Abstract
Inorganic manganese based particles are becoming attractive for molecular and cellular imaging, due to their ability to provide bright contrast on MRI, as opposed to the dark contrast generated from iron based particles. Using a single emulsion technique, we have successfully fabricated pH sensitive, poly(lactic-co-glycolic acid) (PLGA) encapsulated manganese oxide (MnO) nanocrystals. Two classes of particles were fabricated at ~ 140 nm and 1.7 μm, and incorporated 15 to 20 nm MnO nanocrystals with high encapsulation efficiencies. Intact particles at physiological pH cause little contrast in MRI, but following endocytosis into low pH compartments within the cells, the particles erode, and MnO dissolves to release Mn2+. This causes the cells to appear bright on MR images. The magnitude of the change in MRI properties is as high as 35-fold, making it the most dynamic ‘smart’ MRI contrast agent yet reported. Possible applications of these MnO particles include slow release Mn2+, tumor targeting, and confirmation of cell uptake.
Keywords: MRI, manganese, cells, contrast agents, PLGA, nanoparticles, microparticles
The manganese ion (Mn2+) has been a useful MRI contrast agent since the first description of its use in 1979 to delineate cardiac infarct in dogs.1 Mn2+ is almost always delivered as a water-soluble manganese salt and has been delivered by a myriad of routes, including intravenous (i.v.), 2 intraparenchymal (i.p.), 3 or subcutaneous injection,4 directly into the brain 5 or nose, 6 or peripherally. 7 In living systems, water-soluble Mn2+ is handled similarly to Ca2+ and can enter cells via voltage gated calcium channels. 8 Internalized Mn2+ can furthermore travel down active neurons and leap neuronal junctions. 6 These are the basic principles behind dynamic activity induced manganese (AIM) imaging 9 and manganese enhanced MRI (MEMRI).10
Recently, interest has gained in inorganic manganese-based particles for molecular and cellular MRI. 11–16 Particle cores have taken the form of manganese oxides (MnO, Mn3O4, and MnO2) and manganese carbonate (MnCO3). These cores are insoluble in water, and without particle coatings, cores precipitate out of solution. In most cases, particles have very low molar relaxivities, ≤ 0.5 mM−1s−1, owing to the lack of water contact with the manganese atoms, and therefore cause little signal enhancement in MRI.
Inorganic manganese-based particles have been used as both a targeted nanoparticle (NP) for molecular MRI 13 as well as an MRI-based cell tracking T1 agent. 12 In both cases following administration of the NPs, either by i.v. injection 13 or by direct In Vitro cellular incubation, 12 bright contrast was observed by T1 weighted MRI. These results were interpreted as T1 contrast directly attributed to intact manganese particles.
While manganese oxides and carbonates are insoluble in water, they will dissolve slowly in acidic conditions. 11 It is well established that upon internalization into cells, NPs and microparticles (MPs) are shuttled to low pH compartments within the cells. 17,18 Thus, another interpretation of the above results is that bright contrast in manganese particle labeled cells is due to the evolved Mn2+, which has an r1 molar relaxivity of 7 mM−1sec−1, as a result of slow dissolution within low pH endosomes. This is supported by recent studies on dissolution of inorganic manganese cores in low pH buffers giving rise to bright contrast on MR images. 11 Indeed, if this is the case, then delivery of manganese particles to specific cell populations could serve as a slow release reservoir In Vivo. Furthermore, one can envision the use of these kinds of particles to verify endocytosis of delivered particles, as in a targeted MRI experiment, or whether particles simply remain extracellular. Lastly, these kinds of particles might prove useful in reporting on the location of tumors by way of their slightly acidic pH, resulting in evolved Mn2+ and bright contrast in MR images.
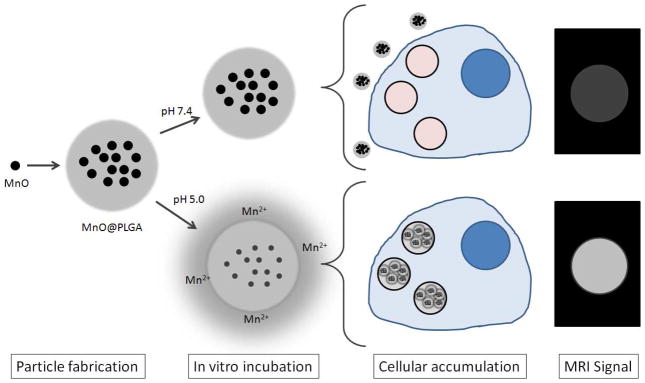
Here we propose a novel paradigm for utilizing MnO nanocrystals for molecular and cellular MRI by encapsulating high quantities of MnO within poly(lactic-co-glycolic acid) (PLGA) NPs and MPs (Figure 1). PLGA is a well described and characterized polymer long used for particle-based drug delivery due to its established safety, biocompatibility, and approval by the FDA. Based on previous success of magnetic cell labeling In Vitro with 150 nm dextran coated iron oxide nanoparticles 19 as well as both In Vitro 20 and In Vivo 21 with inert 1.63 μm sized iron oxide particles, we aimed to produce PLGA encapsulated MnO particles at these two size regimes. We demonstrate how these particles have very low r1 molar relaxivities as intact particles and elicit high r1 molar relaxivities upon dissolution in acidic media.
Figure 1.
Conceptual schematic of MnO encapsulation in PLGA, followed by release of Mn2+ in low pH. This results in increased r1, leading to enhancement in MRI. The In Vitro experiment is a mimic of lysosomal uptake of particles by cells.
15 to 20 nm MnO nanocrystals were fabricated by thermal decomposition of manganese (II) acetylacetonate (MnACAC) in benzyl ether and oleic acid at 300°C under nitrogen for 2 hours, similar to the established protocol of. 22 Upon cooling, the resulting MnO nanocrystals were precipitated in hexane and ethanol, collected by centrifugation, and dried overnight in a vacuum oven. Transmission electron microscopy (TEM) of the MnO cores shows 15–20 nm aggregates of several 5–10 nm cores (Figure 2). Crystal structure and molecular identity of the synthesized MnO nanocrystals were confirmed with powder x-ray diffraction (Supporting Figure 1).
Figure 2.
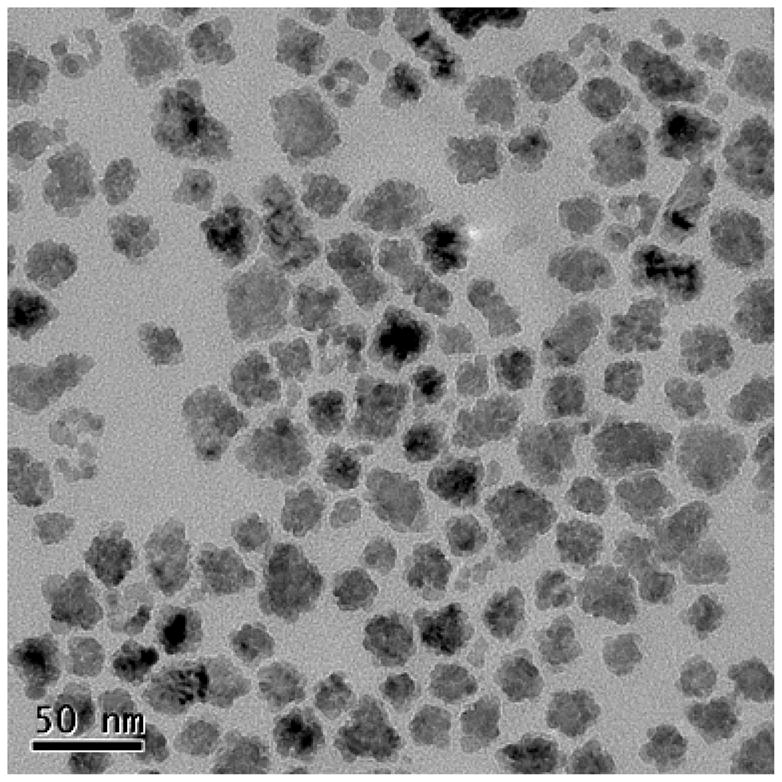
TEM of MnO nanocrystals. Sizes are approximately 15–20 nm.
Next, PLGA encapsulated MnO nanocrystals were fabricated with a single emulsion process, using methylene chloride as the organic solvent and polyvinyl alcohol (PVA) as the stabilizer. Particles were fabricated using either 50% or 100% w/w MnO:PLGA. Control particles were fabricated without MnO. Sonication and homogenization disruption techniques were utilized to form the primary emulsions for NPs and MPs, respectively. Following hardening, particles were washed with deionized water and dried on a lyophilizer. Fluorescent MnO NPs and MPs were fabricated by adding coumarin-6, a green fluorescent dye, at the same time as the MnO nanocrystals were added to the polymer/solvent mixture.
Size of the particles was assessed by scanning electron microscopy (SEM), and representative images of the NPs and the MPs are displayed in Figure 3A and 3B. Average NP and MP diameters were determined to be 140 ± 50 nm and 1.7 ± 0.9 μm, respectively. Supporting Figure 2A,B show histogram distributions of particle sizes. TEM of the intact PLGA encapsulated 100% w/w MnO NPs and MPs shows that the MnO nanocrystals were distributed throughout both the NPs and MPs (Figure 3C and 3D). Total manganese content of the particles was determined using thermogravimetric analysis (TGA), and the curves for all 4 formulations are shown in Supporting Figure 3. As displayed in Table 1, the weight percents and the encapsulation efficiencies (EEs) of MnO for all formulations were high. One reason why EEs may be over 100% could be due to some of the polymer being washed away, while incorporating most/all of the added MnO.
Figure 3.
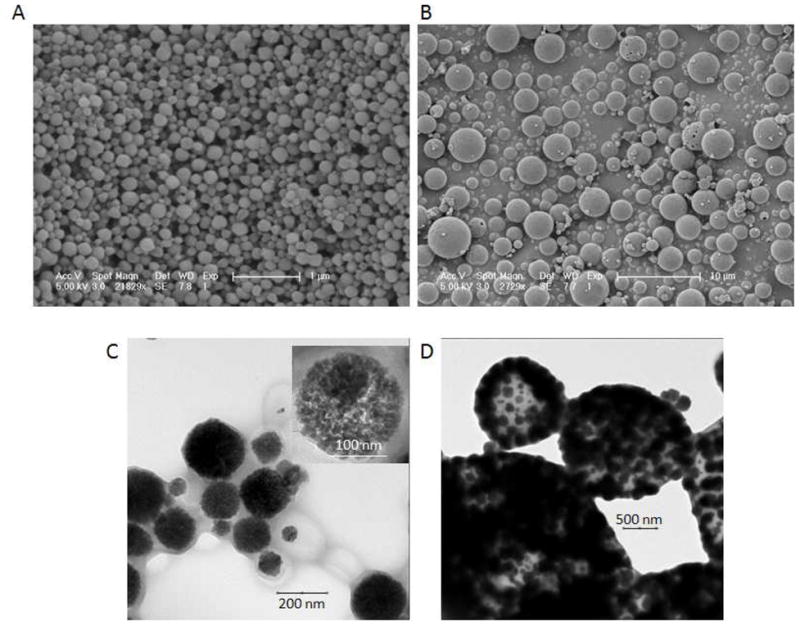
SEM (A,B) and TEM (C,D) of NPs and MPs. A) NPs and B) MPs had average sizes of approximately 140 nm and 1.7 μm, respectively. The scale bar for NPs is 1 μm and MPs is 10 μm. Note how the particles show a regular, spherical morphology. C) TEM of NPs with the insert showing nanocrystals distributed throughout a single particle. D) TEM of MPs also showing nanocrystals disbursed throughout.
Table 1.
Weight percents and encapsulation efficiencies for MnO NPs and MPs
| Particle Type | Wt % (MnO) | EE% |
|---|---|---|
| 50% MnO NP | 52.5 | 157.5 |
| 100% MnO NP | 62.5 | 125 |
| 50% MnO MP | 35 | 105 |
| 100% MnO MP | 46.5 | 93 |
Next, the MRI properties of these particles were measured at 4.0 Tesla. Molar relaxivity, r1 (mM−1sec −1), is defined as the efficiency of a contrast agent to modify the relaxation rate of water protons and is governed by the equation
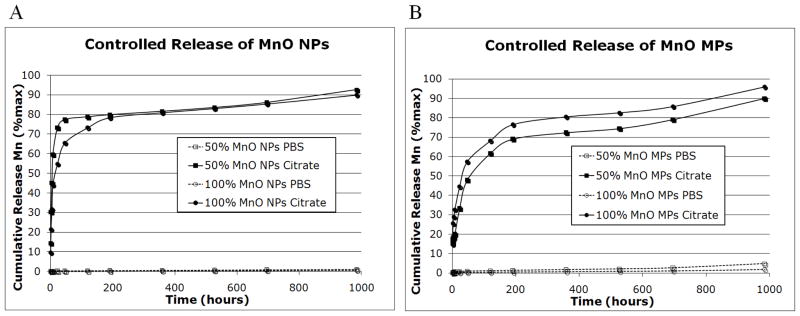
where R1 is the measured relaxation rate, R1,0 is the relaxation rate of the solvent, and [agent] is the concentration of the agent. Intact MnO NPs and MPs had very low r1 of 0.21 mM−1sec−1 and 0.54 mM−1sec−1, respectively. This is due to the lack of direct contact water molecules have with the nanocrystals themselves, as the nanocrystals are both hydrophobic in nature and likely still capped with oleic acid. To assess the dissolution characteristics of the particles in solution, a controlled release experiment was performed. Particles were incubated at 37°C in either phosphate buffered saline (PBS) pH 7.4 or 20 mM citrate buffer pH 5. The two different conditions were chosen to imitate the extracellular space/cytoplasm (PBS) and the acidic environment of endosomes (citrate) within the cell. 18 As discussed previously, it is well known that particles are shuttled to endosomes following cell uptake. 17 As shown in Figure 4, particles in the citrate buffer pH 5 showed significant release of Mn2+ over 41 days, whereas, particles incubated in PBS pH 7.4 did not. In addition, the release of Mn2+ from NPs was faster than MPs, as evidenced by the difference in steepness of the controlled release curves.
Figure 4.
Controlled release of MnO A) NPs and B) MPs in PBS pH 7 (dashed lines) and 20 mM citrate pH 5 (solid lines). Cumulative release of Mn2+ is shown as % release over time in hours. The total duration of the study was 41 days. Note the significant release of Mn2+ from particles incubated in citrate buffer. The 50% w/w MnO formulations are denoted by square data points, while the 100% w/w MnO formulations are denoted by circular data points.
To study dissolution of particles within cells, MnO NPs and MPs were incubated with RG2 cells (a glioblastoma tumor cell line) for 24 hours and evaluated for labeling and evolved T1 MRI contrast. Control cells received only tumor medium. To enable particle visualization by fluorescence microscopy, coumarin-6, a green fluorescent dye, was included in particle formulations at the same time as the MnO. After 24 hours, both NPs and MPs had successfully labeled RG2 cells with green fluorescence. Figure 5A,B display the overlay of the brightfield images of the RG2 cell boundaries with the fluorescence from the MPs. The green fluorescence of the MPs can be seen in some cells in both the 50% w/w (4A) and 100% w/w formulations (4B). In general, 1–5 MPs were observed within cells. An estimate can be made on the mass of Mn per MP based on volume, density of MnO and PLGA, and encapsulation efficiency, yielding ~ 1 pg Mn per 100% MnO MP. Therefore, each cell contains 1–5 pg Mn.
Figure 5.
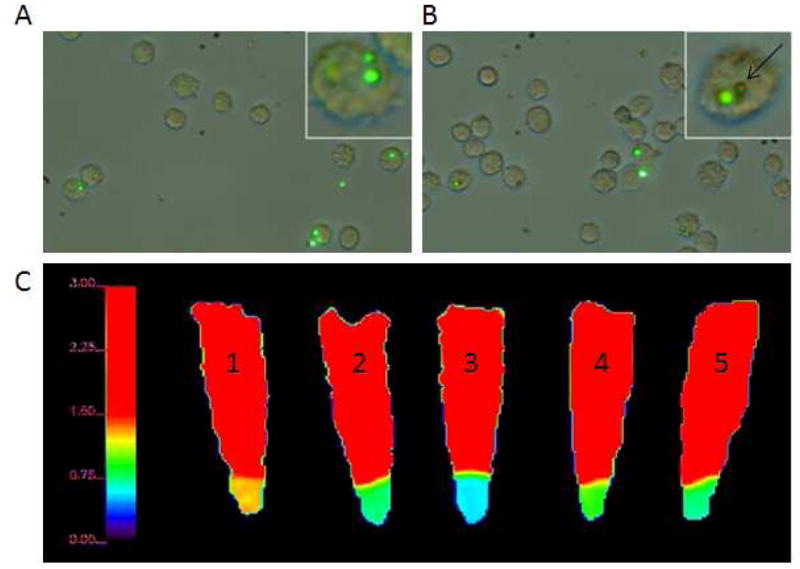
Brightfield and fluorescence microscopy of RG2 cells labeled with A) 50% MnO MPs and B) 100% MnO MPs. The green fluorescence is from the coumarin-6 inside the particles and is overlayed with the light microscopy image of the cells. Some of the MPs did not have robust fluorescence, but are still visible by the light microscopy image to be inside RG2 cells (a representative MP is indicated by a black arrow). C) T1 maps of control and labeled RG2 cell pellets. From left to right: 1) unlabeled RG2 cells, 2) 50% MnO NPs, 3) 100% MnO NPs, 4) 50% MnO MPs, and 5) 100% MnO MPs. The scaling of the T1 map is maximized at 1.50 seconds to enhance the differences of the particle labeled cells.
Cytotoxicity studies were performed following labeling using the well characterized MTS assay. 23 The MTS assay shows that at the concentration used to label RG2 cells (50 μM Mn2+), there is a slight, statistically significant toxic effect elicited by the MnO NPs and MPs, as evidenced from the decreased viability compared to control cells without particles. Yet, there was a slight toxic effect from empty particles as well, so it is difficult to ascertain where the toxicity arises from. However, this In Vitro assay, with particles incubating on cells in a static condition, does not accurately reflect the dynamic conditions In Vivo.
For MRI, after incubation, the cells were washed, trypsinized, resuspended in PBS, and pelleted, after which T1 maps were immediately acquired. As shown in Figure 5C, the T1 values of all the labeled cells were reduced compared to the control. Therefore, R1 was increased with the incubation of particles with the cells, and values are given in Table 2. In addition, the NPs exhibited greater R1 compared to the respective formulations of the MPs. The addition of particles to cells caused an increase in R1, in the following order: 50% MnO MP < 50% MnO NP = 100% MnO MP < 100% MnO NP. These results can be explained through the controlled release experiment, which showed that NPs released Mn2+ at a faster rate than MPs, due to a higher surface area to volume ratio. In addition, the 100% particle formulations contained more encapsulated MnO than the 50% formulations, and were able to cumulatively release more Mn2+, resulting in a larger increase in R1.
Table 2.
T1 and R1 values for control and labeled RG2 cell pellets
| Particle Type | T1 (sec) | R1 (sec−1) |
|---|---|---|
| NONE | 1.29 | 0.78 |
| 50% MNO NPS | 0.805 | 1.24 |
| 100% MNO NPS | 0.594 | 1.68 |
| 50% MNO MPS | 1.02 | 0.98 |
| 100% MNO MPS | 0.805 | 1.24 |
There are two possibilities to explain the positive contrast that resulted following cell uptake of MnO particles: 1) the intact particles themselves were sufficient to cause a significant reduction in T1, or 2) the particles dissolved and released Mn2+, which in turn reduced T1. Both phenomena are likely responsible to some extent. It is well known that internalization of metallic NPs and MPs into cells results in endosomal accumulation. The data presented in Figure 4 shows that particles incubated in citrate buffer pH 5, which mimics the acidic environment of endosomes, released Mn2+ compared to particles incubated in PBS pH 7.4 over the 41 day test period. Indeed, especially for the NPs, significant evolution of Mn2+ occurred within the first 24 hours. The results indicate that MnO particles will not degrade in the extracellular space, but will degrade upon internalization into cellular low pH endosomal compartments, releasing Mn2+ and subsequently causing positive contrast on T1 images.
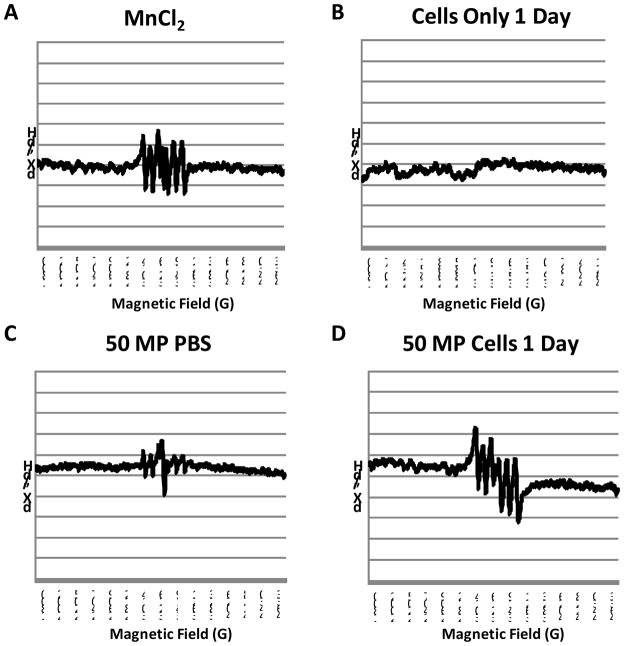
Without a histological marker for Mn2+, electron paramagnetic resonance spectroscopy (EPR) was used to determine whether Mn2+ evolved from MnO intracellularly. Figure 6 shows EPR spectra recorded at room temperature (298 K) for A) MnCl2 (free Mn2+), B) cells alone, C) MnO microparticles in pH 7.4 phosphate buffered saline (no free Mn2+) and D) cells incubated for 24 hours with MnO microparticles. Spectra of all samples are shown in Supporting Figure 5. The spectrum of free Mn2+ shows the six hyperfine lines characteristic of a 55Mn nucleus (I=5/2). The EPR spectrum from cells alone shows no signal. The EPR spectrum of intact particles in PBS shows a very weak Mn2+ signal, possibly due to residual surface-associated Mn2+ in the preparation. However, this weak signal is also present in PBS. The EPR spectrum of the cells incubated with the MnO microparticles clearly shows a robust Mn2+ spectrum, indicative of the intracellular evolution of Mn2+. In interpreting these results, it is crucial to understand that the EPR spectra of Mn2+ changes based on the binding environment of Mn2+. For example, Mn2+ that is free in solution exhibits sharp peaks as seen in the EPR spectrum for MnCl2 in Fig 6A. The EPR spectrum of tightly bound Mn2+ can be very broad due to major changes in its zero-field splitting parameters, and thus undetectable under our experimental conditions. 24
Figure 6.
EPR spectra of A) MnCl2 (free Mn2+), B) cells alone, C) 50% wt MnO MPs in pH 7.4 phosphate buffered saline (no free Mn2+) and D) cells incubated for 24 hours with 50% wt MnO MPs.
The EPR spectra of the cells incubated for 24 hours with the various MnO based particles all exhibit sharp peaks, meaning that the Mn2+ is in a symmetric coordination environment, and is not likely to be tightly bound in these samples. Whether there is a second tightly bound fraction, or whether there is significant Mn remaining as MnO is difficult to ascertain from these studies and is the subject of continuing research. There is also the strong possibility of Mn2+ release during the course of the experiment, which in the case of these particles being useful as a slow Mn2+ release vehicle, is desirable. However, there is confidence that the MRI contrast created by these PLGA encapsulated MnO particles inside cells is coming at least in part from the evolution of Mn2+.
The agents reported here are ‘smart’ contrast agents, having environmentally sensitive properties. The conversion of MnO in acidic environments to Mn2+ yielded a maximal change in r1 of 0.21 mM−1s−1 to 7 mM−1s−1, or nearly 35-fold. ‘Smart’ contrast agents are exciting for MRI as they have been useful for non-invasively probing unique biological and chemical processes within living subjects. In general, however, the dynamic range of the change in molar relaxivity of the agents in response to the biological or chemical stimulus is low. For example, EgadMe, the most recognizable ‘smart’ MRI contrast agent exhibits a 3-fold increase in r1 following enzymatic activation. 25 Various other ‘smart’ contrast agents designed to sense ions similarly exhibit 2.5 to 3-fold increases in r1. 26,27 A ‘smart’ contrast agent to sense pH exhibited a maximal 2-fold increase in r1, however over the pH range of 7.5 to 5.5, used in our study, r1 did not change. The change in r1 reported in the present paper is 10-fold higher than previously reported ‘smart’ contrast agents and are the most dynamic ‘smart’ contrast agents ever reported.
Slow releasing Mn2+ formulations have several potential applications within MRI. Teslascan, a chelate of manganese and the fodipir ligand, is already clinically approved for use in visualizing liver lesions, such as tumors or hepatocellular carcinomas. The chelate is metabolized in the body and transmetallation between zinc and manganese ions occurs to release free Mn2+.28,29 The normal liver tissue preferentially takes up the Mn2+, which causes bright contrast within the hepatocytes, but not within the lesions; lesions tend to not take up the contrast agent and are therefore dark on T1 MRI images. 30,31 An alternative mechanism to slow, persistent delivery of Mn2+ would be to use the types of particles described herein. It is well known that following vascular administration of MPs, significant accumulation in the liver occurs, with Kupfer cell uptake being the dominant mechanism. 32 Therefore, a passive targeting mechanism to the liver is possible, with slow Mn2+ release.
In addition, slowly releasing MnO formulations, such as the ones described here, can be used to selectively target and visualize tumors by increasing the positive contrast of the tumor itself. Increase of the tumor signal on T1 MRI images could result from two processes: 1) the MnO particles are taken up into the tumor cells, degraded within endosomes, and free Mn2+ released 2) tumors have a slightly acidic environment, ranging from pH 5.8 to 7.6 for rats and humans (median pH 7), which in itself, could cause dissolution of the particles and release of the Mn2+ 33. Selectivity to the tumor lesion can be achieved through the addition of ligands on the outside of the particles that target receptors, such as the folate receptor 34 or epidermal growth factor receptor, 35 which are overexpressed on tumor cells.
Furthermore, our MnO particle formulations can be used to verify endocytosis. Particles remaining in the extracellular space will not release free Mn2+, and will therefore, not elicit substantial T1 contrast; positive contrast on T1 images will then only be visible after internalization and dissolution of the MnO particles within endosomal compartments to release the free Mn2+. This could be particularly useful for MRI experiments monitoring immune cell infiltration. 36–39 However, as with all manganese based MRI experiments, desired manganese concentrations must be balanced by potential cytotoxicity. 16,40 Indeed, the particles formulated in this work exhibited some cytotoxicity In Vitro and it remains to be determined whether this In Vitro assay is predicative for In Vivo toxicity.
In conclusion, we have fabricated and characterized PLGA encapsulated MnO NPs and MPs. Intact particles had low r1. Following incubation in acidic media, the r1 increased due to the evolution of free Mn2+. T1 values of cells incubated with particles for 24 hours were decreased relative to unlabeled cells. Analyses were performed demonstrating that dissolution of the MnO to form free Mn2+ occurs following cell internalization into acidic endosomal compartments and likely contributes to changes in relaxation times of labeled cells.
Materials and Methods
Synthesis of MnO cores
Uniform 15 to 20 nm crystals of MnO were synthesized by controlled thermal decomposition of manganese (II) acetylacetonate (MnACAC) similar to the established protocol of. 22 To generate MnO nanocrystals, 2 mmol of MnACAC was dissolved in 20 ml of benzyl ether. To this, 2 ml of oleic acid was added. While stirring, the solution was then rapidly heated to 300°C under nitrogen gas for 2 hours, after which it was allowed to cool to room temperature. The nanocrystals were washed and precipitated by ethanol, collected by centrifugation at 9,000 RPM for 10 minutes, dispersed in hexane, and dried overnight in a vacuum oven after the hexane had evaporated.
PLGA encapsulation
50:50 ester-terminated PLGA, MW = 100–150 kDa with an inherent viscosity range of 0.95–1.2 dl/g, was purchased from Durect Corporation (Lactel Absorbable Polymers, Pelham, AL) and polyvinyl alcohol (PVA, MW = 12–23 kDa; 87–89% hydrolyzed) was obtained from Sigma–Aldrich (St. Louis, MO).
NPs were prepared using a single emulsion technique. 100 mg of PLGA polymer was dissolved in 2 ml of methylene chloride in a glass tube. Either 50 mg or 100 mg of MnO dried nanocrystals were added directly to the tube of the polymer/solvent mixture, which was subsequently sonicated in a water bath and vortexed to disperse the nanocrystals. Control particles were fabricated without MnO. This organic mixture was added dropwise to 4 ml of an aqueous 5% w/v solution of PVA while vortexing at high speed. This mixture was vortexed for an additional 10 s at a high setting. The tube contents were then sonicated for 3 × 10 s at 40% amplitude with a Sonicator 350 cell disruptor (Branson Ultrasonics, Danbury, CT) to create an oil-in-water emulsion. Immediately after sonication, the emulsion was poured into 60 ml of an aqueous 0.3% w/v PVA solution, under rapid stirring with a magnetic stirrer. The resulting nano-sized particles were stirred in solution for 3 h to allow for methylene chloride evaporation. The NPs were then collected by centrifugation at 12,000 RPM for 10 minutes, washed 3 times with deionized water, resuspended in deionized water, flash frozen in liquid nitrogen, and dried on a lyophilizer.
MPs were prepared similarly to NPs except for four differences: 1) The polymer/solvent/MnO nanocrystals mixture was added dropwise to 4 ml of an aqueous 1% w/v solution of PVA while vortexing at high speed. 2) This mixture was sonicated on a T 10 basic ULTRA-TURRAX® homogenizer (IKA, Wilmington, NC) at the power setting 5.5 for 3 × 10 s. 3) This oil-in-water emulsion was poured into 60 ml of an aqueous solution of pure deionized water. 4) After the methylene chloride evaporation step, the MPs were collected by centrifugation at 4,000 RPM for 10 minutes. Washing and freeze drying was similar to NPs.
Fluorescent MnO NPs and MPs were fabricated by adding 500μl of a 2 mg/ml methylene chloride solution of coumarin-6, a green fluorescent dye, to all formulations at the same time as the MnO nanocrystals were added to the polymer/solvent mixture.
Physical characterization of cores and particles
MnO nanocrystals and intact particles were characterized immediately after synthesis. Powder x-ray diffraction (XRD) was performed on ~ 100 mg samples of dry MnO, utilizing a Scintag PAD V diffractometer, in the Department of Geology and Geophysics, Yale University, and was used to identify crystal structure and molecular identity. Transmission electron microscopy (TEM) was performed on MnO nanocrystals and PLGA encapsulated particles dried on carbon coated copper grids. A Tecnai 12 Biotwin system was utilized, operating at 120 kV in the Yale Center for Cellular and Molecular Imaging, to measure the size of MnO nanocrystals and to analyze core distribution within particles. Scanning electron microscopy (SEM) images of the intact, lyophilized particles were taken on a JEOL JXA-8600 Electron Microprobe in the Department of Geology and Geophysics, Yale University. Average particle size was determined through post processing of the SEM images with ImageJ. Total manganese content of the particles was investigated by thermogravimetric analysis (TGA) using the TA Instruments Q50 model (New Castle, DE).
MRI properties of particles
Two different MRI experiments were performed. The first was the measurement of r1 molar relaxivities of intact MnO nano- and microparticles formulated with 100% w/w MnO. Particles were suspended in phosphate buffered saline (PBS) at pH 7.4 and subsequently suspended in 0.5% agarose to achieve Mn2+ concentrations of 2.5, 1.25, 0.625 and 0.313 mM. The last tube contained only agarose and saline. Agarose was necessary to prevent sedimentation during T1 mapping.
MRI was performed at 4.0 T on a Bruker Biospec horizontal bore spectrometer. T1 mapping was generated by the saturation recovery method using a short TE gradient echo sequence. 10 repetition times logarithmically spaced from 25 ms to 5 s were used to acquire images of the tubes. T1 fitting was accomplished offline using the T1 relaxation equation, which was executed by IDL (Boulder, CO).
To measure the longitudinal MRI properties of particles, NPs and MPs were resuspended in two different solutions. The first solution was PBS pH 7.4, mimicking the extracellular pH in tissue and intracellular cytosolic pH. The second solution was 20 mM sodium citrate at pH 5, mimicking the acidic intracellular environment of endosomes. 18
10 mg each of 50% w/w and 100% w/w NPs and MPs were placed into separate 1.5 ml plastic vials, suspended in 1 ml of either the PBS or citrate buffer solution, and incubated with continual 360° inversion in a cell culture incubator at 37°C. At desired time points, the plastic vials were microcentrifuged at 10,000 RPM for 10 minutes to pellet the particles, and the supernatants transferred to other plastic vials for subsequent MRI. At each time point, 1 ml of fresh PBS or citrate buffer was added to the pelleted particles, followed by resuspension and incubation. The following time points were used: 1 hr, 2 hr, 5 hr, 8 hr, 1 d, 2 d, 5 d, 8 d, 15 d, 22 d, 29 d, and 41d post mixing particles in the solutions. MRI was performed on retrieved supernatants as described above, except without agarose. Mn2+ concentrations were calculated from the T1 values using a measured r1 for Mn2+ of 7 mM−1s−1.
Cell labeling and MRI
Rat RG2 glioma cells (American Type Culture Collection, Manassas, VA) were cultured in high glucose DMEM, supplemented with 10% FBS and 1% penicillin streptomycin. To evaluate the ability of the NPs and MPs to label cells, 20 μl of 5 mg/ml coumarin-6 doped, fluorescent MnO NP and MP solutions in media were added to confluent culture dishes of rat RG2 cells and allowed to incubate for 24 hours. Cells were then washed to remove free particles three times in PBS, trypsinized, and centrifuged at 1,000 RPM for 5 minutes. The cell pellet was resuspended in PBS; cells were pipetted onto a slide, coverslipped, and immediately examined on a Leica MZ16FA stereo motorized fluorescence microscope, equipped with a Leica DFC300 FX camera (Leica Microsystems, Cambridge, UK). To analyze whether particles were endocytosed, dual brightfield and green fluorescent photomicrographs were acquired. More than 50 cells were analyzed to determine the range of internalized particles per cell.
To investigate the ability of cells to endocytose particles and then dissolve the MnO nanocrystals, the same amount of nonfluorescent MnO NPs and MPs were added to confluent RG2 cells and incubated for 24 hours. Control cells received only the tumor culture medium. The cells were processed as above except that the resulting cell pellets were resuspended in PBS and transferred to 0.5 ml plastic vials. The vials were spun on a microcentrifuge at 2,000 RPM for 3 minutes to pellet the cells. Cell tubes then underwent T1 mapping as above, and both T1 and R1 values for the cell pellets were determined.
EPR Measurements
All EPR spectra were recorded at room temperature (298 K) on a Bruker ELEXYS E500 EPR spectrometer. Experimental parameters: 9.4 GHz microwave frequency, 1 mW power and 30 G modulation amplitude. Each sample was loaded onto a 10 μL capillary tube, which was then sealed with clay, and placed in an EPR tube (4 mm OD) to record the spectrum.
Cytotoxicity
An MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI) was performed to assess cell cytotoxicity after NP and MP exposure. Briefly, 1 × 104 RG2 cells were added to wells in a 96 well plate and grown to 70% confluency. Then, various concentrations of MnO PLGA NPs and MPs, as well as blank PLGA NPs and MPs, were added to the cells and incubated at 37°C. Control wells had just fresh tumor medium added to the cells. After 48 hours, cells were washed with RG2 medium and then 100 μL of fresh medium was added to each well, followed by 20 μL of the MTS reagent. The plate was incubated for 3.5 hours at 37°C. Viability was quantified using a microplate reader at absorbance wavelengths of 490 nm and 700 nm (700 nm was used as reference). Percent viability was calculated by dividing the absorbance value for each sample well by the average absorbance value of the control wells, multiplied by 100. All samples were assayed in quadruplicate.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DP2 OD004362 and NS052519.
Footnotes
Supporting information available.
X-ray diffraction of MnO cores, histogram analysis of nano- and microparticle diameters, TGA traces of polymer encapsulated MnO nanocrystals, cytotoxicity data and EPR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.Lauterbur PC. Progress in N.m.r. Zeugmatography Imaging. Philos Trans R Soc Lond B Biol Sci. 1980;289:483–487. doi: 10.1098/rstb.1980.0066. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-Enhanced Magnetic Resonance Imaging of Mouse Brain After Systemic Administration of MnCl2: Dose-Dependent and Temporal Evolution of T1 Contrast. Magn Reson Med. 2005;53:640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- 3.Tambalo S, Daducci A, Fiorini S, Boschi F, Mariani M, Marinone M, Sbarbati A, Marzola P. Experimental Protocol for Activation-Induced Manganese-Enhanced MRI (AIM-MRI) Based on Quantitative Determination of Mn Content in Rat Brain by Fast T1 Mapping. Magn Reson Med. 2009;62:1080–1084. doi: 10.1002/mrm.22095. [DOI] [PubMed] [Google Scholar]
- 4.Angenstein F, Niessen HG, Goldschmidt J, Lison H, Altrock WD, Gundelfinger ED, Scheich H. Manganese-Enhanced MRI Reveals Structural and Functional Changes in the Cortex of Bassoon Mutant Mice. Cereb Cortex. 2007;17:28–36. doi: 10.1093/cercor/bhj121. [DOI] [PubMed] [Google Scholar]
- 5.Tucciarone J, Chuang KH, Dodd SJ, Silva A, Pelled G, Koretsky AP. Layer Specific Tracing of Corticocortical and Thalamocortical Connectivity in the Rodent Using Manganese Enhanced MRI. Neuroimage. 2009;44:923–931. doi: 10.1016/j.neuroimage.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pautler RG, Silva AC, Koretsky AP. In Vivo Neuronal Tract Tracing Using Manganese-Enhanced Magnetic Resonance Imaging. Magn Reson Med. 1998;40:740–748. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda K, Wang HX, Suo C, McCombe D, Horne MK, Morrison WA, Egan GF. Retrograde Axonal Tracing Using Manganese Enhanced Magnetic Resonance Imaging. Neuroimage. 2010;50:366–374. doi: 10.1016/j.neuroimage.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Koretsky AP. Manganese Enhanced Magnetic Resonance Imaging. Curr Pharm Biotechnol. 2004;5:529–537. doi: 10.2174/1389201043376607. [DOI] [PubMed] [Google Scholar]
- 9.Aoki I, Tanaka C, Takegami T, Ebisu T, Umeda M, Fukunaga M, Fukuda K, Silva AC, Koretsky AP, Naruse S. Dynamic Activity-Induced Manganese-Dependent Contrast Magnetic Resonance Imaging (DAIM MRI) Magn Reson Med. 2002;48:927–933. doi: 10.1002/mrm.10320. [DOI] [PubMed] [Google Scholar]
- 10.Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-Enhanced Magnetic Resonance Imaging (MEMRI): Methodological and Practical Considerations. NMR Biomed. 2004;17:532–543. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro EM, Koretsky AP. Convertible Manganese Contrast for Molecular and Cellular MRI. Magn Reson Med. 2008;60:265–269. doi: 10.1002/mrm.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K, Hyeon T, van Zijl PC, Bulte JW. MR Tracking of Transplanted Cells With “Positive Contrast” Using Manganese Oxide Nanoparticles. Magn Reson Med. 2008;60:1–7. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan D, Senpan A, Caruthers SD, Williams TA, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Sensitive and Efficient Detection of Thrombus With Fibrin-Specific Manganese Nanocolloids. Chem Commun (Camb ) 2009:3234–3236. doi: 10.1039/b902875g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin J, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Hollow Manganese Oxide Nanoparticles As Multifunctional Agents for Magnetic Resonance Imaging and Drug Delivery. Angew Chem Int Ed Engl. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 15.Huang CC, Khu NH, Yeh CS. The Characteristics of Sub 10 Nm Manganese Oxide T1 Contrast Agents of Different Nanostructured Morphologies. Biomaterials. 2010;31:4073–4078. doi: 10.1016/j.biomaterials.2010.01.087. [DOI] [PubMed] [Google Scholar]
- 16.Choi JY, Lee SH, Na HB, An K, Hyeon T, Seo TS. In Vitro Cytotoxicity Screening of Water-Dispersible Metal Oxide Nanoparticles in Human Cell Lines. Bioprocess Biosyst Eng. 2010;33:21–30. doi: 10.1007/s00449-009-0354-5. [DOI] [PubMed] [Google Scholar]
- 17.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE. Highly Efficient Endosomal Labeling of Progenitor and Stem Cells With Large Magnetic Particles Allows Magnetic Resonance Imaging of Single Cells. Blood. 2003;102:867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 18.Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA. A Model of Lysosomal Metabolism of Dextran Coated Superparamagnetic Iron Oxide (SPIO) Nanoparticles: Implications for Cellular Magnetic Resonance Imaging. NMR Biomed. 2005;18:383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- 19.Bulte JW, Duncan ID, Frank JA. In Vivo Magnetic Resonance Tracking of Magnetically Labeled Cells After Transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro EM, Skrtic S, Koretsky AP. Sizing It Up: Cellular MRI Using Micron-Sized Iron Oxide Particles. Magn Reson Med. 2005;53:329–338. doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro EM, Gonzalez-Perez O, Garcia-Verdugo JM, Alvarez-Buylla A, Koretsky AP. Magnetic Resonance Imaging of the Migration of Neuronal Precursors Generated in the Adult Rodent Brain. Neuroimage. 2006;32:1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin M, O’Brien S. Synthesis of Monodisperse Nanocrystals of Manganese Oxides. Journal of the American Chemical Society. 2003;125:10180–10181. doi: 10.1021/ja0362656. [DOI] [PubMed] [Google Scholar]
- 23.Borenfreund E, Babich H, Martin-Alguacil N. Comparisons of Two in Vitro Cytotoxicity Assays-The Neutral Red (NR) and Tetrazolium MTT Tests. Toxicol In Vitro. 1988;2:1–6. doi: 10.1016/0887-2333(88)90030-6. [DOI] [PubMed] [Google Scholar]
- 24.Reed GH, Ray WJ., Jr Electron Paramagnetic Resonance Studies of Manganese (II) Coordination in the Phosphoglucomutase System. Biochemistry. 1971;10:3190–3197. doi: 10.1021/bi00793a005. [DOI] [PubMed] [Google Scholar]
- 25.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In Vivo Visualization of Gene Expression Using Magnetic Resonance Imaging. Nat Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 26.Li WH, Parigi G, Fragai M, Luchinat C, Meade TJ. Mechanistic Studies of a Calcium-Dependent MRI Contrast Agent. Inorg Chem. 2002;41:4018–4024. doi: 10.1021/ic0200390. [DOI] [PubMed] [Google Scholar]
- 27.Major JL, Parigi G, Luchinat C, Meade TJ. The Synthesis and in Vitro Testing of a Zinc-Activated MRI Contrast Agent. Proc Natl Acad Sci U S A. 2007;104:13881–13886. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toft KG, Hustvedt SO, Grant D, Martinsen I, Gordon PB, Friisk GA, Korsmo AJ, Skotland T. Metabolism and Pharmacokinetics of MnDPDP in Man. Acta Radiologica. 1997;38:677–689. doi: 10.1080/02841859709172400. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt PP, Toft KG, Skotland T, Andersson KK. Stability and Transmetallation of the Magnetic Resonance Contrast Agent MnDPDP Measured by EPR. Journal of Biological Inorganic Chemistry. 2002;7:241–248. doi: 10.1007/s007750100290. [DOI] [PubMed] [Google Scholar]
- 30.Martin DR, Semelka RC, Chung JJ, Balci NC, Wilber K. Sequential Use of Gadolinium Chelate and Mangafodipir Trisodium for the Assessment of Focal Liver Lesions: Initial Observations. Magnetic Resonance Imaging. 2000;18:955–963. doi: 10.1016/s0730-725x(00)00198-3. [DOI] [PubMed] [Google Scholar]
- 31.King LJ, Burkill GJC, Scurr ED, Vlavianos P, Murray-Lyons I, Healy JC. MnDPDP Enhanced Magnetic Resonance Imaging of Focal Liver Lesions. Clinical Radiology. 2002;57:1047–1057. doi: 10.1053/crad.2002.1117. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In Vivo Detection of Single Cells by MRI. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 33.Tannock IF, Rotin D. Acid PH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 34.Kamaly N, Kalber T, Thanou M, Bell JD, Miller AD. Folate Receptor Targeted Bimodal Liposomes for Tumor Magnetic Resonance Imaging. Bioconjug Chem. 2009;20:648–655. doi: 10.1021/bc8002259. [DOI] [PubMed] [Google Scholar]
- 35.Mamot C, Rochlitz C. Targeting the Epidermal Growth Factor Receptor (EGFR)--a New Therapeutic Option in Oncology? Swiss Med Wkly. 2006;136:4–12. doi: 10.4414/smw.2006.10770. [DOI] [PubMed] [Google Scholar]
- 36.Pirko I, Ciric B, Johnson AJ, Gamez J, Rodriguez M, Macura S. Magnetic Resonance Imaging of Immune Cells in Inflammation of Central Nervous System. Croat Med J. 2003;44:463–468. [PubMed] [Google Scholar]
- 37.Hu DE, Kettunen MI, Brindle KM. Monitoring T-Lymphocyte Trafficking in Tumors Undergoing Immune Rejection. Magn Reson Med. 2005;54:1473–1479. doi: 10.1002/mrm.20717. [DOI] [PubMed] [Google Scholar]
- 38.Ahrens ET, Flores R, Xu H, Morel PA. In Vivo Imaging Platform for Tracking Immunotherapeutic Cells. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 39.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In Situ Labeling of Immune Cells With Iron Oxide Particles: an Approach to Detect Organ Rejection by Cellular MRI. Proc Natl Acad Sci U S A. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki I, Takahashi Y, Chuang KH, Silva AC, Igarashi T, Tanaka C, Childs RW, Koretsky AP. Cell Labeling for Magnetic Resonance Imaging With the T1 Agent Manganese Chloride. NMR Biomed. 2006;19:50–59. doi: 10.1002/nbm.1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.