Abstract
Grb2 is a ubiquitously expressed adaptor protein, which activates Ras and MAP kinases in growth factor receptor signalling, while in B-cell receptor (BCR) signalling this role is controversial. In B cell lines it was shown that Grb2 can inhibit BCR-induced Ca2+ signalling. Nonetheless, the physiological role of Grb2 in primary B cells is still unknown. We generated a B-cell-specific Grb2-deficient mouse line, which had a severe reduction of mature follicular B cells in the periphery due to a differentiation block and decreased B-cell survival. Moreover, we found several changes in important signalling pathways: enhanced BCR-induced Ca2+ signalling, alterations in mitogen-activated protein kinase activation patterns and strongly impaired Akt activation, the latter pointing towards a defect in PI3K signalling. Interestingly, B-cell-specific Grb2-deficient mice showed impaired IgG and B-cell memory responses, and impaired germinal centre formation. Thus, Grb2-dependent signalling pathways are crucial for lymphocyte differentiation processes, as well as for control of secondary humoral immune responses.
Keywords: adaptor proteins, B cells, B-cell development, calcium signalling, humoral immune response
Introduction
Adaptor proteins have a critical role in signalling processes in lymphocytes by linking antigen receptor activated protein tyrosine kinase (PTK) cascades to generation of second messengers. Antigen binding to the B-cell receptor (BCR) triggers activation of PTKs and phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) within the tail of Igα/Igβ, which are part of the BCR complex. Phosphorylated ITAMs are bound by the PTK Syk via its Src homology (SH)2 domains. This leads to the activation of Syk and a prominent substrate of Syk is the adaptor protein SH2 domain-containing lymphocyte protein of 65 kDa (SLP65). Phosphorylated SLP65 recruits phospholipase C-γ2 (PLC-γ2) and Bruton's tyrosine kinase via their SH2 domains and this trimolecular complex is translocated to the inner plasma membrane. PLC-γ2 that is activated by these events catalyses the generation of the second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) from the plasma membrane lipid phosphatidyl-4,5-biphosphate (PIP2). IP3 binds to the IP3 receptor (IP3R), which triggers Ca2+ release from the endoplasmatic reticulum and subsequently Ca2+ influx through the plasma membrane. DAG activates protein kinase C and Ras guanine nucleotide releasing protein (RasGRP) and in this way triggers nuclear factor of κ enhancer in B cells (NF-κB) and mitogen-activated protein kinase (MAPK) pathways (Niiro and Clark, 2002; Kurosaki et al, 2010).
The adaptor protein growth factor receptor bound protein 2 (Grb2) consists of one central SH2 domain that is flanked by two SH3 domains. Thus, Grb2 is a classical adaptor protein with no catalytic protein domains. Grb2 was discovered as a protein that is recruited to growth factor receptors via its SH2 domain (Lowenstein et al, 1992). Growth factor receptors are activated and crosslinked by ligand binding and transphosphorylate their intracellular domains with their intrinsic tyrosine kinase activity, which leads to SH2-mediated Grb2 recruitment. Grb2 itself constitutively binds with its two SH3 domains to proline-rich regions of the protein Sos, a guanine nucleotide exchange factor (GEF). Sos-mediated exchange of GDP for GTP activates Ras, which in turn activates the protein kinase Raf and subsequently the extracelluar signal-regulated kinase (Erk), which is one of the MAPK pathways (Buday and Downward, 1993; Egan et al, 1993). Surprisingly, BCR, as well as T-cell receptor (TCR), stimulation does not seem to trigger the Ras/MAPK pathway via Grb2/Sos activation. Instead, it was discovered in the chicken B cell line DT40 and in mice that RasGRPs, which are recruited via DAG to the membrane, are the critical GEFs for Ras activation in BCR signalling (Oh-hora et al, 2003; Coughlin et al, 2005). Although these studies showed that Grb2 and Sos are dispensable for BCR-induced Ras activation, another report showed that RasGRPs and Sos can cooperate in Ras activation (Roose et al, 2007).
Studies in Grb2-deficient DT40 B cells also revealed a role of Grb2 in Ca2+ signalling. Deficiency of Grb2 enhanced BCR-induced Ca2+ responses both from intracellular stores, as well as extracellular Ca2+ influx (Stork et al, 2004). The membrane adaptor protein Dok-3 was found to be responsible for this inhibitory effect of Grb2 in DT40 cells (Stork et al, 2007). Grb2 bound via its SH2 domain to tyrosine phosphorylated Dok-3, which has a typical YxN Grb2-binding motif, and Grb2 was recruited to the membrane by this interaction. Another binding partner of Grb2 is the LAT homologue non-T-cell activation linker (NTAL), which was suggested to counteract this inhibitory pathway by recruiting Grb2 and preventing its inhibitory role on Ca2+ signalling (Stork et al, 2004). Since NTAL is markedly upregulated on mature B cells and since DT40 is an immature B cell line, this suggests a differential regulation of Ca2+ signalling in immature and mature B cells (Neumann et al, 2009). Well-known inhibitory co-receptors such as CD22, CD72 and the FcγRIIb have Grb2-binding sites as well. Whether binding of Grb2 to these inhibitory receptors contributes to the modulation of Ca2+ signalling is unknown. It should be noted that all of these studies have been done in B cell lines so far, the role of Grb2 in primary B-cell Ca2+ signalling is not known. The interactome of Grb2 in B cell lines has recently been examined by a proteomic approach (Neumann et al, 2009). Grb2 binds to a multitude of signalling proteins, apart from the mentioned proteins involved in MAPK and Ca2+ signalling also to several proteins, which are involved in other signalling pathways, for example PI3K signalling. Grb2 interacts to the regulatory P85 subunits of PI3K, as well as to key activators of PI3K signalling, CD19 and BCAP. The functional role of Grb2 in PI3K signalling, a signalling pathway that is important for tonic BCR signalling and survival of B cells (Srinivasan et al, 2009), is completely unknown.
Interestingly, a unique tyrosine motif (ITT for immunoglobulin tail tyrosine) was recently discovered in the cytoplasmic tail of membrane-bound IgG and IgE, which upon antigen binding gets tyrosine phosphorylated and recruits Grb2 (Engels et al, 2009). This Grb2 recruitment leads to enhanced Ca2+ signalling of membrane-bound IgG or IgE, in contrast to IgM that lacks this ITT. In another study it was found that the cytoplasmic tail of IgG is crucial for a rapid IgG clustering and enhanced signalling response; however, the ITT was dispensable for this function (Liu et al, 2010). Mouse models also showed the enhanced signalling capacity of IgG in comparison to IgM (Horikawa et al, 2007; Waisman et al, 2007). Memory B cells have enhanced B-cell responses giving rise to rapid production of high affinity IgG antibodies. However, it is still an open question whether in vivo memory B-cell responses depend on the IgG ITT motif and its Grb2 recruitment.
Although Grb2 has been implicated in several B-cell signalling pathways, most of these studies were done in B cell lines and it is therefore not clear how much of the findings are relevant for physiological B-cell functions. Grb2-deficient mice have been generated, but are embryonic lethal (Cheng et al, 1998). To study the physiological function of Grb2 in B lymphocytes, we generated B-cell-specific Grb2-deficient mice. These mice show defects in B-cell maturation, various changes in signalling pathways and show a defect in secondary IgG responses, as well as in B-cell memory responses.
Results
Defective B-cell maturation in B-cell-specific Grb2−/− mice
In order to study the physiological role of Grb2 in B cells, B-cell-specific Grb2−/− mice were generated. A targeting vector was used for transfection of BALB/c embryonic stem cells, to introduce two loxP sites flanking exon 2 of the grb2 gene. Exon 2 codes for part of the first SH3 domain of Grb2 and its deletion would lead to a non-functional Grb2 protein. We identified correctly targeted ES cell clones by PCR and Southern blot (Supplementary Figure S1A and B), generated chimeric mice and obtained germline transmission with two independently derived clones. The derived Grb2-floxed mice (Grb2fl/fl mice) were mated with mb1-cre mice (Hobeika et al, 2006) to generate mice with a B-cell-specific deletion of grb2 (Grb2fl/fl mb1cre/+ mice). In Grb2fl/fl mb1cre/+ mice, no Grb2 protein was detectable in splenic B cells and only traces were visible in the B220+ bone marrow cells, while the protein was expressed normally in splenic T cells (CD5hi B220−) (Supplementary Figure S1C). Thus, we had obtained a B-cell-specific deletion of the Grb2 protein in Grb2fl/fl mb1cre/+ mice, which are called ‘B-cell-specific Grb2−/− mice’ throughout the text.
B-cell-specific Grb2−/− mice showed no significant changes in pro- and pre-B cells, nor in immature B-cell numbers in the bone marrow; however, there was a tendency towards reduced cell numbers in fractions A, B, D and E (Li et al, 1993) (Figure 1A; Supplementary Table S1). In contrast, we observed a dramatic reduction of recirculating, mature (IgM+B220hi, fraction F) B cells in the bone marrow of B-cell-specific Grb2−/− mice (Figure 1A; Supplementary Table S1). In the periphery, we observed a strong reduction of B cells in the blood of B-cell-specific Grb2−/− mice (Figure 1B). Also, numbers of mature B cells in the spleen were reduced to one-half. In addition, numbers of transitional B cells (T1 and T2) were reduced, whereas marginal zone (MZ) B cells were increased relatively, but not in total numbers (Figure 1C). Similarly, total numbers of B1 cells in the spleen and peritoneal cavity were not changed (Figure 1D; Supplementary Table S1).
Figure 1.
Grb2fl/fl mb1cre/+ mice have decreased numbers of mature B cells in the bone marrow and decreased numbers of transitional and mature B cells in the periphery. (A) Bone marrow cells were stained according to the Hardy fractions A-F and with B220 versus IgM. Typical examples are shown. Bottom right, quantitative analysis of Hardy fractions A-F in absolute cell numbers. (B) Blood cells, (C) splenic cells or (D) peritoneal lavage cells are analysed by the indicated markers and total cell numbers are given for some populations in bar diagrams. *P<0.05; **P<0.01; ***P<0.001. Each experiment was done at least four times. T1, transitional type 1; T2, transitional type 2; MZ, marginal zone; M, mature; FO, follicular B cells.
To address Grb2−/− B-cell development in a competitive situation, we performed an adoptive transfer experiment by injecting a 50:50 mixture of bone marrow cells of CD45.1+ wild-type and Grb2fl/fl mb1cre/+ (CD45.1−) mice into lethally irradiated Rag1−/− mice. All five reconstituted mixed bone marrow chimeras showed a reconstitution with about 20–30% CD45.1+ WT cells and 70–80% CD45.1− mutant cells in non-B-cell populations (Figure 2A and B). This shift from the expected ratio of 50:50 may be explained by different amounts of haematopoietic stem cells in the bone marrow mixture. In contrast, in both pro/pre-B cells and B cells in the bone marrow, there was a strong shift towards 70 or 90% CD45.1+ WT cells, respectively (Figure 2A). The same shift of the reconstitution in favour of wild-type cells was seen in mature follicular B cells in the periphery (90% CD45.1+ WT cells) (Figure 2B). Thus, there was a strong competitive advantage for wild-type B cells compared with Grb2−/− B cells. The preferred reconstitution of wild-type cells started in pro-B-cell population in the bone marrow, and was subsequently increasing in pre-B and immature B cells (Supplementary Figure S2A). MZ B cells or B1 cells in the spleen of reconstituted mice comprised 56 or 44% wild-type CD45.1+ cells, respectively, clearly indicating that these cells are less affected by the Grb2 deficiency (Figure 2B).
Figure 2.
Grb2−/− B cells have a competitive disadvantage to wild-type B cells in vivo. B-cell subsets (left) in the bone marrow (A) and spleen (B) of mixed bone marrow chimaeras, generated by injection of Grb2fl/fl mb1cre/+ (CD45.2) and wild-type (CD45.1) bone marrow cells (1:1 mixture) into RAG1−/− recipients, analysed 7–8 weeks after transfer. Gated populations were separately analysed for percentage of CD45.1+ cells in histograms (middle). Numbers in histograms indicate percent of CD45.1+ cells±s.d. Statistical analysis of percentages of CD45.1+ cells within several gated populations is given in addition in bar diagrams (right). Five recipient mice were analysed which all showed similar results. T, T cells; non-B, non-B cells; pre-B, pre-B cells; immat. B, immature B cells; mat. B, mature B cells; B1, B1 cells; B2, B2 cells; MZ B, marginal zone B cells; Foll. B, follicular B cells.
Lower B-cell survival of Grb2−/− B cells and impaired BAFF-R upregulation
In order to examine whether the reduced number of B cells in the periphery of B-cell-specific Grb2−/− mice is a result of a proliferation defect, we performed an in vivo BrdU experiment by feeding mice BrdU in the drinking water. We observed no difference in BrdU incorporation in mature B cells (IgMloIgDhi) or T2 B cells (IgMhiIgDhi) from B-cell-specific Grb2−/− mice and control mice. However, we found a severe reduction of BrdU-positive cells among the T1/MZ B cells (IgMhiIgDlo) of B-cell-specific Grb2−/− mice (Figure 3A). T1 cells are short lived, recent emigrants from the bone marrow, so the lower BrdU incorporation in Grb2−/− mice could indicate lower proliferation of their precursors in the bone marrow. We also determined BrdU incorporation in bone marrow pre-B and immature B cells, but were not able to detect differences between the two groups of mice due to BrdU incorporation rates of 95% even after 2 days of feeding BrdU (data not shown). We next performed a BrdU pulse–chase experiment to examine the turnover of B cells. Both immature and mature Grb2−/− B cells showed a lower decline of BrdU labelling in the chase phase, indicating a lower turnover of Grb2−/− B cells (Figure 3B).
Figure 3.
Grb2−/− B cells show a lower turnover rate, a reduced survival rate in vitro and impaired BAFF-R upregulation. (A) BrdU incorporation of splenic B cells in mice, which were fed for indicated time periods with BrdU in drinking water. BrdU+ cells are shown in line diagrams for indicated populations. One typical example of three independent experiments is shown. Statistics are summarized results of five mice each. (B) Mice were fed for 21 days with BrdU (pulse) followed by indicated time points without BrdU (chase). Statistical analysis could only be done for day 73, because only at this time point there were three mice per group. At all other time points there were two control and three Grb2−/− mice. (C) Spontaneous apoptosis rates of sorted immature and mature B cells (shown on top before sorting) in medium without cytokines for indicated time periods measured by intracellular DAPI stainings. Shown are summarized results of three independent experiments. (D) BAFF-R expression is shown for immature (HSAhiB220lo) or mature (HSAloB220hi) B cells and mean fluorescence intensities (mfi) quantified from five mice each. *P<0.05; **P<0.01; ***P<0.001, P-values are only shown for significant differences.
The reduced number of peripheral Grb2−/− B cells could also be due to lower survival. In order to test this, we sorted immature or mature splenic B cells, cultivated them in vitro without cytokines and measured spontaneous apoptosis by intracellular DAPI staining. Both immature and mature B cells of B-cell-specific Grb2−/− mice showed slightly increased spontaneous apoptosis at early time points in vitro (Figure 3C). One important survival pathway for B cells is the BAFF/BAFF-receptor (BAFF-R) pathway (Sasaki et al, 2004). Normally, B cells do upregulate BAFF-R expression at the transition from the immature to the mature stage (Hsu et al, 2002). Strikingly, B cells from B-cell-specific Grb2−/− mice failed to upregulate BAFF-R expression during this transition (Figure 3D), suggesting that a failed BAFF response may contribute to lower survival of mature Grb2−/− B cells in vivo.
Increased Ca2+, but decreased JNK and PI3K signalling in B-cell-specific Grb2−/− mice
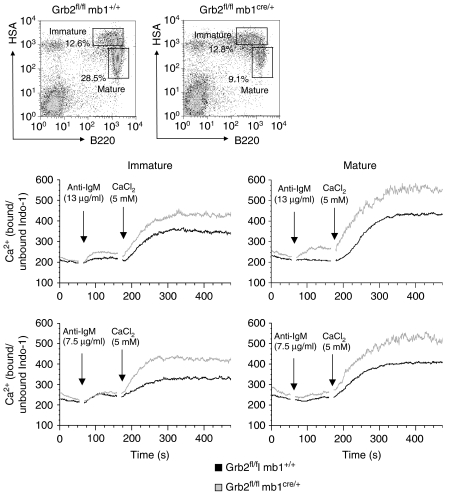
Grb2 is implicated in several signalling pathways. We first examined the BCR-induced Ca2+ response in Grb2−/− B cells, since Grb2 was reported to modulate Ca2+ responses (Stork et al, 2007). Modulation of Ca2+ signalling may be different in immature and mature B cells (Neumann et al, 2009) and since these populations were changed in their relative proportions in B-cell-specific Grb2−/− mice, we measured Ca2+ responses in immature and mature splenic B cells separately. In both B-cell populations of B-cell-specific Grb2−/− mice, the intracellular and extracellular Ca2+ signal was clearly increased (Figure 4). This shows that Grb2 has an inhibitory role on BCR-induced Ca2+ signalling.
Figure 4.
Enhanced Ca2+ signalling in B cells of Grb2fl/fl mb1cre/+ mice. Extracellular and intracellular calcium mobilization of Indo-1 loaded splenic B cells of Grb2fl/fl mb1cre/+ and control mice, stimulated with anti-IgM (Fab2) in Ca2+-free medium and then CaCl2 was added at indicated time point. Immature and mature B cells were analysed separately as indicated by HSA/B220 staining. Data represent typical results of four independent experiments.
In growth factor receptor signalling pathways, Grb2 is closely linked to MAPK signalling by recruiting the GEF Sos to Ras (Lowenstein et al, 1992; Buday and Downward, 1993; Egan et al, 1993). To study the role of Grb2 on MAPK signalling in B cells, we stimulated splenocytes of B-cell-specific Grb2−/− mice with anti-IgM and measured MAPK activation. The MAPK Erk and p38 phosphorylation patterns were normal, whereas JNK phosphorylation was attenuated by 30–70% in B cells of B-cell-specific Grb2−/− mice (Figure 5A). Interestingly, despite a normal Erk activation Grb2−/− B cells showed a tendency for lower Ras activity both before and a lower Ras activity after BCR stimulation (Figure 5B).
Figure 5.
Grb2−/− B cells show impaired Ras, JNK and AKT and CD22 signalling, but increased Syk activation. (A–C) Splenic B cells of Grb2fl/fl mb1cre/+ and control mice were stimulated for indicated time points with anti-IgM (Fab2) antibodies. (A, C) Lysates were analysed by western blot with phospho-specific antibodies. CD22 phosphorylation was analysed after CD22 IP from sorted mature B cells, blotted with anti-phospotyrosine antibody (B) Active Ras was precipitated with Raf1-binding domain and blotted with anti-Ras antibody. (A–C) One typical example of three experiments is shown. Diagrams below western blots show quantification of band intensities (phospho-specific bands divided by loading controls) in arbitrary units, summarized from at least three experiments each. *P<0.05; **P<0.01.
Proximal BCR signalling was studied by analysing Syk activation. Grb2−/− B cells had a higher Syk activation than wild-type cells after 5 min BCR stimulation (Figure 5C). Syk is upstream of PLC-γ2, which is a key regulator of Ca2+ mobilization (Scharenberg et al, 2007). Hence, a negative influence of Grb2 on Syk could contribute to the inhibitory role of Grb2 on Ca2+ mobilization. Ca2+ mobilization is negatively regulated by inhibitory receptors such as CD22 (Nitschke, 2009). It has been shown that CD22 inhibition is higher in mature than in immature B cells (Gross et al, 2009). We therefore looked for tyrosine phosphorylation of CD22 in sorted mature B cells and found a slightly impaired tyrosine phosphorylation of CD22 in Grb2−/− mature B cells at early time points after stimulation, but the difference was not significant (Figure 5C). Finally, we analysed the activation of Akt, which is downstream of PI3K signalling, a pathway important for tonic BCR survival signals (Pogue et al, 2000; Srinivasan et al, 2009). Interestingly, the Akt activation was strongly reduced in Grb2−/− B cells (Figure 5C).
Higher anti-IgM-induced proliferation and higher IgM secretion in B-cell-specific Grb2−/− mice
After anti-IgM and LPS stimulation, immature B cells (HSAhiB220lo) from B-cell-specific Grb2−/− mice showed higher proliferation rates than those of control mice. In contrast, Grb2-deficient mature B cells (HSAloB220hi) did not respond significantly different from control cells. Neither immature nor mature B cells of B-cell-specific Grb2−/− mice showed altered proliferation rates after anti-CD40 stimulation (Supplementary Figure S3). Consistent with higher proliferation rates, immature Grb2−/− B cells showed a higher upregulation of the activation marker B7.2 (CD86) after anti-IgM or LPS stimuli (Supplementary Figure S4A). In contrast, MHC-II was similarly upregulated on both Grb2−/− and control B cells (Supplementary Figure S4B). Interestingly, IgM titers in the serum of B-cell-specific Grb2−/− mice were about 10-fold increased while all other Ig isotypes were similar to controls (Figure 6A). This was due to a higher number of IgM-secreting cells in bone marrow and spleen (Figure 6B). We also observed a strong increase of IgMhi plasma cells in the red pulp of the spleen of B-cell-specific Grb2−/−mice, while there were no changes in primary follicles nor MZs visible (Figure 6C).
Figure 6.
Grb2fl/fl mb1cre/+ mice show higher serum IgM levels and higher numbers of IgM-secreting plasma cells. (A) Serum Ig levels of Grb2fl/fl mb1cre/+ and control mice were measured by ELISA. (B) Antibody-secreting cell numbers were determined by ELISPOT. Data represent typical results of three analyzed mice per group. (C) Spleen sections stained with anti-IgM (green) and MOMA (red). IgM+ plasma cells are indicated by arrows. Original magnification, × 5. Data represent typical results of three analysed mice per group. **P<0.01; ***P<0.001.
Impaired responses to a TI-2 antigen, impaired germinal centre formation and impaired memory B-cell responses in B-cell-specific Grb2−/− mice
B-cell-specific Grb2−/− mice were immunized with the thymus-independent type 2 (TI-2) antigen TNP-ficoll. Despite an increased TNP-specific IgM level before immunization, the IgM response was normal. In contrast, the IgG3 response to TNP-ficoll was clearly impaired in B-cell-specific Grb2−/− mice (Figure 7A). Next, we immunized mice with sheep red blood cells in order to study germinal centre (GC) formation. Interestingly, sheep red blood cell immunization led to an impaired number and smaller sizes of GCs, as detected by histology and quantification of PNA+ GCs (Figure 7B). Responses to thymus-dependent (TD) antigens were tested by immunizing mice with human cytomegalovirus (CMV)-derived virus-like particles (VLPs). VLPs are non-infectious enveloped hCMV particles (Weisel et al, 2010). While the VLP-specific IgM and primary IgG response was normal, the secondary IgG response was strongly impaired in B-cell-specific Grb2−/− mice (Figure 8A). VLPs from hCMV were chosen as an antigen, because after an adoptive memory B-cell transfer, recipient mice mount a memory antibody response, which is independent on T cell help (Hebeis et al, 2004). Thus, 90 days after the secondary immunization, splenic B cells were purified by complement-mediated T-cell lysis and anti-CD19 beads. The relative amount of IgG-positive cells within the splenic B-cell population of VLP immunized mice was determined by FACS. Similar percentages of IgG+ cells were detected in immunized control and B-cell-specific Grb2−/− mice (Figure 8B) and thus similar amounts of B cells were transferred into RAG1−/− recipients. Seven days after transfer, recipient mice were challenged with VLPs. While both types of recipient mice mounted similar IgM responses (not shown), the memory IgG responses of B-cell-specific Grb2−/− mice were strongly defective (Figure 8B and C). Furthermore, 21 days after VLP challenge, those RAG1−/− recipient mice, which had received Grb2−/− memory B cells, had a strongly reduced number of IgG-secreting cells (Figure 8D). VLPs should be devoid of viral DNA. To fully exclude that remaining traces of viral DNA stimulated TLR9, and that a defective B-cell memory response in B-cell-specific Grb2−/− mice may be due to lower TLR9 responses, Grb2−/− B cells were stimulated with TLR7 and TLR9 ligands. Grb2−/− B cells mounted even a higher response to these ligands (Supplementary Figure S5). Also, the defective IgG response was not due to a general class switching defect, as Grb2−/− B cells showed normal Ig class switch upon stimulation in vitro (Supplementary Figure S6). Taken together, these results indicate that Grb2 is crucial for secondary IgG responses to virus-derived TD antigens and is particularly important for B-cell memory responses.
Figure 7.
Grb2fl/fl mb1cre/+ mice have impaired TI type 2 responses and impaired germinal centre formation. (A) Mice were immunized with TNP-ficoll and anti-TNP IgM and IgG3 responses were determined by ELISA. (B) (Left) Spleen sections stained with PNA (green), IgM (red) and IgD (blue), 10 days after immunization with sheep red blood cells. Original magnification, × 5. (Right) Number of germinal centres per total area and percentage of germinal centres per total area are shown by bar diagrams. Data represent typical results of three analyzed mice per group. *P<0.05.
Figure 8.
Grb2fl/fl mb1cre/+ mice have impaired IgG secondary and memory responses to hCMV-derived virus-like particles. (A) Anti-virus-like particles (VLP)-specific IgM and IgG levels after immunization with hCMV VLPs. (B) In all, 90 days after the secondary immunization, B cells were purified by complement-mediated T-cell lysis and anti-CD19 beads and stained with anti-IgG. Not preimmunized mice: control mice without VLP immunizations. (C) In all, 2 × 106 cells each from (B) were i.v. injected into RAG1−/− mice. Seven days after transfer, recipient mice were immunized with VLPs again and VLP-specific IgG was measured. (D) In all, 21 days after VLP immunization (28 days after adoptive transfer), the number of IgG-secreting cells of RAG1−/− recipient mice from the spleen was determined by ELISPOT. *P<0.05; **P<0.01; ***P<0.001.
Discussion
The reduced numbers of immature and mature follicular B cells in peripheral lymphoid organs of B-cell-specific Grb2−/− mice could be caused by a decreased survival in peripheral lymphoid organs, as indicated by lower survival rates of these cells when analysed in vitro. On the other hand, there is also a lower input into the pool of newly formed peripheral B cells, as the BrdU experiments show. Possible mechanisms for lower survival could be the impaired BAFF-R upregulation and impaired PI3K/Akt signalling in Grb2−/− B cells. Both BAFF responses (Yan et al, 2001; Hsu et al, 2002; Sasaki et al, 2004), as well as PI3K signalling induced by tonic BCR signals (Srinivasan et al, 2009) are crucial for survival of peripheral B cells. Normally, BAFF-R expression increases when transitional B cells reach the mature B-cell stage (Hsu et al, 2002). In a recent model, it has been proposed that BCR signalling is needed to induce BAFF-R signal transmission (Stadanlick and Cancro, 2008). Grb2 could be a possible candidate for this pathway and the defective BAFF-R upregulation in B-cell-specific Grb2−/− mice could be due to impaired BCR signalling. Follicular B cells are stronger affected by the Grb2 deficiency than transitional B cells. Therefore, we cannot exclude that a differentiation block also contributes to the lower numbers of follicular B cells in the spleen.
We interpret the lower BrdU incorporation of Grb2−/− T1 B cells and the remaining higher BrdU levels of Grb2−/− B cells in the chase phase to be a consequence of an attenuated proliferation rate of pre-B cells in the bone marrow or of a lower exit rate of immature B cells from the bone marrow. This leads to a lower turnover of both the immature and mature B-cell population in the spleen. Bone marrow precursor cells were not strongly affected in B-cell-specific Grb2−/− mice. However, in a competitive situation of mixed bone marrow chimeras, there seems to be a more severe differentiation block, beginning at the pro-B-cell stage in the bone marrow. The pro-B-cells are the first population where the mb1 gene (coding for Igα) is expressed and where the Cre-mediated grb2 deletion can occur. The competitive disadvantage at this stage, even before pre-BCR expression, may be explained by defective growth factor signalling due to the Grb2 deficiency. Interestingly, the B-cell defect of B-cell-specific Grb2−/− mice does not affect MZ or B1 B-cell populations. This is remarkable, because these cell populations are often very sensitive in mice with signal protein deficiencies that have impaired BCR signalling (Niiro and Clark, 2002). Similarly to follicular B cells, MZ B cells are usually very much dependent on BAFF signalling (Fletcher et al, 2006). The relative B-cell paucity in the spleen of B-cell-specific Grb2−/− mice and the normal supply of BAFF may lead to a situation where MZ B cells win the competition against follicular B cells for BAFF supply, even if the BAFF receptor is not fully upregulated on mature B cells.
Grb2 seems to be an inhibitor of BCR-induced Ca2+ signalling, as our results in primary B cells clearly show. Mechanistically, Grb2 recruitment to the membrane adaptor Dok-3 may be responsible for this function, as has been implicated from DT40 cell line studies (Stork et al, 2007) and Dok-3-deficient mice (Ng et al, 2007). An inhibition of Syk activation, as found here in this study, or a block of activation of the inhibitory co-receptor CD22, which also has a Grb2-binding site (Nitschke, 2009), may contribute to this mechanism. The inhibitory role of Grb2 on Ca2+ signalling is similar in immature and mature B cells, which was unclear so far (Neumann et al, 2009).
Cell line studies of Grb2−/− or Sos−/− DT40 B cells showed a normal BCR-induced Ras and MAPK Erk activation (Oh-hora et al, 2003; Stork et al, 2004). In contrast, RasGRP3−/− DT40 cells had a defective Ras/Erk activation, indicating that the GEF RasGRP3 is crucial for Ras/Erk activation in B cells (Oh-hora et al, 2003). Both RasGRP1 and RasGRP3 have been shown to be crucial for Ras activation in murine primary B cells (Coughlin et al, 2005). However, a recent study showed an interplay of RasGRPs and Sos for full Ras activation in lymphocytes (Roose et al, 2007). Our results confirm that Grb2, and therefore the classical Grb2/Sos activation pathway, are dispensable for Erk activation. But, interestingly, Grb2−/− B cells nevertheless show an impairment of Ras activation, showing that Grb2 contributes to its activity. In contrast to Erk, JNK activation is impaired Grb2−/− B cells, suggesting an important activatory role of Grb2 for JNK signalling. Previous studies indicated that Ras can regulate the activation of Rho GTPases, which in turn can activate other MAPK members, such as JNK (Nimnual et al, 1998). It has also been suggested that for Erk a lower threshold of activation is sufficient, while JNK activation needs a stronger signal (Gong et al, 2001). Thus, the slightly impaired Ras activity in Grb2−/− B cells may still activate Erk, but may be not sufficient for JNK activation. Our results from B cells of B-cell-specific Grb2−/− mice are remarkably similar to thymocytes of haploid insufficient Grb2+/− mice. Also in those mice, which express reduced amounts of Grb2, impaired Ras activity combined with normal Erk, but deficient JNK activation has been reported (Gong et al, 2001). Similarly, thymocytes of T-cell-specific Grb2−/− mice have normal Erk, but impaired JNK activation (Jang et al, 2010), suggesting a similar MAPK regulation in T cells and B cells.
Akt is an important survival factor in B cells and its impaired activation in B cells of B-cell-specific Grb2−/− mice may provide a possible explanation for the reduced numbers of transitional and mature B cells. A reduced activation of Akt in DT40 cells for example leads to increased BCR-induced cell death (Pogue et al, 2000). Activation of Akt is completely abrogated in B cells, when B cells are treated with PI3K inhibitors, strongly indicating, that Akt is downstream of PI3K (Niiro and Clark, 2002). Since PI3K is activated by the adaptor protein BCAP, the CD19 co-receptor and by CD19-bound Vav and since Grb2 can bind to all of these phosphorylated proteins with its SH2 domain (Neumann et al, 2009), we propose the model that Grb2 association to these proteins is important for triggering the PI3K/Akt pathway.
B-cell-specific Grb2−/− mice have up to 10-fold increased serum IgM levels. Natural IgM is usually produced by B1 cells, as well as by MZ B cells (Martin et al, 2001), two populations which are not increased in B-cell-specific Grb2−/− mice. These mice also have a higher number of IgM-secreting plasma cells. Since Grb2−/− B cells show higher proximal BCR signalling and a higher upregulation of activation markers they could be preactivated and therefore could differentiate more readily to IgM producing plasma cells. While IgM antibody responses in B-cell-specific Grb2−/− mice are normal, these mice show a clear immune defect in switched IgG responses, both to a TI-2 antigen, as well as to a virus-derived particulate antigen. Similarly, the response to sheep red blood cells is diminished as detected by impaired GC formation.
hCMV-derived VLPs are known to generate a strong secondary response dominated by IgG (Weisel et al, 2010). While there was a clear defective secondary IgG response in B-cell-specific Grb2−/− mice to these virus particles, the soluble protein antigen NP-KLH given with adjuvant (alum) did not show defects in IgG responses (not shown). This difference may be explained by the structure of the antigen (particulate versus soluble) and the absence and presence of adjuvants (VLPs were injected in PBS, while NP-KLH was given as alum-precipitated protein, which leads to ongoing long antigen supply). Importantly, Grb2−/− memory B cells, which were transferred without T cells, clearly show that the defective memory B-cell IgG response is B-cell intrinsic. The memory B cells in our cell transfer system were transferred as total splenic B cells. In the future it will be important to determine the amount of antigen-specific memory B cells from challenged Grb2-deficient mice and transfer the same amount of antigen-specific memory B cells from both types of mice. Can the defective memory response be explained by the particular structure of the VLPs? VLPs are non-infectious particles and should not contain any viral DNA. The higher CpG-induced proliferation rates of Grb2−/− B cells in response to three TLR ligands (including CpG) exclude that defective TLR signalling contributes to the defective B-cell memory responses in the mutant mice.
The recently detected ITT motif in the cytoplasmic tail of IgG and IgE receptors, which enhances signalling and recruits Grb2 (Engels et al, 2009) could well explain the defective IgG secondary responses of B-cell-specific Grb2−/− mice. The significance of the Grb2-binding ITT sequences was so far controversial (Liu et al, 2010) and it was unclear whether this mechanism is responsible for enhanced B-cell memory responses, since in vivo experiments were lacking. Failing Grb2 recruitment to IgG tails may potentially affect activation, survival or antigen presentation of switched memory B cells. Since the restimulation of VLP-specific memory B cells functions without helper T cells (Hebeis et al, 2004), we think we can exclude defective antigen presentation as a mechanism. We found similar numbers of IgG+ B cells in immunized control and B-cell-specific Grb2−/− mice (before transfer), but lower IgG-secreting cells after challenge (after adoptive transfer). Thus, Grb2 is crucial for stronger activation/enhanced signalling of IgG memory B-cell responses or for their prolonged survival and these functions are most likely performed by binding to a specific motif in the tail of IgG. The crucial role of Grb2 in humoral immune responses indicates that this well-known adaptor protein may offer a completely new potential as a target for therapies. This is conceivable in cases of systemic autoimmune diseases where IgG autoantibody production could be blocked by Grb2 inhibitors.
Materials and methods
Generation of B-cell Grb2-deficient mice
We generated a targeting vector, containing a floxed second exon of the grb2 gene and flanking arms by PCR cloning. The short arm (∼1 kb) was cloned into the pRAPIDflirt vector (Hovelmeyer et al, 2007) by introduced NotI and BamHI sites. It was amplified with the following primers: Grb-in2S-Not, 5′-AGT GAT GCG GCC GCC ACC ACG CCT GAT GCT TCT A-3′ and Grb-in2AS-Bam, 5′-AGT GAG GAT CCT GGG CTT GTG GAA GGC TTA-3′ (restriction sites underlined). The long arm (∼5.3 kb) was cloned by one introduced FseI and one already present XhoI site and was amplified with the following primers: Grb-in3S-Fse, 5′-AGT GAC GGC CGG CCT TAG CCC ATA GAC ATT A-3′ and Grb-in4AS 5′-GTC ACC GTA AGT GCA GCA GA-3′. The central part with the floxed second exon (∼0.5 kb) was cloned by introduced SdaI and SalI sites and amplified with the following primers: Grb-in2S-Sda, 5′-AGT GAC CCT GCA GGC CCA GAC AAC ATA ATT GTC T-3′ and Grb-in3AS-Sal, 5′-AGT GAC GTC GAC CGC CAG AGC ACT AGA ATT A-3′. BALB I embryonic stem cells (Noben-Trauth et al, 1996) (derived from BALB/c mice) were transfected with the target vector by electroporation. Clones were screened for homologous integration by PCR and verified by Southern blot with an external probe (generated with primers 5′-ACC CAT AGC CAC TGC GTC AT-3′ and 5′-GAC ATC CAC GGT ATG CCA AG-3′). Two positive clones were identified and injected into blastocysts. Those were transferred into pseudo-pregnant females and gave germline transmission successfully. Mice carrying the correct mutation in their germline were identified with PCR, bred to homozygosity and crossed with mb1-cre mice (Hobeika et al, 2006). Age-matched control and B-cell-specific Grb2−/− mice of 8–14 weeks were used for analysis, except for adoptive transfer experiments, where 20-week-old mice were used as donors and recipients.
Flow cytometry
Single-cell suspensions of bone marrow, spleen, lymph nodes, peritoneal cavity and blood were stained in PBS buffer containing 0.1% (wt/vol) BSA and 0.05% (wt/vol) sodium azide for 30 min at 4°C. Saturating concentrations of 2.4G2 (our hybridoma) assured the blocking of Fc receptors. The following antibodies (conjugated to biotin, FITC, PE, PE-Cy5, APC) were used: anti-B220 (RA3-6B2, BD Biosciences), anti-CD43 (S7, BD Biosciences), anti-HSA (CD24, M1/69, BD Biosciences), anti-BP-1 (6C3, eBioscience), anti-IgM (11/41, eBioscience), anti-IgD (11-26C, our hybridoma), anti-CD5 (53-7-3, BD Biosciences), anti-CD23 (B3B4, eBioscience), anti-CD21 (7G6, our hybridoma), anti-CD25 (PC61.5, eBioscience), anti-c-kit (CD117, 2B8, eBioscience), AA4.1 (eBioscience), anti-CD45.1 (A20, BD Biosciences), anti-CD45.2 (104, BD Biosciences), anti-B7.2 (CD86, G4, eBioscience), anti-MHC-II-Ab (I-Ab, AF6-120.1, BD Biosciences), anti-BAFF-R (eBio7H22-E16, eBioscience). Biotinylated antibodies were further stained with streptavidin PE-Cy5 or streptavidin PE-Cy5.5. Detection of cell surface marker expression was performed with a flow cytometer (FACSCalibur) and analysed with CellQuest Software (BD Bioscience). Typically living lymphocytes, judged by forward and side scatter parameters, were gated for analysis. Total cell numbers were obtained by counting after tryptan blue staining of the cells.
Adoptive transfer
For bone marrow reconstitution, we injected 106 cells containing Grb2−/− bone marrow cells and CD45.1+ wild-type bone marrow cells in a ratio of 1:1 intravenously into lethally irradiated (10 Gy) Rag1−/− mice (BALB/c background). Cells were given after 10–24 h of rest. Before the injection, donor bone marrow cells had been stained with anti-CD4 (RL174.2), anti-CD8 (3.168.1) and anti-B220 (RA3-3A1; all from American Type Culture Collection) and subsequently incubated with baby rabbit complement (Cedarlane) for 45 min at 37°C to kill B and T cells. In all, 7–8 weeks after transfer, reconstituted mice were killed and their splenic and bone marrow cells were analysed by flow cytometry. Successful reconstitution of lymphocytes was monitored by continuous blood analysis. The contribution of donor cells in several lymphocyte populations was analysed with anti-CD45.1 (BD Biosciences) in combination with other markers.
BrdU incorporation measurements
BrdU was administered to mice in drinking water containing 1 mg/ml of BrdU and 1% (wt/vol) sucrose (Roth). For pulse–chase experiments, animals were fed with BrdU in the drinking water for 21 days, followed by normal drinking water without BrdU. Bone marrow and splenic cells were isolated and stained with various antibodies (labelled with PE or PE-Cy5). After staining, cells were fixed, permeabilized and stained intracellular with FITC-conjugated anti-BrdU. We used the FITC BrdU Kit (BD Biosciences) for this method.
Cell-cycle analysis
Splenic single-cell suspensions of Grb2−/−(B) mice and wild-type control mice were stained for HSA and B220 surface markers and treated for 10 min at 25°C with 2% (wt/vol) paraformaldehyde. After a washing step with PBS, cells were incubated overnight at 4°C in 1 ml 70% methanol in PBS. Cells were washed twice and incubated in 600 μl 4,6-diamino-2-phenylindole (DAPI) solution (10 μg/ml DAPI, 0.1% Tween and 10 μg/ml RNAse A) for 30 min at 25°C. DAPI staining was measured with an LSRII equipped with an ultraviolet Laser and FACSDiva software (Becton Dickinson). The analysis of the data was done with FlowJo (Tristar) and CellQuest (BD Bioscience).
Proliferation assays
Splenic immature (HSAhiB220lo) and mature (HSAloB220hi) B cells were sorted with a MoFlo cell sorter (BeckmanCoulter) and cultivated in a density of 5 × 105 cells/ml. After 48 h of stimulation with different concentrations (as indicated in the figures) of anti-IgM, LPS, anti-CD40, R848 (InvivoGen, San Diego, CA) or CpG (oligodeoxy-nucleotide 1826, InvivoGen) proliferation was measured by incorporation of [3H]thymidine (5 μCi/ml for anti-IgM, LPS, anti-CD40 or 1 μCi/ml for R848 and CpG) during the final 8 h of culture for LPS and IL-4 or the final 12 h for CpG and R848.
Calcium measurement in primary mouse cells
In all, 107 splenocytes were loaded with Indo-1 as described (Stork et al, 2004). Cells were washed, stained extracellulary with anti-B220 and anti-HSA and stimulated with F(ab′)2 anti-IgM (Dianova). The emission maximum of Indo-1 shifts from a wavelength of about 475 mm in Ca2+-free medium to about 400 nm in a Ca2+-saturated medium. To discriminate between intracellular and extracellular Ca2+ mobilization, we performed anti-IgM stimulation for 2 min in CaCl2-free Krebs Ringer in the presence of 0.5 mM EGTA, and subsequently restored the extracellular Ca2+ concentration to 1 mM. Changes in the ratio of fluorescence intensities at 400 and 475 nm were measured on an LSRII cytometer (Becton Dickinson). Equal loading of the samples with Indo-1 was controlled by treating cells with 100 nM ionomycin (Sigma). Data were analysed with FlowJo (Tristar). Immature B cells were gated as HSAhiB220lo, mature B cells as HSAloB220hi.
BCR stimulation and immunoblot analysis
For T-cell depletion, splenocytes were pretreated on ice with anti-CD4 and anti-CD8 IgM antibodies and treated with complement (see above). For stimulation, B cells were incubated with 10 μg/ml F(ab′)2 anti-IgM (Dianova) or anti-κ (Biozol) for 3, 15 or 50 min at 37°C. Cells were quickly sedimented and lysed in 5 × 106 cells/ml of ice-cold lysis buffer (1 M Tris, pH 7.5, 0.5 mM EDTA, 5 M NaCl, 1% Brij 58) with protease and phosphatase inhibitors. Proteins of the lysed cells were resolved on a 10% SDS–polyacrylamide gel and transferred to a nitrocellulose transfer membrane (Pall). For detection of phosphorylated and unphosphorylated proteins, we used the following antibodies: anti-Grb2 (3F2), anti-phospho-JNK (pTPpY) (Promega), anti-JNK (p46/54), anti-phospho-Erk1/2 (p44/42) (Thr202/Tyr204), anti-pErk1/2 (p44/42), anti-phospho-p38, (Thr180/Tyr182), anti-p38, anti-phospho-PLC-γ2 (Tyr759), anti-PLC-γ2, anti-phospho-Syk (Tyr525/526), anti-Syk, anti-phospho-Akt (Ser473), anti-Akt (all from Cell Signalling) and anti-Actin (Cedarlane). Active Ras was precipitated by adding recombinant Raf1-binding domain and quantification by anti-Ras antibody (which detects all isoforms of Ras) (Millipore). CD22 immunoprecipitation and phosphotyrosine blot was done as described (Waisman et al, 2007). Proteins were visualized by anti-rabbit IgG HRP-conjugated antibody (Cell Signalling) or anti-mouse IgG HRP-conjugated antibody (Bio-Rad) and ECL detection system (Amersham).
Immunizations
B-cell-specific Grb2−/− mice and control mice were immunized intraperitoneally once with TNP-ficoll (10 μg/mouse) for T-independent responses. Blood samples were taken on days 0, 5, 7, 11 and 14. VLPs also known as dense bodies (non-infectious enveloped particles consisting mainly of tegument proteins contained in a viral envelope with all glycoproteins embedded) of HCMV strain AD 169 were isolated from cell culture supernatant as described (Talbot and Almeida, 1977; Weisel et al, 2010). Cells were human foreskin fibroblasts. In all, 2 μg VLPs (in PBS) were injected intravenously into mice at day 0 for primary and on day 32 for secondary immunization. Blood samples were taken on days 0, 4, 7, 10, 32, 36, 39 and 42. Adoptive B-cell transfer into RAG1−/− mice was done 90 days after secondary immunization. Splenic B cells were isolated by anti-CD4 and anti-CD8-mediated complement lysis, followed by anti-CD19 beads purification. B-cell purity was at least 98.5%. B cells were stained with anti-IgG1, anti-IgG2a and anti-IgG2b (all FITC conjugated). In all, 2 × 106 B cells were transferred i.v. per recipient mouse. After 7 days, recipient mice were immunized with 2 μg VLPs, blood samples were taken at days 5, 7, 10 and VLP-specific antibodies were measured by ELISA.
ELISA and ELISPOT assays
We measured immunoglobulin serum titers from naive and immunized mice by standard ELISA methods (Gerlach et al, 2003). Maxisorb plates (Nunc) were coated with antigen (10 μg/ml TNP-BSA) for detection of TNP-specific Igs in the sera of immunized mice. Polysorb plates (Nunc) were coated with 1.5 μg/ml CMV VLPs. As standard, we used a sample of pooled sera of immunized mice, which was applied to every plate. For detection of Igs in the sera of naive mice, we coated polysorb plates (Nunc) with isotype-specific Abs (Southern Biotechnology Associates, Brimingham, AL) and used monoclonal Ig isotype antibodies as standard (Southern Biotechnology Associates). We applied sera as serial dilutions of 1:3 in dilution reagent (1% (wt/vol) BSA, 0.05% (wt/vol) sodium azide in PBS) and incubated plates for 2 h at 37°C. Alkaline-phosphatase-conjugated secondary antibodies were added for detection (Southern Biotechnology Associates).
In vitro class switch analysis
After erythrocyte lysis, spleen cells (2.5 × 105/ml) were stimulated with 10 μg/ml LPS and 10 ng/ml IL-4 for 6 days. Ig levels of supernatants were determined by ELISA. For reverse transcription (RT)–PCR, cells (2 × 106/ml) were stimulated for 60 h. Trizol (Invitrogen) was used to extract mRNA from the cells and cDNA was generated with the Reverse Transcriptase II kit (Invitrogen) by using 1 μg of total RNA and 1 μg of poly d(T)18 (Fermentas) in a 25-μl reaction volume. In all, 1 μl of it was used as a template for RT–PCR. The γ1 circle transcript PCR was performed as described in Kinoshita et al (2001).
Histology
Mice were injected intraperitoneally with 109 sheep red blood cells and were killed after 10 days to collect the spleens. Spleens were embedded in Tissue Tek (Sakura Finetek) and were frozen at −80°C. Cryostat sections of 4–8 μm were cut, rehydrated in PBS, incubated for 30 min with blocking solution (10% FCS, 0.1% (wt/vol) BSA, 1:100 dilution of Fc Block (CD16–CD32, BD Pharmigen), 10% rat/mouse/goat serum) and washed in 0.05% Tween-20 in PBS. We stained sections for 60 min with the following primary antibodies (conjugated to biotin, FITC or Cy5): MOMA-1 (BMA), IgM (Jackson ImmunoResearch), IgD (eBioscience), PNA (Vector Laboratories). As secondary antibody, streptavidin Cy3 (GE Healthcare) was used. Slides were covered with Mowiol (Calbiochem) and analysed with an Axioplan 2 (Zeiss) using OpenLab v2.2.6 software (Improvision).
Statistical analysis
Student's t-test was used for all statistical analysis except for determining significances of differences in Ig levels, where Mann–Whitney U-test was used.
Supplementary Material
Acknowledgments
We thank S Angermüller, A Urbat and U Appelt for technical help. We thank Dr A Gessner for help with thymidine assays, M Wöhner for i.v. injections, as well as Dr E Hobeika and Dr M Reth for mb1-cre mice. This work was supported by the DFG (Ni 549/8-1 and GK 592).
Author contributions: JA wrote the paper and performed experiments; DR and AM performed experiments; TW contributed to VLP immunizations, histology experiments and discussions; LN wrote the paper and supervised the experiments.
Footnotes
The authors declare that they have no conflict of interest.
References
- Buday L, Downward J (1993) Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell 73: 611–620 [DOI] [PubMed] [Google Scholar]
- Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, Pawson T (1998) Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell 95: 793–803 [DOI] [PubMed] [Google Scholar]
- Coughlin JJ, Stang SL, Dower NA, Stone JC (2005) RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol 175: 7179–7184 [DOI] [PubMed] [Google Scholar]
- Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA (1993) Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363: 45–51 [DOI] [PubMed] [Google Scholar]
- Engels N, Konig LM, Heemann C, Lutz J, Tsubata T, Griep S, Schrader V, Wienands J (2009) Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol 10: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Fletcher CA, Sutherland AP, Groom JR, Batten ML, Ng LG, Gommerman J, Mackay F (2006) Development of nephritis but not sialadenitis in autoimmune-prone BAFF transgenic mice lacking marginal zone B cells. Eur J Immunol 36: 2504–2514 [DOI] [PubMed] [Google Scholar]
- Gerlach J, Ghosh S, Jumaa H, Reth M, Wienands J, Chan AC, Nitschke L (2003) B cell defects in SLP65/BLNK-deficient mice can be partially corrected by the absence of CD22, an inhibitory coreceptor for BCR signaling. Eur J Immunol 33: 3418–3426 [DOI] [PubMed] [Google Scholar]
- Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC (2001) Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol 2: 29–36 [DOI] [PubMed] [Google Scholar]
- Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL (2009) Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol 182: 5382–5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M, Winkler TH (2004) Activation of virus-specific memory B cells in the absence of T cell help. J Exp Med 199: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M (2006) Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA 103: 13789–13794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC (2007) Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med 204: 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovelmeyer N, Wunderlich FT, Massoumi R, Jakobsen CG, Song J, Worns MA, Merkwirth C, Kovalenko A, Aumailley M, Strand D, Bruning JC, Galle PR, Wallach D, Fassler R, Waisman A (2007) Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J Exp Med 204: 2615–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP (2002) Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol 168: 5993–5996 [DOI] [PubMed] [Google Scholar]
- Jang IK, Zhang J, Chiang YJ, Kole HK, Cronshaw DG, Zou Y, Gu H (2010) Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc Natl Acad Sci USA 107: 10620–10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K, Harigai M, Fagarasan S, Muramatsu M, Honjo T (2001) A hallmark of active class switch recombination: transcripts directed by I promoters on looped-out circular DNAs. Proc Natl Acad Sci USA 98: 12620–12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Shinohara H, Baba Y (2010) B cell signaling and fate decision. Annu Rev Immunol 28: 21–55 [DOI] [PubMed] [Google Scholar]
- Li YS, Hayakawa K, Hardy RR (1993) The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med 178: 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK (2010) Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity 32: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J (1992) The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70: 431–442 [DOI] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF (2001) Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14: 617–629 [DOI] [PubMed] [Google Scholar]
- Neumann K, Oellerich T, Urlaub H, Wienands J (2009) The B-lymphoid Grb2 interaction code. Immunol Rev 232: 135–149 [DOI] [PubMed] [Google Scholar]
- Ng CH, Xu S, Lam KP (2007) Dok-3 plays a nonredundant role in negative regulation of B-cell activation. Blood 110: 259–266 [DOI] [PubMed] [Google Scholar]
- Niiro H, Clark EA (2002) Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol 2: 945–956 [DOI] [PubMed] [Google Scholar]
- Nimnual AS, Yatsula BA, Bar-Sagi D (1998) Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science 279: 560–563 [DOI] [PubMed] [Google Scholar]
- Nitschke L (2009) CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev 230: 128–143 [DOI] [PubMed] [Google Scholar]
- Noben-Trauth N, Kohler G, Burki K, Ledermann B (1996) Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res 5: 487–491 [DOI] [PubMed] [Google Scholar]
- Oh-hora M, Johmura S, Hashimoto A, Hikida M, Kurosaki T (2003) Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-gamma2 to Ras in B cell receptor signaling. J Exp Med 198: 1841–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue SL, Kurosaki T, Bolen J, Herbst R (2000) B cell antigen receptor-induced activation of Akt promotes B cell survival and is dependent on Syk kinase. J Immunol 165: 1300–1306 [DOI] [PubMed] [Google Scholar]
- Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A (2007) Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol 27: 2732–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M (2004) TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol 173: 2245–2252 [DOI] [PubMed] [Google Scholar]
- Scharenberg AM, Humphries LA, Rawlings DJ (2007) Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol 7: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K (2009) PI3 kinase signals BCR-dependent mature B cell survival. Cell 139: 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadanlick JE, Cancro MP (2008) BAFF and the plasticity of peripheral B cell tolerance. Curr Opin Immunol 20: 158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork B, Engelke M, Frey J, Horejsi V, Hamm-Baarke A, Schraven B, Kurosaki T, Wienands J (2004) Grb2 and the non-T cell activation linker NTAL constitute a Ca(2+)-regulating signal circuit in B lymphocytes. Immunity 21: 681–691 [DOI] [PubMed] [Google Scholar]
- Stork B, Neumann K, Goldbeck I, Alers S, Kahne T, Naumann M, Engelke M, Wienands J (2007) Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells. EMBO J 26: 1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P, Almeida JD (1977) Human cytomegalovirus: purification of enveloped virions and dense bodies. J Gen Virol 36: 345–349 [DOI] [PubMed] [Google Scholar]
- Waisman A, Kraus M, Seagal J, Ghosh S, Melamed D, Song J, Sasaki Y, Classen S, Lutz C, Brombacher F, Nitschke L, Rajewsky K (2007) IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig alpha/beta. J Exp Med 204: 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel FJ, Appelt UK, Schneider AM, Horlitz JU, van Rooijen N, Korner H, Mach M, Winkler TH (2010) Unique requirements for reactivation of virus-specific memory B lymphocytes. J Immunol 185: 4011–4021 [DOI] [PubMed] [Google Scholar]
- Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM (2001) Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol 11: 1547–1552 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.