Abstract
Newly formed glutamatergic synapses often lack postsynaptic AMPA-type glutamate receptors (AMPARs). Aside from ‘unsilencing’ the postsynaptic site, however, the significance of postsynaptic AMPAR insertion during synapse maturation remains unclear. To investigate the role of AMPAR in synapse maturation, we used RNA interference (RNAi) to knockdown AMPARs in cultured hippocampal neurons. Surprisingly, loss of postsynaptic AMPARs increased the occurrence of presynaptically inactive synapses without changing the release probability of the remaining active synapses. Additionally, heterologous synapses formed between axons and AMPAR-expressing HEK cells develop significantly fewer inactive presynaptic terminals. The extracellular domain of the AMPAR subunit GluA2 was sufficient to reproduce this effect at heterologous synapses. Indeed, the retrograde signalling by AMPARs is independent of their channel function as RNAi-resistant AMPARs restore synaptic transmission in neurons lacking AMPARs despite chronic receptor antagonist treatment. Our findings suggest that postsynaptic AMPARs perform an organizational function at synapses that exceeds their standard role as ionotropic receptors by conveying a retrograde trans-synaptic signal that increases the transmission efficacy at a synapse.
Keywords: AMPA receptors, glutamatergic synaptogenesis, hippocampal neurons, presynaptic maturation, retrograde signalling
Introduction
In the developing brain, glutamatergic synapses are formed at individual contact sites between an axon and a dendrite. Initial axodendritic contact is followed by a series of signalling events leading to the differentiation of presynaptic and postsynaptic compartments. A number of synaptic cell adhesion molecules participate in signalling during synaptogenesis by promoting the accumulation of both presynaptic and postsynaptic specializations (Biederer et al, 2002; Varoqueaux et al, 2006; Aoto et al, 2007; Chubykin et al, 2007; Ko et al, 2009; Linhoff et al, 2009).
Among the first steps of presynaptic differentiation is the recruitment of active zone components and synaptic vesicles to the presynaptic terminal (Ahmari et al, 2000; Friedman et al, 2000). Although detection of neurotransmitter release from growing axons of young neurons suggests that much of the exocytic machinery may be present before synapse formation occurs (Young and Poo, 1983; Matteoli et al, 1992; Krueger et al, 2003), the machinery involved in synaptic vesicle release and recycling is further modified upon axodendritic contact (Kraszewski et al, 1995; Coco et al, 1998; Verderio et al, 1999). The functional maturation of a presynaptic terminal requires the formation of both the readily releasable pool (RRP) through docking and priming of vesicles at the active zone, and of the reserve pool through additional vesicle accumulation adjacent to the active zone (Renger et al, 2001; Mozhayeva et al, 2002). Throughout development, presynaptic function is continuously adjusted by changes in active zone molecular composition, vesicle release probability, and the organization of vesicle pools (Ziv and Garner, 2004; Jin and Garner, 2008).
Dendritic growth and postsynaptic differentiation are highly regulated processes during neuronal development (Biederer, 2005; Chen and Firestein, 2007; Carrel et al, 2009). Postsynaptic events at nascent synapses include the recruitment of scaffolding proteins and the clustering of neurotransmitter receptors juxtaposed to the presynaptic terminal. In the hippocampus, both NMDA-type glutamate receptors (NMDARs) and AMPA-type glutamate receptors (AMPARs) are present at mature glutamatergic synapses, whereas in younger neurons, a large fraction of synapses contain only NMDARs but not AMPARs (Isaac et al, 1995; Liao et al, 1995; Durand et al, 1996; Gomperts et al, 1998; Petralia et al, 1999; Pickard et al, 2000). These postsynaptically silent synapses indicate that recruitment of AMPARs to synapses is a discrete step during synapse maturation that is regulated by specific mechanisms. Does synapse maturation end with the insertion of AMPARs into postsynaptically silent synapses or is there additional synapse development following AMPAR insertion? It has been well established that modulation of AMPAR cycling into and out of synapses underlies various forms of synaptic plasticity (Song and Huganir, 2002; Bredt and Nicoll, 2003). Despite recognition of the central role for AMPARs in regulating synaptic strength, it remains unclear whether and how AMPARs influence synapse development.
Here, we provide evidence that insertion of postsynaptic AMPARs represents a significant step in development towards establishing the functional maturity of a synapse. AMPAR knockdown in hippocampal neurons and experiments with heterologous synapses suggest that AMPARs provide a trans-synaptic instructive signal during synapse development that promotes the competency for glutamate release at presynaptic terminals.
Results
Knockdown of AMPARs in developing hippocampal neurons
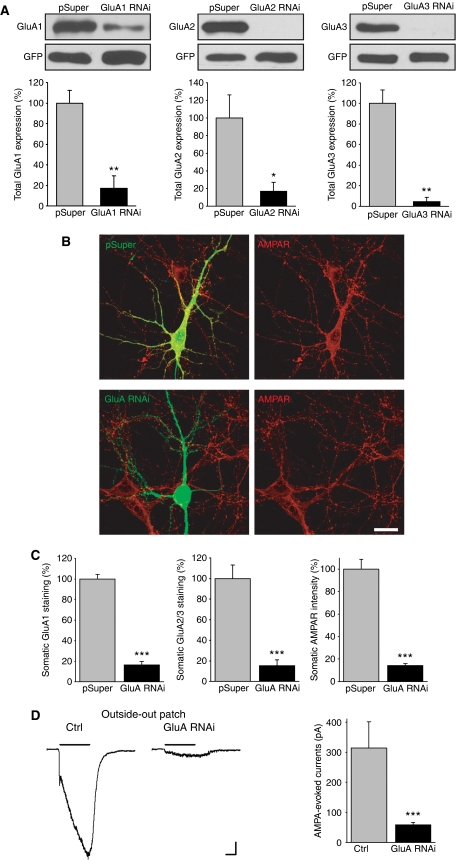
To determine whether the loss of postsynaptic AMPARs affects synapse maturation, we used plasmid-based RNA interference (RNAi) to knockdown the expression of the GluA1, GluA2, and GluA3 AMPAR subunits in young cultured hippocampal neurons that exhibit ongoing synaptogenesis. Given that the majority of AMPARs expressed in hippocampal pyramidal neurons are GluA1/2 or GluA2/3 heteromers (Wenthold et al, 1996; Lu et al, 2009), we did not include a short hairpin RNA (shRNA) for the GluA4 subunit in these experiments. We first established the knockdown efficiency of each GluA-shRNA in HEK293 cells (Figure 1A), and then confirmed the knockdown efficiency in hippocampal neurons (Figure 1B–D). In all subsequent experiments we co-expressed the GluA1–3 shRNAs in neurons and we identify this group as GluA RNAi. The total AMPAR immunostaining in the soma of transfected neurons was dramatically reduced by GluA RNAi after 5 days (Figure 1B and C). Consistent with this observation, AMPA-evoked currents from somatic outside-out patches, which reflects extrasynaptic AMPAR density on the somatic surface, were diminished by >75% (Figure 1D). Thus, these shRNAs effectively suppress AMPAR expression.
Figure 1.
GluA RNAi in cultured hippocampal neurons leads to a loss of functional AMPARs. (A) AMPAR knockdown efficiencies of GluA1–3 shRNAs were determined by co-expression with GFP-tagged GluA1–3 in HEK293 cells (n=3 experiments/group; *P<0.05; **P<0.005). (B) Immunostaining of all three AMPAR subunits (red) in neurons after 5 days expression of pSuper empty-vector or GluA RNAi. GluA RNAi=GluA1+GluA2+GluA3 RNAi. Scale bar: 20 μm. (C) Average intensity of GluA1, GluA2/3, and all three subunits GluA1, GluA2, and GluA3 in the soma of GluA RNAi neurons compared with pSuper controls (n=8–10; ***P<1 × 10−4). The average immunofluorescence intensity was normalized to neighbouring untransfected neurons to account for variability in immunostaining. (D) Example traces and quantification of somatic outside-out patch recordings from control and GluA RNAi neurons. AMPAR currents were evoked with a 3-s application of AMPA (100 μm) in the presence of cyclothiazide (100 μm) (n=11 neurons/group; ***P<1 × 10−5). Scale bars: 50 pA, 1 s.
GluA RNAi effectively weakens synaptic transmission by decreasing the number of postsynaptic AMPARs
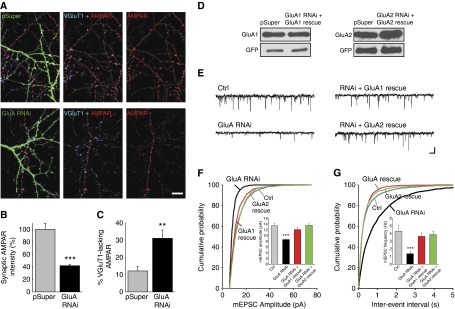
In dendrites, GluA RNAi depleted intracellular and extrasynaptic pools of AMPARs to below detection. Interestingly, we observed residual AMPARs clustered at synaptic sites as identified by labelling of VGluT1, a marker for glutamatergic presynaptic terminals (Figure 2A). Quantification of AMPAR immunofluorescence co-localized with VGluT1 puncta revealed a reduction in the number of synaptic AMPARs to ∼40% of control neurons (Figure 2B). Moreover, GluA RNAi significantly increased the proportion of glutamatergic synapses lacking AMPARs (Figure 2C).
Figure 2.
Reduced miniature excitatory synaptic transmission in AMPAR knockdown neurons. (A) Synaptic AMPARs in neurons expressing pSuper or GluA-shRNAs were identified by co-localization of AMPAR puncta, comprised of total GluA1, GluA2, and GluA3 immunostaining (red), with VGluT1 (blue). Scale bar: 10 μm. (B) Quantification of the average synaptic AMPAR immunoreactivity. (n=9–10 cells/group; ***P<1 × 10−5). (C) Percentage of AMPAR-lacking synapses identified by VGluT1 puncta devoid of AMPAR immunostaining (n=9–10 cells/group; **P<0.005). (D) The expression levels of GFP-tagged GluA1 and GluA2 rescue constructs were unaffected by the corresponding GluA-shRNAs in HEK293 cells. (E) Example traces of mEPSCs from dissociated cultured hippocampal neurons at 12 DIV. Scale bars: 10 pA, 200 ms. (F, G) Cumulative probability plot of mEPSC amplitude and frequency. (Inset) Average mEPSC amplitude and frequency (n=26–39 cells/group; ***P<1 × 10−5).
To investigate the impact of the GluA RNAi on synaptic function, we performed whole-cell patch-clamp recordings of miniature EPSCs (mEPSCs) on neurons (Figure 2E). We found a reduction in the average mEPSC amplitude, indicative of the removal of postsynaptic AMPARs (Figure 2F). We also observed a significant decrease in the frequency of mEPSCs, which is likely due to the increased prevalence of postsynaptically silent synapses (Figure 2G). These impairments could be reversed to the level of control neurons with the co-expression of RNAi-resistant constructs of GluA1 or the unedited form of GluA2 (GluA2Q) (Figure 2D–G), indicating that our results are not due to an off target effect of GluA RNAi.
Loss of postsynaptic AMPARs impairs both AMPAR- and NMDAR-mediated synaptic transmission
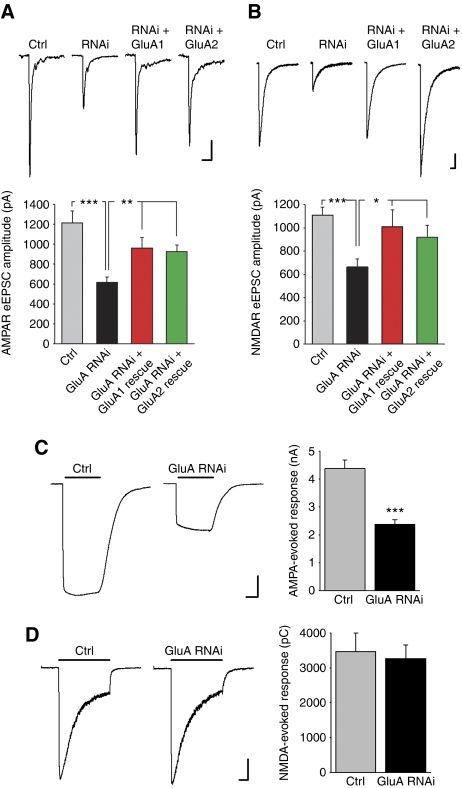
We next performed recordings of postsynaptic responses to action potential-evoked presynaptic vesicle release elicited by extracellular local field stimulation (Maximov et al, 2007). An input–output curve revealed that the AMPAR-mediated evoked EPSC (eEPSC) amplitude was reduced in GluA RNAi neurons at all stimulus intensities while the eEPSCs in both control and GluA RNAi neurons increased with the strength of the stimulus intensity and plateaued at a stimulus higher than 4 mA (Supplementary Figure S1). For subsequent experiments, we used a 6-mA stimulus to excite all presynaptic axons thereby eliciting the maximum synaptic response in neurons. When all active synapses are engaged, the degree of reduction in the synaptic AMPAR response (Figure 3A) corresponds well to the magnitude of synaptic AMPAR depletion assessed by immunocytochemistry (Figure 2B). Co-expression of either the GluA-shRNA insensitive GluA1 or GluA2 restored the AMPAR eEPSC amplitude to a level comparable to that found in untransfected neurons (Figure 3A).
Figure 3.
Reduced NMDAR-mediated synaptic responses in neurons after AMPAR knockdown. (A) Representative traces (each an average of five eEPSCs) and quantification of AMPAR-mediated eEPSCs in cultured hippocampal neurons elicited by extracellular local field stimulation (n=12–28 cells/group; **P<0.005; ***P<1 × 10−4). Scale bars: 200 pA, 50 ms. (B) Representative traces (each an average of five eEPSCs) and quantification of NMDAR-mediated eEPSCs recorded at −60 mV in external solution with CNQX (10 μM), glycine (20 μm), and zero magnesium (n=11–25 cells/group; *P<0.05; ***P<1 × 10−4). Scale bars: 200 pA, 200 ms. (C) Representative traces and quantification of whole-cell AMPA-evoked (3 s, 100 μm) currents from cultured neurons in the presence of cyclothiazide (100 μm) (n=11 cells/group; ***P<1 × 10−5). Scale bars: 1 nA, 1 s. (D) Representative traces and quantification of whole-cell NMDA-evoked (3 s, 1 mM) currents from cultured neurons (n=16 cells/group; P>0.7). Scale bars: 0.5 nA, 0.5 s.
Since mature glutamatergic synapses contain both AMPA- and NMDARs, we next examined NMDAR-mediated evoked synaptic responses in AMPAR knockdown neurons. Surprisingly, loss of AMPARs caused a significant decrease in the amplitude of NMDAR eEPSCs (Figure 3B). This decrease was almost as large as the reduction in AMPAR-mediated eEPSCs, and could be restored by co-expression of either the GluA1 or GluA2 rescue construct (Figure 3B). Thus, knockdown of AMPARs in cultured neurons leads to an unexpected concomitant decrease of NMDAR-mediated synaptic responses, suggesting that the insertion of postsynaptic AMPARs is important for establishing functionally mature excitatory synapses beyond the role of AMPARs in sensing glutamate.
The decline in NMDAR-mediated synaptic transmission could occur if AMPAR knockdown leads to an equivalent loss of synaptic NMDARs. To address this possibility, we assayed the totality of functional receptors that were expressed on the neuronal surface by whole-cell patch-clamp recordings of agonist-evoked currents. Unlike AMPA-evoked whole-cell currents, which were greatly reduced upon AMPAR knockdown (Figure 3C), NMDA-evoked whole-cell currents were not altered (Figure 3D). Since both published works (Rosenmund et al, 1995; Thomas et al, 2006) and our results after the blockade of synaptic NMDARs with the irreversible open channel blocker MK-801 (Supplementary Figure S2) indicate that a large proportion (roughly 80%) of surface NMDARs are located at synapses, the sustained NMDA-evoked surface response suggests that most likely, synaptic NMDAR abundance is unaltered by AMPAR knockdown.
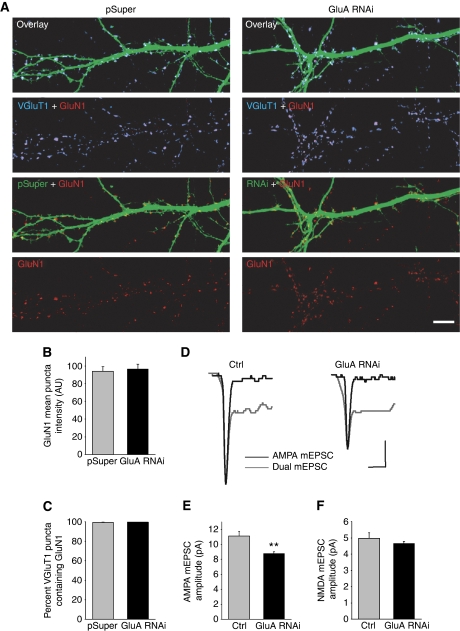
We took two additional approaches to assess synaptic NMDARs. We immunolabelled neurons with antibodies to GluN1, the essential subunit for all NMDARs, and to VGluT1, and quantified the amount of GluN1 that co-localized with VGluT1 puncta (Figure 4A). The intensity of synaptic GluN1 puncta was comparable between control and GluA RNAi neurons, and nearly all glutamatergic synapses contained NMDARs with no apparent difference in the number of synapses containing NMDARs (Figure 4B and C). Next, we performed dual component mEPSC recordings of NMDAR- and AMPAR-mediated events to measure the NMDAR mEPSC amplitude (Gomperts et al, 1998, 2000). Despite a significant decrease in AMPAR mEPSC amplitude, there was no difference in NMDAR mEPSC amplitude with GluA RNAi compared with control neurons (Figure 4D–F). Collectively, these results suggest that an alteration in NMDAR expression is not responsible for the reduction in NMDAR-mediated synaptic transmission.
Figure 4.
GluA RNAi does not alter the number of synaptic NMDARs. (A) Immunolabelling of the NMDAR subunit GluN1 (red) and VGluT1 (blue) on dendrites of neurons expressing either pSuper or GluA RNAi. Scale bar: 10 μm. (B, C) Synaptic GluN1 expression identified as GluN1 puncta co-localized with VGluT1. The mean intensity of synaptic GluN1 immunoreactivity (B), and the percentage of NMDAR-containing glutamatergic synapses (C), were analysed (n=10 cells/group; P>0.6). (D) Representative traces of dual component mEPSCs (grey trace). The AMPAR-only mEPSCs recorded from the same cells after APV perfusion is shown scaled to the peak of the dual component mEPSC (black trace). Scale bars: 4 pA, 20 ms. (E, F) Quantification of the mean AMPAR mEPSC amplitude (E) (n=18 cells/group; **P<0.002), and the mean NMDAR mEPSC amplitude (F). The scaled average AMPAR mEPSC was subtracted from the average dual component mEPSC to determine the NMDAR mEPSC amplitude (n=18 cells/group; P>0.3).
A deficiency in postsynaptic AMPARs does not alter the number of glutamatergic synapses
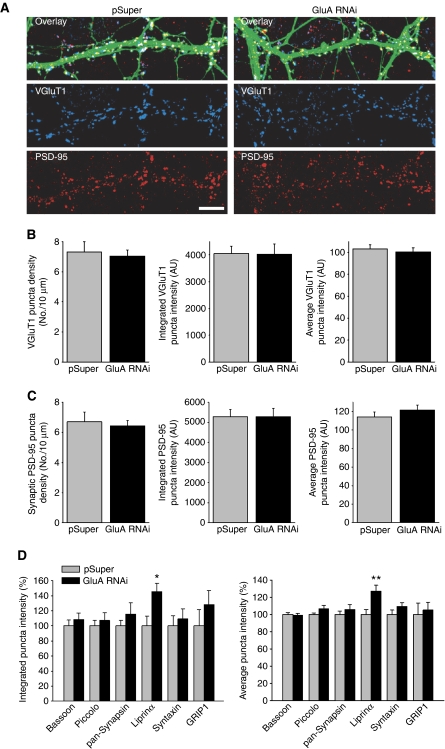
Having found no alteration in synaptic NMDARs, we considered the possibility that dendritic growth was hindered by AMPAR knockdown. Both Sholl analysis and a quantification of the total length of dendrites for each neuron revealed no significant differences between control and GluA RNAi neurons (Supplementary Figure S3). The growth of dendritic spines is a structural hallmark of excitatory synapse maturation in hippocampal pyramidal neurons (Yoshihara et al, 2009). We measured the density of spines in control and GluA RNAi neurons, but found no difference (number of spines per 10 μm dendrite: pSuper, 3.65±0.66; GluA RNAi, 3.76±0.45; n=15 neurons/group). Reduced postsynaptic AMPAR expression also did not alter the density of glutamatergic presynaptic terminals on dendrites of pyramidal neurons (Figure 5A and B). Moreover, immunostaining for PSD-95, a postsynaptic scaffold, revealed that AMPAR knockdown had no effect on the number of PSD-95 puncta co-localized with VGluT1 puncta (Figure 5A and C). This confirms that postsynaptic AMPARs do not have a major role in determining how many excitatory synapses are formed, and rules out a reduction in synapse density as an explanation for the reduction in NMDAR-mediated synaptic transmission. Finally, we analysed the mean puncta intensity as well as the integrated puncta intensity of VGluT1, PSD-95, GRIP-1, bassoon, piccolo, synapsin, and syntaxin1 immunostaining at excitatory synapses and we observed no changes with GluA RNAi (Figure 5B–D), suggesting that the expression and localization of most synaptic proteins are not generally perturbed by loss of AMPARs. Interestingly, we found that synaptic expression of liprin-α is increased by postsynaptic AMPAR knockdown (Figure 5D). Liprin-α is believed to be present at both presynaptic and postsynaptic sites, and it has been linked to AMPAR trafficking (Wyszynski et al, 2002) as well as presynaptic vesicle release (Olsen et al, 2005; Zurner and Schoch, 2009) at mammalian synapses. Whether AMPARs regulate presynaptic vesicle release through modulating liprin-α localization and function remains to be determined.
Figure 5.
Reduction in postsynaptic AMPARs does not alter synapse density. (A) Immunostaining for VGluT1 (blue) and PSD-95 (red) at 12 DIV, 5 days after pSuper or GluA-shRNAs transfection. Scale bar: 10 μm. (B) Quantification of VGluT1 localization at synapses (n=12–15 cells/group; P>0.6). (C) Quantification of PSD-95 at synapses (n=12–15 cells/group; P>0.6). (B, C) The integrated puncta intensity is a measure of the sum of the pixel intensities within each puncta. The average puncta intensity is a measure of the average pixel intensity within each puncta. (D) Analysis of synaptic localization of several additional presynaptic and postsynaptic proteins (n=9–14 cells/group; *P<0.05; **P<0.005).
GluA RNAi does not decrease the probability of presynaptic vesicle release
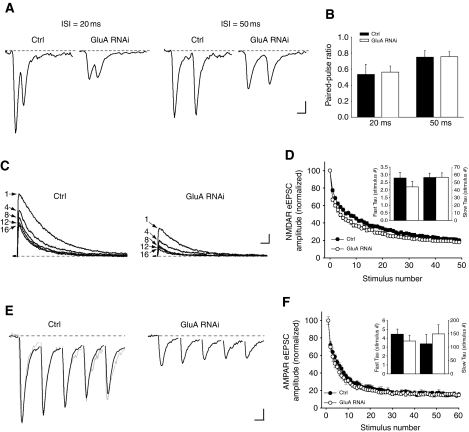
We next wondered whether the knockdown of postsynaptic AMPARs at developing synapses might weaken presynaptic function by decreasing the probability of vesicle release. Changes in vesicle release probability often lead to altered paired-pulse ratio (PPR) of evoked synaptic responses (Katz and Miledi, 1968; Zucker and Regehr, 2002). We did paired recordings from two synaptically connected neurons, in which a single action potential is elicited in a presynaptic neuron by current injection and the AMPAR-mediated eEPSC is recorded from its target postsynaptic neuron, either untransfected or expressing GluA-shRNAs. There was no difference in the PPR measured from control and GluA RNAi neurons (Figure 6A and B).
Figure 6.
Loss of postsynaptic AMPARs does not alter the release probability at synapses. (A) Representative traces (each an average of five responses) of AMPAR EPSCs recorded from a postsynaptic neuron in paired recordings. The EPSCs were elicited by current injection to a synaptically connected presynaptic neuron in whole-cell current clamp recording mode. Scale bars: 20 pA, 20 ms. (B) Quantification of PPR from paired recordings at a 20- and 50-ms ISI (n=10–14 cells/group; P>0.8). (C) Example traces of NMDAR eEPSCs recorded at +40 mV in the presence of 10 μM MK-801, showing the progressive block of responses at each designated stimulus number. Scale bars: 200 pA, 100 ms. (D) Quantification of the NMDAR eEPSC amplitude in the presence of MK-801 at consecutive stimuli normalized to the amplitude of the first response. (Inset) Rate of response decay fitted with a double exponential equation (n=22–24 cells/group; P>0.2). (E) Example traces of the first five AMPAR eEPSCs in response to a 20-Hz stimulation (black traces). The response from the GluA RNAi neuron was scaled and superimposed onto the control response (grey trace). Each trace is an average of three individual 20 Hz trains of eEPSCs recorded from one neuron. Scale bars: 200 pA, 20 ms. (F) The amplitude of each successive response was normalized to the size of the first AMPAR eEPSC. (Inset) Time constants of the response decay fitted with a double exponential equation (n=13 cells/group; P>0.3).
In addition to PPR, changes in synaptic release probability can be evaluated by analysing the rate of the progressive block of NMDAR eEPSCs with the irreversible open channel blocker MK-801 (Hessler et al, 1993; Rosenmund et al, 1993). Indeed, decreasing the external Ca2+ from 2 to 1 mM to reduce the release probability dramatically slowed the rate of NMDAR eEPSC blockade, thereby validating this approach (Supplementary Figure S4). However, the rate of NMDAR eEPSC blockade in GluA RNAi neurons was not significantly different from control neurons (Figure 6C and D), arguing against the possibility that a lower release probability at synapses underlies the reduced synaptic NMDAR responses following AMPAR knockdown.
Finally, using a high-frequency stimulus train (60 pulses at 20 Hz), we monitored the rate of vesicle depletion from synapses (Dobrunz and Stevens, 1997). GluA RNAi in neurons did not alter the rate of AMPAR eEPSC depression during the stimulus train (Figure 6E and F). Consistent with our previous results, this suggests that the synaptic release probability is unchanged and that the loss of postsynaptic AMPARs does not affect the rate of vesicle depletion from active presynaptic terminals.
Diminished size of the RRP following AMPAR knockdown
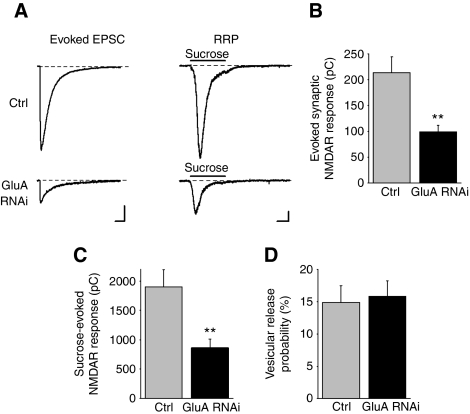
The decrease in synaptic transmission by GluA RNAi could be a consequence of an increase in presynaptically inactive synapses that lack fusion-competent vesicles. To examine this possibility, we applied hypertonic sucrose to neurons to estimate the size of the RRP (Rosenmund and Stevens, 1996) and we recorded NMDAR-mediated responses because postsynaptic NMDARs were unaltered by GluA RNAi (Figure 4). Before measuring the RRP, eEPSCs were recorded from each cell (Figure 7A and B). Indeed, AMPAR knockdown significantly reduced the charge transfer of the sucrose-evoked NMDAR current (Figure 7A and C). Given that the charge of the NMDAR eEPSC represents the amount of vesicle release at presynaptic terminals in response to a single action potential, we divided it by the RRP charge to estimate the probability of release per vesicle (Fernandez-Chacon et al, 2001). Loss of AMPARs had no effect on the vesicular release probability at presynaptic terminals (Figure 7D). The RRP size at an individual synapse affects its synaptic release probability (Dobrunz and Stevens, 1997). If AMPAR knockdown caused a uniform reduction in the RRP size at each synapse, we would expect to see a decrease in synaptic release probability. However several approaches to measure release probability revealed no change after GluA RNAi (Figure 6A–F). Instead, the reduction in RRP by AMPAR knockdown can be attributed to an increased number of inactive presynaptic terminals that are deficient in fusion-competent vesicles, whereas remaining active presynaptic terminals are functionally normal with no alteration in synaptic release probability.
Figure 7.
AMPAR knockdown decreases the RRP size among all excitatory synapses. (A) Representative traces of NMDAR eEPSCs (left) and sucrose-evoked (3 s of 0.5 M sucrose) NMDAR responses (right). For each neuron, five NMDAR eEPSCs were recorded to generate an average response to extracellular stimulation, which was followed by a single application of sucrose to estimate the RRP size. (B) Average charge transfer of NMDAR eEPSCs elicited by extracellular field stimulation. (C) Average sucrose-evoked NMDAR responses from the same neurons in (B) (n=22–23 cells; **P<0.005). (D) The vesicular release probability estimated for each neuron by calculating the charge transfer of the average NMDAR eEPSC as a percentage of the total sucrose-evoked current (n=22–23 cells; P>0.7).
GluA RNAi increases the number of inactive glutamatergic terminals
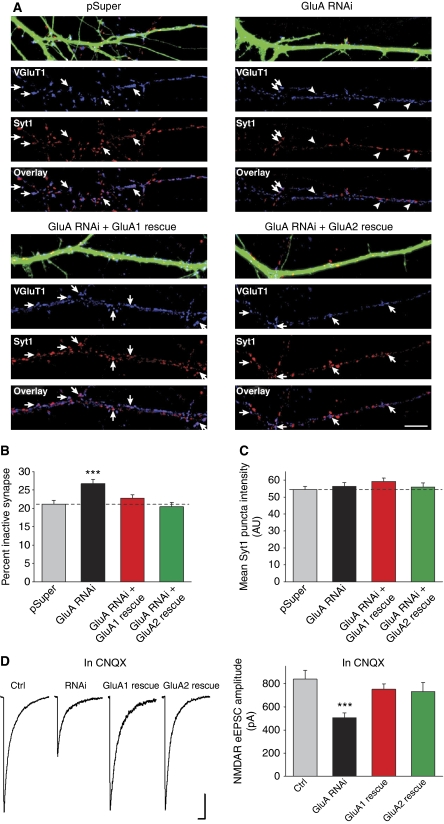
To visualize whether neurons with AMPAR knockdown have more functionally inactive presynaptic terminals, we used an antibody that recognizes the intraluminal domain of synaptotagmin 1 (Syt1) to directly monitor presynaptic vesicle cycling at individual synapses (Matteoli et al, 1992; Malgaroli et al, 1995). The Syt1 antibody was applied to live neurons in the culture media and the differential uptake of the antibody driven by endogenous network activity enabled us to assess vesicle fusion at individual synapses. Postfixation immunostaining for VGluT1 and GAD-65 revealed that 65% of Syt1 antibody uptake occurred at glutamate release sites whereas 35% occurred at GABA release sites (Supplementary Figure S5A and B). Therefore, a combination of Syt1 antibody uptake and VGluT1 immunostaining allows us to specifically monitor vesicle release from glutamatergic terminals. VGluT1 puncta co-localized with Syt1 labelling represent active glutamate release sites and VGluT1 puncta lacking Syt1 labelling represent inactive glutamate release sites.
We found that GluA RNAi increased the number of inactive glutamatergic presynaptic terminals observed after the 20-min period of Syt1 labelling (Figure 8A and B). This effect is specifically due to the loss of AMPARs because the number of inactive synapses was reduced back to control levels with the co-expression of either the GluA1 or GluA2 rescue constructs (Figure 8A and B). Although we might expect to observe more Syt1-lacking synapses after AMPAR knockdown given the striking reduction in NMDAR-mediated synaptic transmission (Figures 3B and 7), dynamic vesicle trafficking between presynaptic terminals within minutes (Darcy et al, 2006; Westphal et al, 2008; Staras et al, 2010) may lead to an underestimate of the fraction of synapses lacking active vesicle cycling observed with GluA RNAi due to mobile Syt1-labelled vesicles transported from active to inactive terminals during the 20-min Syt1 antibody incubation.
Figure 8.
GluA RNAi increases the number of functionally inactive glutamatergic presynaptic terminals. (A) The Syt1 antibody uptake (red) and postfixation immunostaining of VGluT1 (blue) were performed. Active glutamatergic presynaptic terminals exhibit Syt1 immunostaining (arrows), whereas inactive terminals do not (arrowheads). (B) Quantification of the number of functionally inactive glutamatergic synapses on transfected neurons (n=18–30 neurons/group; ***P<0.001). (C) Quantification of the mean intensity of Syt1 immunostaining at active glutamatergic synapses (n=18–30 neurons/group; P>0.1). (D) Representative traces and quantification of NMDAR eEPSCs recorded from neurons treated with CNQX (10 μM) throughout the 5 days of construct expression (n=16–33 cells/group; ***P<0.001). Scale bars: 200pA, 200 ms.
In agreement with our assessment of presynaptic release probability, the average intensity of Syt1 antibody uptake at VGluT1 puncta was not changed, suggesting that the amount of vesicle release at active glutamatergic terminals was unaltered by GluA RNAi (Figure 8C). We found no difference in the amount of vesicle cycling at neighbouring untransfected neurons adjacent to the transfected neuron for each of the groups (Supplementary Figure S5C and D), confirming that the global network activity was unaffected by AMPAR knockdown. Finally, the neurons were immunostained with the Syt1 antibody after fixation and permeabilization and we found that almost all glutamatergic terminals contained Syt1 (pSuper: 97.11±0.63%; GluA RNAi: 96.56±0.56%; n=10/group) and the mean Syt1 puncta intensity was no different between control and GluA RNAi neurons (pSuper: 100.61±5.63 A.U.; GluA RNAi: 110.87±5.94 A.U.; n=10/group).
To probe the mechanism by which postsynaptic AMPARs affect presynaptic function, we asked whether retrograde signalling mediated by AMPARs is activity dependent, that is, whether receptor activation is required for promoting presynaptic function. We repeated the rescue of NMDAR synaptic transmission following GluA RNAi while blocking all AMPAR activity with an AMPAR antagonist CNQX. CNQX was added to the culture concurrently with transfection of the GluA-shRNAs and the RNAi-resistant GluA1 or GluA2, and it was maintained in the culture for 5 days until the time of recording. CNQX treatment for 5 days did not change NMDAR eEPSCs recorded from untransfected neurons (Supplementary Figure S6). Strikingly, the NMDAR eEPSC amplitude of GluA RNAi neurons was successfully rescued by GluA1 or GluA2 co-expression despite the complete blockade of AMPAR activity by CNQX (Figure 8D), suggesting that postsynaptic depolarization by AMPARs is not required for maintaining presynaptic function. Therefore, it is improbable that the activity-dependent release of a retrograde messenger is responsible for this AMPAR-mediated signal transduction during synapse development.
Heterologous synapses reveal a distinct role for AMPARs in the induction of synaptic vesicle release at a subset of presynaptic terminals
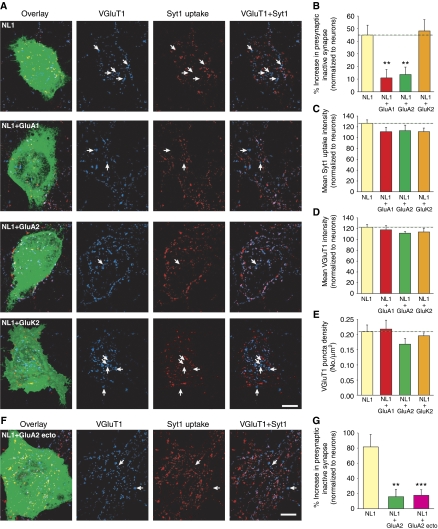
To investigate the specific role of AMPARs in modifying presynaptic function at newly formed synapses, we took advantage of the heterologous synapse formation assay (Scheiffele et al, 2000; Biederer et al, 2002). Expression of the postsynaptic cell adhesion molecule neuroligin 1 (NL1) in heterologous cells induces presynaptic differentiation in contacting neuronal axons. We expressed NL1 with or without AMPARs in HEK293 cells, and co-plated them with young dissociated hippocampal neurons. Three days after co-plating, we used the Syt1 antibody uptake assay with VGluT1 immunostaining to evaluate inactive presynaptic sites at heterologous synapses in the absence or presence of postsynaptic AMPARs.
Consistent with previous reports, the expression of NL1 in HEK293 cells induced glutamatergic presynaptic differentiation, as manifested by the accumulation of VGluT1 puncta on the cells (Figure 9A). Due to the strong synaptogenic effect of NL1 expression, some co-cultured HEK293 cells developed significant overlap of synaptic contacts to the extent that individual synapses could not be clearly resolved. These cells were excluded from our analysis. To evaluate presynaptic maturation at heterologous synapses in comparison to neuronal synapses, and to account for variability in culture density and immunostaining, we normalized quantifications of both VGluT1 and Syt1 immunostaining at each HEK293 cell to the corresponding quantifications made from neighbouring neuronal synapses.
Figure 9.
Postsynaptic AMPARs participate directly in trans-synaptic retrograde signalling to influence glutamate release at a subset of presynaptic terminals. (A) Images of heterologous synapse formation between HEK293 cells and neurons. HEK293 cells were transfected with NL1 alone, or NL1 with either a GFP-tagged AMPAR subunit, GluA1 or GluA2, or a GFP-tagged kainate receptor subunit, GluK2 and co-plated with hippocampal neurons. Glutamatergic presynaptic terminals were identified by VGluT1 puncta (blue). Synaptic vesicle cycling at each terminal was measured by Syt1 antibody uptake (red). Functionally inactive presynaptic terminals were identified as VGluT1 puncta that lack Syt1 immunofluorescence (arrows). Scale bar: 10 μm. (B) Quantification of functionally inactive presynaptic terminals. The fraction of inactive glutamatergic terminals on each HEK293 cell was calculated and normalized to the fraction of inactive terminals at neighbouring neuronal synapses (n=24–27 cells; **P<0.005). (C) Mean Syt1 uptake intensity at heterologous synapses (normalized to the mean Syt1 uptake intensity at neighbouring neuronal synapses; n=24–27 cells/group; P>0.05). (D) Mean VGluT1 puncta intensity at heterologous synapses (normalized to the VGluT1 intensity at neighbouring neuronal synapses, n=24–27 cells/group; P>0.05). (E) Density of glutamatergic synaptic contacts made onto HEK293 cells (n=24–27 cells/group; P>0.1). (F) Image of heterologous synapse formation on a HEK293 cell co-expressing NL1 and GluA2 ecto. Similar to (A), arrows indicate functionally inactive presynaptic terminals. Scale bar: 10 μm. (G) Quantification of functionally inactive presynaptic terminals formed on HEK293 cells expressing NL1 alone, NL1+GluA2, or NL1+GluA2 ecto (n=27–31; **P<0.005; ***P<0.001).
As expected, HEK293 cells expressing GluA1 or GluA2 without NL1 did not show any significant accumulation of synaptic contacts (VGluT1 puncta density (No./μm2): NL1, 0.157±0.017; GluA1 alone, 0.025±0.004; GluA2 alone, 0.024±0.009; n=8 cells/group; P<1 × 10−5). Interestingly, although HEK293 cells expressing either NL1 alone or NL1 together with AMPARs potently induced formation of excitatory synapses from contacting axons, HEK293 cells expressing NL1 alone exhibited a greater proportion of inactive glutamatergic terminals than HEK293 cells expressing both NL1 and AMPARs (Figure 9A and B). This effect was specific to AMPARs, because co-expression of the kainate receptor subunit GluK2 with NL1 did not reduce the fraction of inactive glutamatergic terminals at heterologous synapses. Co-expression of AMPARs with NL1 did not alter the size of the HEK293 cells (Supplementary Figure S7A); therefore, the contact area for crossing axons is not influenced by AMPAR expression. The mean intensity of Syt1 antibody uptake at active glutamatergic terminals was similar between heterologous synapses with or without AMPARs (Figure 9C). Likewise, the mean VGluT1 puncta intensity (Figure 9D) and the density of glutamatergic presynaptic terminals (Figure 9E, see also Supplementary Figure S7B) were comparable between HEK293 cells that expressed AMPARs and those that did not. From these results, it is evident that although AMPARs are not required for the recruitment of actively recycling vesicles to all glutamatergic presynaptic terminals, they appear to promote presynaptic function by reducing the number of functionally inactive terminals.
A direct trans-synaptic interaction mediates AMPAR-induced presynaptic vesicle release
Given that AMPAR channel activity was not required for the retrograde effect of AMPAR on presynaptic function (Figure 8D), we next tested an alternative signalling mechanism: a direct trans-synaptic interaction between the AMPAR ectodomain and an unidentified component of the presynaptic membrane. We generated a chimeric AMPAR construct, GluA2 ecto, which consists of the GFP-tagged GluA2 extracellular domain fused to the transmembrane domain of the interleukin-2 receptor (Tac) (Standley et al, 2000), with the intracellular GluA1 C-terminal domain to promote synaptic targeting. While the GluA2 ecto protein was not efficiently exported from the endoplasmic reticulum in neurons (Supplementary Figure S8B) making it inadequate for rescue experiments, when expressed in HEK293 cells, GluA2 ecto was trafficked robustly to the cell surface (Supplementary Figure S8A). In the co-culture heterologous synapse system, GluA2 ecto behaved similarly to the full-length GluA2 in that when co-expressed with NL1 it greatly reduced the number of inactive presynaptic terminals compared with NL1-alone expression (Figure 9F and G). Taken together, our results point to a transduction mechanism that involves a direct trans-synaptic interaction between the postsynaptic AMPAR extracellular domain and an unknown presynaptic protein, which makes a subset of presynaptic terminals functional by recruiting a releasable pool of synaptic vesicles.
The trans-synaptic effect of AMPARs on presynaptic development does not require N-cadherin
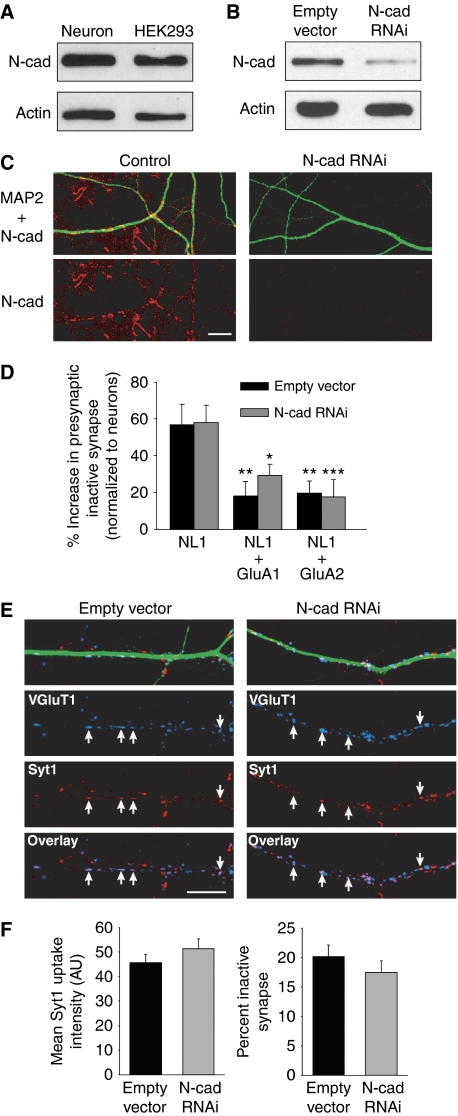
N-cadherin is localized at both the presynaptic and postsynaptic membranes and its signalling regulates the structural and functional development of synapses (Arikkath and Reichardt, 2008; Mysore et al, 2008). Reports of its interaction with AMPARs make N-cadherin a potential candidate for the presynaptic binding partner of AMPARs that promotes the functional maturation of glutamatergic terminals (Nuriya and Huganir, 2006; Saglietti et al, 2007). To test this we used a previously reported shRNA construct (Saglietti et al, 2007), delivered by lentivirus, to knockdown N-cadherin expression in neurons. The lentivirus infection of N-cadherin shRNA achieved a knockdown efficiency of 94% in hippocampal cultures (Figure 10B and C).
Figure 10.
N-cadherin is not required for AMPAR retrograde signalling in presynaptic maturation. (A) Immunoblot of endogenous N-cadherin from lysate of 14 DIV hippocampal culture and HEK293 cells. (B) N-cadherin RNAi delivered with lentivirus had a 94% knockdown efficiency in hippocampal cultures (n=3 experiments). (C) Representative images of N-cadherin knockdown in neuronal dendrites (red: N-cadherin; green: MAP2). Scale bar: 10 μm. (D) Quantification of the number of inactive glutamatergic synapses formed on HEK293 cells normalized to neighbouring neuronal synapses (n=13–15 cells/group; *P<0.05; **P<0.01; ***P<0.005). (E) Representative images of 12 DIV neurons labelled with Syt1 antibody uptake (red) and postfixation VGluT1 immunostaining (blue). Neuronal dendrites were identified by MAP2 immunostaining (green). Arrows indicate active glutamate release sites. Scale bar: 10 μm. (F) Quantification of the mean Syt1 puncta intensity at active synapses and the percent of inactive presynaptic terminals (n=18 cells/group; P>0.2).
We first evaluated heterologous synapses using the Syt1 antibody uptake assay on HEK293 cells co-plated with control or N-cadherin RNAi neurons. Since HEK293 cells express endogenous N-cadherin (Figure 10A) (Hogan et al, 2004; Okuda et al, 2007), the heterologous synapse assay allows us to examine the specific effect of presynaptic N-cadherin knockdown on synaptic function. The loss of presynaptic N-cadherin failed to block the observed effect of AMPARs on presynaptic function at heterologous synapses (Figure 10D), suggesting that presynaptic N-cadherin is not required for trans-synaptic AMPAR signalling. We next analysed synaptic vesicle release at neuronal synapses lacking both presynaptic and postsynaptic N-cadherin. In contrast to the increased number of functionally inactive synapses with AMPAR knockdown (Figure 8A and B), we did not observe a change in the fraction of inactive synapses with N-cadherin RNAi nor did we find any effect of N-cadherin RNAi on the mean intensity of Syt1 antibody uptake at active synapses (Figure 10E and F). Together, these results argue that N-cadherin is not the binding partner of AMPARs required for the functional maturation of presynaptic terminals.
Discussion
A novel role for AMPARs in synapse maturation
In this study, we describe an unexpected finding: RNAi-mediated knockdown of postsynaptic AMPAR expression in young neurons not only decreased AMPAR-mediated synaptic currents, but also caused a dramatic, corresponding decrease in NMDAR-mediated currents. This overall weakening of synaptic transmission was accompanied by a reduction in the RRP size and an increase in the number of inactive glutamatergic terminals. In a second set of experiments, we showed that postsynaptic AMPARs reduce the number of functionally inactive presynaptic terminals at heterologous synapses via a mechanism that does not require glutamate-activated postsynaptic currents, but is fully mediated by the ectodomain of AMPARs. Importantly, the GluA1 and GluA2 rescue experiments in neurons and the expression of these homomeric AMPARs at heterologous synapses reveal that the trans-synaptic effect of AMPARs is not subunit specific. Based on these key observations, supported by ancillary control experiments that validated the specificity of these results, we propose that AMPARs contribute to functional synapse maturation, and that they operate, at least in part, by mediating a retrograde trans-synaptic signal carried out by an interaction between the ectodomain of AMPAR subunits and an unknown presynaptic component.
Glutamatergic synapse maturation is marked by discrete events occurring both at presynaptic and postsynaptic sites; the process entails the organization of the presynaptic active zone, the accumulation of postsynaptic scaffold proteins and receptors, the upregulation of synaptic vesicle cycling, and finally the modification of vesicle release efficacy and postsynaptic sensitivity to glutamate (Ziv and Garner, 2001). Changes in the probability of vesicle release at presynaptic terminals have been documented throughout development at different synapses in the CNS (Bolshakov and Siegelbaum, 1995; Choi and Lovinger, 1997; Iwasaki and Takahashi, 2001; Mori-Kawakami et al, 2003). In our study, however, modulation of presynaptic release probability does not seem to be the step regulated by postsynaptic AMPAR insertion, as we failed to detect a difference in synaptic release probability following AMPAR knockdown. Presynaptic function is also dependent on the number of recycling vesicles at the terminal, including both RRP vesicles capable of immediate exocytosis upon excitation and vesicles in the reserve pool, which can be recruited for release during prolonged stimulation (Sudhof, 2000). Indeed, immature presynaptic terminals that lack an RRP have been reported at newly formed synapses in dissociated hippocampal cultures (Mozhayeva et al, 2002). Thus, our results indicate that developmental restructuring of vesicle pools in presynaptic terminals may be triggered by the insertion of postsynaptic AMPARs that trans-synaptically activate RRP formation. Previous studies have established the postsynaptic silent synapse as an immature stage in synapse development (Gomperts et al, 1998; Petralia et al, 1999; Pickard et al, 2000). We have shown here that additional presynaptic functional maturation can proceed after postsynaptic silent synapses are switched on.
Influence of AMPARs on functional synapse development
Previous attempts to understand how AMPARs influence synaptic function in the hippocampus entailed the use of knockout mice deficient in either the GluA1 subunit (Zamanillo et al, 1999), the GluA2 subunit (Jia et al, 1996), or both the GluA2 and GluA3 subunits (Meng et al, 2003). Importantly, these studies provided insight into AMPAR subunit-specific effects on synaptic strength, but no changes in synapse maturation were identified. In our study, we observed that simultaneous knockdown of all three AMPAR subunits impairs presynaptic function, and show that either GluA1 or GluA2 expression is sufficient to restore presynaptic function, suggesting that the effect of AMPARs on synapse maturation is not subunit specific. The lack of subunit specificity of the AMPAR effect indicates that these AMPAR subunits share the same presynaptic partner, and that it is the common extracellular domains of the AMPAR subunits that mediate the interaction. Compensation by remaining AMPARs in the subunit-specific knockouts could explain why there is no apparent impairment in synapse maturation. In another study, RNAi was used to acutely knockdown GluA2 expression in hippocampal neurons, demonstrating a specific role for this subunit in promoting spine formation (Passafaro et al, 2003; Saglietti et al, 2007). By contrast, we did not observe a change in spine density on pyramidal neurons expressing all three GluA-shRNAs during a stage of rapid synaptogenesis. GluA2 may be primarily required for spine stabilization and maintenance after synapse maturation is established, which occurs at a later stage in the lifetime of a synapse that was not addressed in our study.
More recently, a conditional knockout of the GluA1, GluA2, and GluA3 subunits was generated, which resulted in a virtually complete loss of postsynaptic AMPAR-mediated responses recorded from Schaffer collateral synapses in the hippocampus (Lu et al, 2009). This study found no change in NMDAR eEPSCs following the conditional knockout of AMPARs. At present, we have no ready explanation for the discrepancy between our results and those of Lu et al (2009), although it should be noted that our experiments were performed in very different systems. Specifically, their analysis may not be sensitive enough to detect the observed effect of AMPAR deficiency on presynaptic vesicle release. This may only be observable if the RRP size is directly tested, which was not done by Lu et al (2009). Moreover, homeostatic compensation after prolonged loss of AMPARs (up to 3 weeks in the Lu et al (2009) study) could further mask the direct effect of AMPAR removal observed 5 days after shRNA-mediated acute knockdown. A fundamental difference in experimental approach could thus underlie this discrepancy in results following the loss of postsynaptic AMPARs.
Mechanism underlying functionally inactive presynaptic terminals
The synaptic phenotype we observe upon AMPAR knockdown consists of a decrease in both AMPAR- and NMDAR-mediated synaptic responses, without a change in postsynaptic NMDAR expression, or in presynaptic release probability. Importantly, the significant decrease in hypertonic sucrose-evoked currents signified a loss of synaptic vesicles from the RRP. The synaptic phenotype is most consistent with the notion that a subset of synapses lack a presynaptic RRP, as opposed to a uniform decrease in RRP size at all synapses. This is because the RRP size at a synapse influences the synaptic release probability (Dobrunz and Stevens, 1997), which was unchanged in our experiments. Although we do not know how precisely postsynaptic AMPARs influence the presynaptic RRP, for example what its presynaptic molecular interaction partners may be, our results are compelling in delineating this pathway.
Previous studies have implicated several presynaptic candidates that mediate the availability of releasable vesicles at a synapse (referred to as priming factors). These include Munc13-1 and its homologs (Augustin et al, 1999); RIM proteins (Schoch et al, 2002), and SNARE and SM proteins (Rizo and Rosenmund, 2008). Our analysis of several presynaptic active zone proteins suggest that liprin-α, a protein implicated in both AMPAR trafficking and presynaptic vesicle release at mammalian synapses (Wyszynski et al, 2002; Olsen et al, 2005), may be an additional factor in regulating presynaptic development.
Our evaluation of presynaptic function at heterologous synapses revealed that HEK293 cells expressing NL1 alone had more inactive glutamatergic terminals but simultaneously maintained terminals with active vesicle release akin to neighbouring neuronal synapses. This suggests that AMPARs are only required for the functional maturation of a subset of presynaptic terminals. Why do postsynaptic AMPARs influence presynaptic function at only a distinct population of synapses? For now, the reason for this disparate effect on synapses remains unclear. At excitatory synapses, significant heterogeneity exists in presynaptic morphology (Schikorski and Stevens, 1997), properties of vesicle release (Hessler et al, 1993; Rosenmund et al, 1993; Murthy et al, 1997; Moulder et al, 2007) and molecular composition (Reid et al, 1997; Atwood and Karunanithi, 2002; Rosenmund et al, 2002; Altrock et al, 2003). Accordingly, it is conceivable that a subset of presynaptic terminals, perhaps with distinct molecular composition, is more susceptible to remain at an immature developmental stage in the absence of postsynaptic AMPARs. A thorough investigation of the molecular and/or structural identity of immature presynaptic terminals may elucidate the selective effect of AMPARs on synapse maturation.
Implications for trans-synaptic signalling by postsynaptic AMPARs
Activity-dependent signalling by BDNF has been reported to rapidly unsilence immature glutamatergic terminals of hippocampal neurons (Shen et al, 2006; Cabezas and Buno, 2010). However, prolonged CNQX treatment during the rescue of synaptic transmission with AMPAR knockdown showed that postsynaptic excitation by AMPARs was not required for the functional maturation of presynaptic terminals. Instead, additional experiments using heterologous synapses revealed that the ectodomain of the AMPARs was sufficient for promoting vesicle release at glutamatergic terminals. To date only a few proteins have been identified that interact with AMPARs extracellularly, including both Narp and NP1 from the neuronal pentraxin family (O’Brien et al, 2002; Xu et al, 2003; Sia et al, 2007) and N-cadherin (Saglietti et al, 2007). Our findings suggest that while presynaptic N-cadherin may affect other aspects of synapse development, it does not mediate the retrograde signalling by postsynaptic AMPARs uncovered by our study. Ongoing work to elucidate the complexity of signalling events during synapse development may provide insight regarding the AMPAR trans-synaptic binding partner that mediates presynaptic terminal maturation.
Materials and methods
Please see online Supplementary data for additional Materials and methods.
Immunocytochemistry
Coverslips were fixed in 4% PFA then incubated in blocking solution containing 0.3% Triton X-100 and 2% NGS. Primary antibodies were added to the cells followed by flourophore-conjugated secondary antibodies. For the Syt1 antibody uptake assay, live cells were incubated with the Syt1 antibody in neurobasal media for 20–30 min, then washed thoroughly and fixed. For immunostaining of the GluN1 subunit, neurons were fixed in methanol for 10 min at −20°C following the fixation in 4% PFA.
Lentivirus transfer vector and virus production
The N-cadherin shRNA was cloned into a lentivirus transfer vector, JHUMCS, derived from the original L307 vector (Soden and Chen, 2010) but lacking the IRES-GFP sequence. HEK293 cells were transfected with the transfer vector and three helper plasmids, pRSV-REV, pIVS-vesicular stomatitis G protein (VSVg), and pMDL gag/pol. Transfected HEK293 cells were grown in neurobasal media and 2 days later the media was collected, spun at 2000 r.p.m. for 5 min, and passed through a 0.45-μm filter. The lentivirus was applied to neuronal cultures at 4 DIV and the media was replaced after 24 h.
Image acquisition and quantification
For fluorescent image analysis, the cells were chosen randomly from three or more cover slips per group. Fluorescent images were acquired with an Olympus (Tokyo, Japan) FV1000 BX61WI laser-scanning confocal microscope, using an Olympus Plan Apochromat × 60 oil objective (numerical aperture (NA), 1.42; working distance (WD), 0.15) or an Olympus U-Plan Apochromat × 100 oil objective (NA, 1.40; WD, 0.12), with sequential acquisition setting at 1024 × 1024 pixel resolutions. Laser power and photomultipliers were set such that no detectable bleedthrough occurred between different channels. Digital images of the cells were captured with Fluoview Imaging software (Olympus). For each image, 8–10 sections were taken, and brightest point projections were made. Identical settings for laser power, photomultiplier gain, and offset were used in each experiment. Pixel intensities for the brightest samples were just below saturation, except when contours of the cell or of the neuronal processes had to be clearly determined (e.g. saturated pixels at the centre of HEK cells to detect peripheral fluorescence or at the soma of neurons to detect dendritic signals). For the analysis of synaptic proteins, images from the same experiment were thresholded identically by intensity to exclude the diffuse/intracellular pool. To reduce the effect of background staining on synaptic co-localization analysis, VGluT1 puncta smaller than 0.4 μm2 were excluded from analysis. Sholl analysis was performed using the ImageJ plugin (http://www-biology.ucsd.edu/labs/ghosh/software/). Image quantification was performed by experienced investigators who were blind to the experimental conditions.
Electrophysiological recordings
Whole-cell patch-clamp recordings were made at room temperature with 3–7 MΩ patch pipettes filled with an internal solution containing (in mM) 140 CsCl, 2 MgCl2, 5 EGTA, 10 HEPES, 0.3 Na3-GTP, 4 Na2-ATP, pH=7.35. Cultures were continuously superfused with external solution (in mM) 119 NaCl, 26 NaHCO3, 2.5 KCl, 10 glucose, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4. Recordings of mEPSCs were done in the presence of tetrodotoxin (1 μM) and picrotoxin (100 μM). Miniature responses were analysed with the Mini Analysis Program from Synaptosoft.
The recording method for evoked synaptic response using extracellular stimulation in dissociated cultures was adopted from Maximov et al (2007). AMPAR-mediated eEPSCs were recorded in external solution containing picrotoxin (100 μM). NMDAR-mediated eEPSCs were recorded in external solution containing CNQX (10 μM), picrotoxin (100 μM), and glycine (20 μM) but lacking Mg2+. QX-314 (10 mM) was added to the internal solution used in recordings of all eEPSCs. Cells were held at −60 mV. Local extracellular field stimulation was applied using a concentric bipolar electrode (FHC) placed 50 μm from the cell soma. A current injection of 6 mA with 1 ms duration was sufficient to evoke reliable postsynaptic currents, and the stimulus was kept constant during each experiment. Recordings of NMDAR eEPSCs with MK-801 (10 μM) were done at a +40 mV holding potential in external solution containing CNQX (10 μM), picrotoxin (100 μM), and glycine (20 μM).
Whole-cell AMPAR currents and currents from somatic outside-out patches were recorded in external solution containing picrotoxin (100 μM) and tetrodotoxin (1 μM). Whole-cell AMPAR or NMDAR currents were evoked with a local 3 s application of AMPA (100 μM) with cyclothiazide (100 μM) or NMDA (1 mM), respectively. To estimate the size of the RRP of vesicles, 0.5 M sucrose was locally applied to each neuron for 3 s. The NMDA- and sucrose-evoked responses were recorded at −60 mV in the presence of CNQX (10 μM), picrotoxin (100 μM), and glycine (20 μM) but lacking Mg2+ and the internal solution contained QX-314 (10 mM).
Supplementary Material
Acknowledgments
We thank Huili Wang and Christine Nam for technical support and other members of the Chen Laboratory for discussion and comments on this manuscript. This work was supported by National Institute of Mental Health Grants MH069792 and MH091193, David and Lucile Packard Foundation, and WM Keck Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahmari SE, Buchanan J, Smith SJ (2000) Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci 3: 445–451 [DOI] [PubMed] [Google Scholar]
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fassler R, Richter K, Boeckers TM, Potschka H, Brandt C, Loscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brandstatter JH, Garner CC et al. (2003) Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron 37: 787–800 [DOI] [PubMed] [Google Scholar]
- Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, Chen L (2007) Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation. J Neurosci 27: 7508–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikkath J, Reichardt LF (2008) Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci 31: 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood HL, Karunanithi S (2002) Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci 3: 497–516 [DOI] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N (1999) Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400: 457–461 [DOI] [PubMed] [Google Scholar]
- Biederer T (2005) Progress from the postsynaptic side: signaling in synaptic differentiation. Sci STKE 2005: pe9. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC (2002) SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297: 1525–1531 [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA (1995) Regulation of hippocampal transmitter release during development and long-term potentiation. Science 269: 1730–1734 [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40: 361–379 [DOI] [PubMed] [Google Scholar]
- Cabezas C, Buno W (2010) BDNF is required for the induction of a presynaptic component of the functional conversion of silent synapses. Hippocampus; doi: 10.1002/hipo.20754 [DOI] [PubMed] [Google Scholar]
- Carrel D, Du Y, Komlos D, Hadzimichalis NM, Kwon M, Wang B, Brzustowicz LM, Firestein BL (2009) NOS1AP regulates dendrite patterning of hippocampal neurons through a carboxypeptidase E-mediated pathway. J Neurosci 29: 8248–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Firestein BL (2007) RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci 27: 8378–8386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM (1997) Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA 94: 2665–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC (2007) Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54: 919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco S, Verderio C, De Camilli P, Matteoli M (1998) Calcium dependence of synaptic vesicle recycling before and after synaptogenesis. J Neurochem 71: 1987–1992 [DOI] [PubMed] [Google Scholar]
- Darcy KJ, Staras K, Collinson LM, Goda Y (2006) Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci 9: 315–321 [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF (1997) Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008 [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A (1996) Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381: 71–75 [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature 410: 41–49 [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE (2000) Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron 27: 57–69 [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, Nicoll RA (2000) Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J Neurosci 20: 2229–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Rao A, Craig AM, Malenka RC, Nicoll RA (1998) Postsynaptically silent synapses in single neuron cultures. Neuron 21: 1443–1451 [DOI] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R (1993) The probability of transmitter release at a mammalian central synapse. Nature 366: 569–572 [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y (2004) Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol 24: 6690–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC (1995) Evidence for silent synapses: implications for the expression of LTP. Neuron 15: 427–434 [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T (2001) Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol 534 (Pt 3): 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J (1996) Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron 17: 945–956 [DOI] [PubMed] [Google Scholar]
- Jin Y, Garner CC (2008) Molecular mechanisms of presynaptic differentiation. Annu Rev Cell Dev Biol 24: 237–262 [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R (1968) The role of calcium in neuromuscular facilitation. J Physiol 195: 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Sudhof TC (2009) LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 64: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P (1995) Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci 15: 4328–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger SR, Kolar A, Fitzsimonds RM (2003) The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron 40: 945–957 [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R (1995) Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375: 400–404 [DOI] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM (2009) An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron 61: 734–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62: 254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A, Ting AE, Wendland B, Bergamaschi A, Villa A, Tsien RW, Scheller RH (1995) Presynaptic component of long-term potentiation visualized at individual hippocampal synapses. Science 268: 1624–1628 [DOI] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Perin MS, Sudhof TC, De Camilli P (1992) Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol 117: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Pang ZP, Tervo DG, Sudhof TC (2007) Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J Neurosci Methods 161: 75–87 [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z (2003) Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron 39: 163–176 [DOI] [PubMed] [Google Scholar]
- Mori-Kawakami F, Kobayashi K, Takahashi T (2003) Developmental decrease in synaptic facilitation at the mouse hippocampal mossy fibre synapse. J Physiol 553 (Pt 1): 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Taylor AA, Shin W, Gillis KD, Mennerick S (2007) Vesicle pool heterogeneity at hippocampal glutamate and GABA synapses. J Neurosci 27: 9846–9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayeva MG, Sara Y, Liu X, Kavalali ET (2002) Development of vesicle pools during maturation of hippocampal synapses. J Neurosci 22: 654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF (1997) Heterogeneous release properties of visualized individual hippocampal synapses. Neuron 18: 599–612 [DOI] [PubMed] [Google Scholar]
- Mysore SP, Tai CY, Schuman EM (2008) N-cadherin, spine dynamics, and synaptic function. Front Neurosci 2: 168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Huganir RL (2006) Regulation of AMPA receptor trafficking by N-cadherin. J Neurochem 97: 652–661 [DOI] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P (2002) Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci 22: 4487–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y (2007) beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci USA 104: 13479–13484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen O, Moore KA, Fukata M, Kazuta T, Trinidad JC, Kauer FW, Streuli M, Misawa H, Burlingame AL, Nicoll RA, Bredt DS (2005) Neurotransmitter release regulated by a MALS-liprin-alpha presynaptic complex. J Cell Biol 170: 1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M (2003) Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature 424: 677–681 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R (1999) Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci 2: 31–36 [DOI] [PubMed] [Google Scholar]
- Pickard L, Noel J, Henley JM, Collingridge GL, Molnar E (2000) Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci 20: 7922–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Clements JD, Bekkers JM (1997) Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures. J Neurosci 17: 2738–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger JJ, Egles C, Liu G (2001) A developmental switch in neurotransmitter flux enhances synaptic efficacy by affecting AMPA receptor activation. Neuron 29: 469–484 [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C (2008) Synaptic vesicle fusion. Nat Struct Mol Biol 15: 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL (1993) Nonuniform probability of glutamate release at a hippocampal synapse. Science 262: 754–757 [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL (1995) Synaptic NMDA receptor channels have a low open probability. J Neurosci 15: 2788–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, Rhee JS (2002) Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron 33: 411–424 [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF (1996) Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16: 1197–1207 [DOI] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, Sheng M, Passafaro M (2007) Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 54: 461–477 [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101: 657–669 [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF (1997) Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci 17: 5858–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC (2002) RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 415: 321–326 [DOI] [PubMed] [Google Scholar]
- Shen W, Wu B, Zhang Z, Dou Y, Rao ZR, Chen YR, Duan S (2006) Activity-induced rapid synaptic maturation mediated by presynaptic cdc42 signaling. Neuron 50: 401–414 [DOI] [PubMed] [Google Scholar]
- Sia GM, Beique JC, Rumbaugh G, Cho R, Worley PF, Huganir RL (2007) Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron 55: 87–102 [DOI] [PubMed] [Google Scholar]
- Soden ME, Chen L (2010) Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci 30: 16910–16921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–588 [DOI] [PubMed] [Google Scholar]
- Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ (2000) PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron 28: 887–898 [DOI] [PubMed] [Google Scholar]
- Staras K, Branco T, Burden JJ, Pozo K, Darcy K, Marra V, Ratnayaka A, Goda Y (2010) A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron 66: 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2000) The synaptic vesicle cycle revisited. Neuron 28: 317–320 [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL (2006) Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol 95: 1727–1734 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N (2006) Neuroligins determine synapse maturation and function. Neuron 51: 741–754 [DOI] [PubMed] [Google Scholar]
- Verderio C, Coco S, Bacci A, Rossetto O, De Camilli P, Montecucco C, Matteoli M (1999) Tetanus toxin blocks the exocytosis of synaptic vesicles clustered at synapses but not of synaptic vesicles in isolated axons. J Neurosci 19: 6723–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci 16: 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW (2008) Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 320: 246–249 [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M (2002) Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron 34: 39–52 [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P (2003) Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39: 513–528 [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D (2009) Dendritic spine formation and stabilization. Curr Opin Neurobiol 19: 146–153 [DOI] [PubMed] [Google Scholar]
- Young SH, Poo MM (1983) Spontaneous release of transmitter from growth cones of embryonic neurones. Nature 305: 634–637 [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811 [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC (2001) Principles of glutamatergic synapse formation: seeing the forest for the trees. Curr Opin Neurobiol 11: 536–543 [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC (2004) Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci 5: 385–399 [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405 [DOI] [PubMed] [Google Scholar]
- Zurner M, Schoch S (2009) The mouse and human Liprin-alpha family of scaffolding proteins: genomic organization, expression profiling and regulation by alternative splicing. Genomics 93: 243–253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.