutions were prepared in chloroform/methanol (1:1, v/v) and stored under nitrogen at −20°C. Phospholipids concentration was determined with a lipid phosphorus assay according to Rouser et al, 1970. The lysine form of UDP-MurNAc-pentapeptide was purified from Staphylococcus simulans as previously described (Kohlrausch and Holtje, 1991). This compound was labelled with NBD chloride at the lysine of the pentapeptide moiety residue following the same procedure as reported before for the labelling of UDP-MurNAc-pentapeptide with pyrenesulphonylchloride (Breukink et al, 2003). The synthesis and purification of the cell wall precursor NBD-labelled Lipid II was performed as described (Breukink et al, 2003; van Dam et al, 2007). Undecaprenyl phosphate was extracted from Laurus nobilis leaves (Breukink et al, 2003) and phosphorylated as described (Breukink et al, 2003). All other chemicals were obtained from Sigma.
Synthesis of vancomycin-TMR
Fluorescently labelled vancomycin was used as the acceptor in the FRET assay (see below). To this end, vancomycin was coupled via its carboxyl-terminus to TMR (Molecular Probes). In brief, the labelling procedure consisted of assembling a reaction comprising 1000 nmol vancomycin HCl (MP Biomedicals), 250 nmol TMR, 300 nmol 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and 300 nmol 1-hydroxy-7-azobenzotriazole in DMF in a total volume of 250 μl, followed by overnight incubation under nitrogen in the dark at room temperature. The vacuum-desiccated mixture was then dissolved in 60 μl water/acetonitrile/trifluoro acetic acid (82:18:0.09). After separation on a RP-C18 HPLC column, the fractions containing labelled vancomycin were collected and pooled. Using a rotary evaporator the residual acetonitrile and trifluoro acetic acid were removed and the remaining sample was dehydrated on a freeze dryer. The resulting dried material was dissolved in 250 μl water. The overall yield of the reaction was estimated to be 54%.
Expression and purification of recombinant proteins
The expression and purification of His-tagged KcsA were carried out as described previously (van Dalen et al, 2002). His-tagged MraY from B. subtilis was purified as published earlier (Bouhss et al, 2004). The strains and plasmids used in this study are listed in Supplementary Table S1.
Purified His-tagged SecYEG from E. coli (Van der Does et al, 1998) was a generous gift from Dr Ilja Küsters and Prof Dr Arnold Driessen (Molecular Microbiology, University of Groningen, The Netherlands).
The E. coli strain used for the expression of MviN as well as purified His-tagged MviN from E. coli (to be published elsewhere) were a generous gift from Prof Dr Dominique Mengin-Lecreulx (Institut de Biochimie et Biophysique Moléculaire et Cellulaire, Université Paris-Sud, France).
The purification of recombinant FtsW was carried out as follows: E. coli strain C43(DE3) harbouring the plasmid pDML2400 (encoding N-terminal His6-FtsW) was grown at 37°C in LB medium supplemented with 50 μg/ml of kanamycin to an OD600 of 0.6. Expression of the proteins was induced with 1 mM IPTG. Growth was continued for 5 h. Cells were collected by centrifugation and resuspended in a buffer containing 50 mM Tris–HCl, pH 8.0, 50 mM NaCl, 1 mM PMSF and complete EDTA-free protease inhibitors (Roche, Germany). The bacterial cells were lysed by passing twice through a cell disruptor (pressure setting 2 kbar) and subsequently centrifuged for 10 min 12 000g at 4°C. The membranes were pelleted by centrifugation at 200 000g for 45 min at 4°C, and then resuspended and washed in a buffer comprising 20 mM Tris–HCl, pH 8.0, 500 mM NaCl, 10% glycerol and complete EDTA-free protease inhibitors. After centrifugation, the pellet was resuspended in 50 mM Hepes, pH 7.5, 500 NaCl and 10% glycerol. To solubilize the membranes the resulting mixture was incubated with 0.5% LAPAO (Anatrace) for 1 h at 4°C. The proteins, recovered in the supernatant after centrifugation at 200 000g for 45 min at 4°C, were purified by Ni-affinity chromatography.
The purification procedure consisted of incubating the solubilized membranes with 5 ml of Ni Sepharose High Performance beads (GE Healthcare) for 1–2 h at 4°C. The beads were equilibrated with 50 mM Hepes, pH 7.5, 500 mM NaCl, 10 mM imidazole and 0.1% LAPAO. On-column detergent exchange was then carried out. The column was extensively washed with a 50 mM Hepes, pH 7.5, 300 mM NaCl, 10% glycerol, 1 mM n-dodecyl-β-D-maltopyranoside (DDM, Anatrace) and 10 mM imidazole buffer. The proteins were eluted with a stepwise increasing concentration of imidazole ranging from 20 to 250 mM in a 50 mM Hepes, pH 7.5, 300 mM NaCl and 1 mM DDM buffer. His-tagged FtsW was recovered in fractions eluting between 100 and 200 mM imidazole. Fractions containing FtsW were pooled and concentrated using Amicon ultra-4 centrifugal filter devices 10K NMWL (Millipore) to remove imidazole. The sample was then subjected to a second purification step using a Ni-charged HiTrap chelating column of 1 ml. Washing and eluting of the proteins were subsequently carried out as described above. Integrity and purity of the protein was assessed by SDS–PAGE and immunoblotting. The protein concentration of the isolated His-tagged protein was determined with BCA reagent kit, according to the manufacturer's protocol (Pierce, Rockford, IL, USA), and densitometry of the Coomassie Brilliant Blue stained gel using bovine serum albumin as standard.
Dithionite reduction assay
Preparation of proteoliposomes. For the dithionite reduction assay, LUVs containing fluorescently labelled Lipid II were prepared. This was achieved by mixing 60 mol% DOPC, 25 mol% DOPE, 15 mol% DOPG from stock solutions. For the incorporation of Lipid II, a 0.1 mol% of NBD-Lipid II was added to the mixture. Lipids were dried by nitrogen stream, followed by vacuum desiccation for 2 h. The obtained lipid films were hydrated with a buffer composed of 10 mM Hepes-KOH, pH 8, 100 mM NaCl, 5 mM KCl and 1 mM MgSO4 to a lipid–phosphate (LPi) concentration of ∼5 mM. Subsequently, the vesicle suspension was subjected to 10 cycles of freezing and thawing. Finally, unilamellar vesicles were obtained after 10 rounds of extrusion through 200 nm membrane filters (Anotop 10, Whatman, Maidstone UK).
Reconstitution of proteins into proteoliposomes. The proteoliposomes were incubated with proteins. For reconstitution of proteins 350 μl LUVs (5 mM LPi) were first solubilized with 8 mM Triton X-100, followed by the addition of a purified protein. The final concentration of the proteins was adjusted to a protein–phospholipid molar ratio of ∼1:20 000. This was followed by incubation with gentle agitation for 1 h at 4°C. Samples were then supplemented with 100 mg/ml Bio-Beads SM-2 Adsorbent (Bio-Rad Laboratories Inc.) to remove the detergent. After 2 h of incubation at 4°C, another 100 mg/ml fresh Bio-Beads suspension was added to the micelle solution and a second incubation overnight at 4°C was undertaken. Thereafter, a third incubation with 100 mg/ml fresh Bio-Beads was performed for 2 h. Subsequently, the vesicles were collected by ultracentrifugation at 435 000g for 30 min and resuspended in 200 μl 10 mM Hepes-KOH, pH 8, 100 mM NaCl, 5 mM KCl and 1 mM MgSO4.
Flop and fluorescence measurement. The in-to-out translocation (flop) of NBD-Lipid II in LUVs and proteoliposomes was measured by determining the percentage of NBD fluorescence that is not available for reduction by 8 mM sodium dithionite, added from a 1 M stock solution in 1 M Tris, pH 11 (Kol et al, 2001; van Dam et al, 2007). When required, excess of dithionite was removed by supplementing the mixture with a molar excess of 20 mM potassium ferricyanide (III), (Sigma-Aldrich, Germany), added from a 1 M stock solution in water. After quenching, 10 μl of a 10% (w/v) solution of Triton X-100 in water was added to permeabilize the membrane and make all remaining NBD-label available for dithionite reduction.
Measurements were performed at 20°C in 1.25 ml buffer in a quartz cuvette using an SLM Aminco SPF 500C fluorimeter. The excitation and emission wavelengths were adjusted to 481 and 534 nm, respectively. Emission spectra were recorded between 500 and 620 nm.
FRET-based translocation assay
Translocation of NBD-Lipid II in isolated E. coli membrane vesicles was measured according to the method published by van Dam et al, 2007 with slight modifications. In this assay, FRET was used as a means of measuring Lipid II transport across the cytoplasmic membrane. To this end, RSO vesicles were isolated from various E. coli strains as indicated and prepared in 50 mM potassium phosphate buffer (pH 7.5) containing 10 mM NaCl and 1 mM MgSO4 as described (Kol et al, 2001). NBD-UDP-MurNAc-pentapeptide (250 pmol) and UDP-GlcNAc (10 nmol) were incorporated in the membrane vesicles (125 nmol lipid Pi) by performing two steps of freezing and thawing on ice in a volume of 45 μl 50 mM potassium phosphate buffer (pH 7.5) comprising 10 mM NaCl and 1 mM MgSO4. This procedure resulted in the incorporation of 4% of the NBD-UDP-MurNAc-pentapeptide and UDP-GlcNAc in the vesicles, as determined using radiolabelled versions of the precursors. NBD-Lipid II synthesis occurred during the incubation of the samples on ice. After washing, the membrane pellet was resuspended into 1.25 ml of the same buffer, and fluorescence spectra were recorded between 500 and 620 nm in the presence or absence of 68 pmol vancomycin-TMR at 14°C at different time points during 30 min.
Supplementary Material
Acknowledgments
We thank Mandy Lutters for synthesis of Lipid II. This work was supported by the European Commission EUR-INTAFAR (KSHM-CT-2004-512138) network and by ZonMW (project nr 20500001).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bouhss A, Trunkfield AE, Bugg TDH, Mengin-Lecreulx D (2008) The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev 32: 208–233 [DOI] [PubMed] [Google Scholar]
- Bouhss A, Crouvoisier M, Blanot D, Mengin-Lecreulx D (2004) Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J Biol Chem 279: 29974–29980 [DOI] [PubMed] [Google Scholar]
- Breukink E, van Heusden HE, Vollmerhaus PJ, Swiezewska E, Brunner L, Walker S, Heck AJR, de Kruijff B (2003) Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J Biol Chem 278: 19898–19903 [DOI] [PubMed] [Google Scholar]
- Breukink E, de Kruijff B (2006) Lipid II as a target for antibiotics. Nat Rev Drug Discov 5: 321–332 [DOI] [PubMed] [Google Scholar]
- Boyle DS, Khattar MM, Addinall SG, Lutkenhaus J, Donachie WD (1997) ftsW is an essential cell-division gene in Escherichia coli. Mol Microbiol 24: 1263–1273 [DOI] [PubMed] [Google Scholar]
- Cabeen MT, Jacobs-Wagner C (2007) Skin and bones: the bacterial cytoskeleton, cell wall, and cell morphogenesis. J Cell Biol 179: 381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Blaauwen T, de Pedro MA, Nguyen-Distèche M, Ayala JA (2008) Morphogenesis of rod-shaped sacculi. FEMS Microbiol Rev 32: 321–344 [DOI] [PubMed] [Google Scholar]
- Höltje JV (1998) Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev 62: 181–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay A, Dworkin J (2009) Bacillus subtilis homologs of MviN (MurJ), the putative Escherichia coli Lipid II flippase, are not essential for growth. J Bacteriol 191: 6020–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraipont C, Alexeeva S, Wolf B, van der Ploeg R, Schloesser M, den Blaauwen T, Nguyen-distèche M (2011) The integral membrane FtsW protein and peptidoglycan synthase PBP3 form a sub-complex in Escherichia coli. Microbiology 157: 251–259 [DOI] [PubMed] [Google Scholar]
- Henrichfreise B, Schiefer A, Schneider T, Nzukou E, Poellinger C, Hoffman TJ, Johnston KL, Moelleken K, Wiedemann I, Pfarr K, Hoerauf A, Sahl HG (2009) Functional conservation of the lipid II biosynthesis pathway in the cell wall-less bacteria Chlamydia and Wolbachia: why is lipid II needed? Mol Microbiol 73: 913–923 [DOI] [PubMed] [Google Scholar]
- Inoue B, Murata Y, Takahashi H, Tsuji N, Fujisaki S, Kato J (2008) Involvement of an essential gene, mviN, in murein synthesis in Escherichia coli. J Bacteriol 190: 7298–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlrausch U, Holtje JV (1991) One-step purification procedure for UDP-N-acetylmuramyl-peptide murein precursors from Bacillus cereus. FEMS Microbiol Lett 62: 253–257 [DOI] [PubMed] [Google Scholar]
- Kol MA, de Kroon AI, Rijkers DT, Killian JA, de Kruijff B (2001) Membrane-spanning peptides induce phospholipid flop: a model for phospholipid translocation across the inner membrane of E coli. Biochemistry 40: 10500–10506 [DOI] [PubMed] [Google Scholar]
- Kol M, van Dalen A, de kroon AI, de Kruijff B (2003) Translocation of phospholipids is facilitated by a subset of membrane-spanning proteins of the bacterial cytoplasmic membrane. J Biol Chem 278: 24586–24593 [DOI] [PubMed] [Google Scholar]
- Matsuhashi M (1994) Utilization of lipid-linked precursors and the formation of peptidoglycan in the process of cell growth and division: membrane enzymes involved in the final steps of peptidoglycan synthesis and the mechanism of their regulation. In Bacterial Cell Wall, Ghuysen J-M, Hakenbeck R (eds) pp 55–71. Amsterdam: Elsevier Science BV [Google Scholar]
- Mercer K, Weiss DS (2002) The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J Bacteriol 184: 904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JC, Sleight RG (1991) Fluorescence assay for phospholipid membrane asymmetry. Biochemistry 30: 11819–11827 [DOI] [PubMed] [Google Scholar]
- Pastoret S, Fraipont C, den Blaauwen T, Wolf B, Aarsman MEG, Piette A, Thomas A, Brasseur R, Nguyen-Distèche M (2004) Functional analysis of the cell division protein FtsW of Escherichia coli. J Bacteriol 186: 8370–8379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G, Fkeischer S, Yamamoto A (1970) Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5: 494–496 [DOI] [PubMed] [Google Scholar]
- Ruiz N (2008) Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proc Natl Acad Sci USA 105: 15553–15557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Frank CG, Menon AK (2008) Distinct flippases translocate glycerophospholipids and oligosaccharide diphosphate dolichols across the endoplasmic reticulum. Biochemistry 47: 7937–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Menon AK (2009) Flipping lipids: why an’ what's the reason for? ACS Chem Biol 4: 895–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam V, Sijbrandi R, Kol M, Swiezewska E, de Kruijff B, Breukink E (2007) Transmembrane transport of peptidoglycan precursors across model and bacterial membranes. Mol Microbiol 64: 1105–1114 [DOI] [PubMed] [Google Scholar]
- Van Dalen A, Hegger S, Killian JA, de Kruijff B (2002) Influence of lipids on membrane assembly and stability of the potassium channel KcsA. FEBS Lett 525: 33–38 [DOI] [PubMed] [Google Scholar]
- Van der Does C, Manting EH, Kaufmann A, Lutz M, Driessen AJ (1998) Interaction between SecA and SecYEG in micellar solution and formation of the membrane-inserted state. Biochemistry 37: 201–210 [DOI] [PubMed] [Google Scholar]
- Vehring S, Pakkiri L, Schröer A, Alder-Baerens N, Herrmann A, Menon AK, Pomorski T (2007) Flip-flop of fluorescently labelled phospholipids in proteoliposomes reconstituted with Saccharomyces cerevisiae microsomal proteins. Eukaryotic cell 6: 1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W, Bertsche U (2008) Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochem Biophys Acta 1778: 1714–1734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.