Abstract
In cortical pyramidal neurons, the axon initial segment (AIS) is pivotal in synaptic integration. It has been asserted that this is because there is a high density of Na+ channels in the AIS. However, we found that action potential–associated Na+ flux, as measured by high-speed fluorescence Na+ imaging, was about threefold larger in the rat AIS than in the soma. Spike-evoked Na+ flux in the AIS and the first node of Ranvier was similar and was eightfold lower in basal dendrites. At near-threshold voltages, persistent Na+ conductance was almost entirely axonal. On a time scale of seconds, passive diffusion, and not pumping, was responsible for maintaining transmembrane Na+ gradients in thin axons during high-frequency action potential firing. In computer simulations, these data were consistent with the known features of action potential generation in these neurons.
In neocortical pyramidal cells, as in many CNS neurons, the AIS has a pivotal integrative role because it has a lower threshold for action potential generation than the soma and dendrites1 and thus controls both the transformation of dendrosomatic synaptic input into spike output and the backpropagation of action potentials into the dendrites. The properties of Na+ channels in the AIS are reported to differ from those in other regions of the cell. For example, several studies have found that the activation voltage of Na+ channels is 6–14 mV more hyperpolarized in the axon than in the soma2–4. Moreover, the AIS has been implicated as the primary source of persistent Na+ current (INaP)5,6.
One major subject of disagreement is whether the density of Na+ channels is substantially greater in the AIS than in the soma. An early computational study7 found that, in an anatomically correct compartmental model of a pyramidal neuron with identical Na+ channel properties in all regions, Na+ channel density in the AIS must be orders of magnitude higher in the AIS than in the soma to simulate the lower axonal threshold. However, subsequent patch recordings from the AIS suggested that the density in the two regions is similar2,3,8. More recently, in the study in which patch recordings indicated equal densities, the authors3 presented several arguments why this result might not be correct and asserted that the true Na+ channel density in the AIS is much higher than in the soma or dendrites. A similar conclusion was reached on the basis of recordings from blebs that form when cortical axons are cut4.
We used high-speed fluorescence imaging of the Na+ indicator sodium-binding benzofuran isophthalate (SBFI)9,10 to quantitatively describe the Na+ dynamics that accompany subthreshold depolarizations and single and multiple action potential generation in axons, dendrites and somata of layer 5 neocortical neurons. Our measurements of Na+ flux in axon, soma and basal dendrites suggest that the ratios of Na+ channel densities in these regions is approximately 3:1:0.3. These results provide evidence for the axonal location of the subthreshold persistent Na+ conductance and indicate that diffusion is the main removal mechanism following Na+ entry in the AIS and first node of Ranvier.
RESULTS
Na+ transients in the axon decay faster than in the soma
In 197 layer 5 pyramidal neurons, we recorded changes in SBFI fluorescence during single or multiple action potentials elicited by brief somatic current injections in the soma and nearby axon and basal dendrites. All the fluorescence transients were blocked by bathapplied tetrodotoxin (1 µM, n = 4). During experiments, axons were distinguished from the other fine processes because they emerged from the soma opposite the apical dendrite and had distinctive Na+ transients (see below). We examined 36 neurons live in a two-photon microscope after the physiological experiment. In these cells, axons were readily distinguished from basal dendrites by their lack of spines (Fig. 1a), confirming the determination that had been made during recording. These reconstructions were used to measure the dimensions of the axon, soma and dendrites, which were later used to estimate the relative Na+ fluxes in the different compartments.
Figure 1.
Time course of action potential–induced [Na+]i changes is different in different compartments. (a) Reconstruction of 58 optical sections taken at 1-µm intervals through part of a layer 5 pyramidal neuron filled with 2 mM SBFI. (b) Left, the same neuron as seen during the fluorescence imaging experiment with a NeuroCCD-SMQ camera. The rectangles and arrows indicate the regions from which fluorescence measurements were obtained. Middle, averaged Na+ transients (n = 20) elicited by a single action potential were only prominent in the axon. Between 0–30 µm, the transients peaked sharply at the time of the spike, whereas the rise was more gradual between 30 and 40 µm. Right, averaged Na+ transients (n = 10) elicited by a train of ten spikes (33 Hz) were detected in soma, basal and apical dendrites, although they were larger in the axon. In the proximal axon, [Na+]i grew throughout the duration of the train; immediately after the train, the [Na+]i declined rapidly. In soma and dendrites, [Na+]i stayed at a nearly steady level after the end of the spike train.
In a representative neuron, averaged Na+ transients (n = 20) elicited by single action potentials were prominent only in the axons and were poorly or not at all detectable in soma and dendrites (Fig. 1b; see also Supplementary Movie 1). The greatest change in axonal SBFI fluorescence occurred 10–30 µm from the soma. The intracellular Na+ concentration ([Na+]i) grew rapidly (10–90% rise time less than 10 ms) and decayed rapidly with a time constant of 200–600 ms. At distances beyond 35–50 µm, in the presumed myelinated region of the axon11, Na+ transient amplitudes were smaller and their peaks were progressively delayed (Fig. 1b). The amplitudes and time courses of the fluorescence transients are expected to accurately reflect the entry and removal of Na+ from the cell, as Na+ is not substantially buffered by either components of the cytoplasm or the Na+ indicator (see Online Methods).
When trains of three or more action potentials were generated, we also detected Na+ signals in the soma and basal and apical dendrites, although they remained largest in the proximal axon (Fig. 1b). The most notable difference was in the rates of Na+ clearance. In the soma and dendrites, [Na+]i either stayed at a plateau level or decayed slowly after the end of the spike train; [Na+]i recovery was often not complete even 10 s following the cessation of stimuli. In contrast, [Na+]i rapidly declined immediately on termination of the stimuli in the proximal axon.
Rapid Na+ removal in axons is not due to active transport
We used two experimental manipulations to decrease the activity of the pump. First, we cooled the slices from 33 °C to 23 °C. This had only a minimal effect on the decay time constant of the Na+ transients in the proximal axon (τ = 0.42 ± 0.16 s at 33 °C versus 0.54 ± 0.10 s at 23 °C, P > 0.05, n = 4; Fig. 2).
Figure 2.
Active transport cannot account for the rapid Na+ clearance in the axon. Left, the decay of axonal Na+ transients ~20 µm from the soma elicited by a train of ten action potentials was not affected by heating of the slice from 22 °C to 32 °C. Thin traces are superimposed best fits to a single exponential at 22 °C, 32 °C and on return of the temperature back to 22 °C. The difference was undetectable. Right, blockade of Na+/K+-ATPase with bath-applied oubain (100 µM) had little effect on axonal Na+ clearance. Thin traces are superimposed best fits of decay of the Na+ transients in control, during oubain application and following washout.
Second, bath-applied oubain (100 µM), which blocks the Na+/K+-ATPase12, had little or no effect on axonal Na+ clearance (τ = 0.34 ± 0.18 s in control versus 0.37 ± 0.21 s with oubain, P > 0.05, n = 3). Therefore, in the time frame of our experiments, active transport is not responsible for the dynamics of Na+ clearance from the axons.
Na+ dynamics reflects localized Na+ influx and diffusion
We next examined whether spike-evoked Na+ entry and subsequent axial diffusion is sufficient to account for the time course and observed rapid Na+ clearance in the axons by comparing experimentally observed Na+ transients with the results of numerical simulations. We built a simplified compartmental model that encompassed the fundamental morphological features of a layer 5 pyramidal neuron. In the model, the first 35–50 µm of the axonal length were assumed to be uncovered by the myelin sheath11 and to possess a uniform Na+ channel density about threefold higher than that of the soma (see Online Methods). The subsequent, myelinated segment possessed no Na+ channels. In addition to Hodgkin-Huxley type Na+ and K+ conductances, the model incorporated longitudinal diffusion of Na+ ions between neighboring compartments with a diffusion coefficient of 0.6 µm2 ms−1 (ref. 13); no mechanism for Na+ extrusion was included. Thus, the spatio-temporal patterns of the simulated [Na+]i transients solely reflected transmembrane influx into the ‘active’ compartments that possess Na+ channels (soma and AIS) and Na+ diffusion into compartments that either possess no Na+ channels (myelinated segments) or are large sinks because they have a relatively small surface-to-volume ratio (soma). In all of the active locations in the model, [Na+]i grew throughout the action potential train; it continued to grow after the termination of the train in the passive myelinated internodes, reflecting diffusion of Na+ from the adjacent AIS (Fig. 3). The initial parts of the decay time constants of the simulated Na+ transients were fastest at the most distal and proximal extremes of the AIS and increased toward its center. Within several tens of milliseconds, however, the rate of Na+ diffusion from the AIS into the myelinated internodes slowed, reflecting the progressive decrease in the steepness of the Na+ concentration gradient. In contrast, the steep axo-somatic Na+ gradient persisted, as [Na+]i in the relatively immense soma remained low and nearly constant.
Figure 3.
Axonal Na+ transients reflect localized Na+ influx into the AIS followed by diffusion to the soma and first myelinated internode. (a) Experimentally observed (left) and simulated (right) changes in [Na+]i elicited by a single action potential. (b) Similar [Na+]i changes elicited by ten action potentials at the indicated locations. The changes peaked after the end of the spike train (dashed line) at the dark blue and pink locations. (c) The same [Na+]i changes plotted as a function of distance from the soma for three different times after the last spike. Dots are average values (n = 9). (d) Time-to-peak of the Na+ transients elicited by a single action potential versus distance from the axon hillock (n = 5). Red continuous line calculated from a simplified model, assuming a 50-µm AIS. (e) [Na+]i changes elicited by ten action potentials in pyramidal neurons (L5) and Purkinje neurons (PC). Left, time-to-peak of the Na+ transients in pyramidal neurons (black, n = 9) and in Purkinje neurons (blue, n = 5; see Supplementary Fig. 1) versus distance from the soma. Continuous lines are fitted from the model, which assumed an AIS length of 38 µm for pyramidal cells (red) and 15 µm for Purkinje cells (blue). Right, effect of AIS length on width of the axonal Na+ transients 10 µm from the hillock of pyramidal (black, n = 8) and Purkinje neurons (blue, n = 5). Points are at the AIS lengths for the two cell types (15 µm for Purkinje cells and 40 µm for pyramidal cells). The red continuous line is the half-width of the simulated transients.
The model closely mimicked experimentally observed changes in fluorescence during a single action potential (Fig. 3a) as well as action potential trains (Fig. 3b,c). Amplitudes of [Na+]i elevations were largest in the AIS, where the time-to-peak was fastest and uniform throughout the segment (Fig. 3a,b). In the first myelinated segment, in which simulated Na+ transients only reflected longitudinal Na+ diffusion from the AIS, time-to-peak grew and amplitude declined as a function of the distance from the site of Na+ influx (Fig. 3a,b,d).
Finally, we compared the Na+ transients in cortical neurons to those in cerebellar Purkinje cells (Supplementary Fig. 1), which possess a much shorter AIS of only 15 µm (ref. 14). Both the spatial distribution of the times-to-peak of the Na+ transients along the axonal length and the half-width at a distance of 10 µm from the hillock differed significantly between the two types of neurons (P < 0.05; Fig. 3e). The difference was completely predicted by diffusion models by changing the AIS length of 38 µm in cortical neurons to 15 µm for Purkinje cells (Fig. 3e).
Relative spike-evoked Na+ influx in different compartments
Because the dynamics of the Na+ transients for short time periods can be entirely described in terms of influx and diffusion, we can use the fluorescence measurements to estimate the relative fluxes of Na+ ions into the different compartments during action potential generation. However, inference of spike-evoked Na+ influx per unit membrane area (QNa) from the imaging data requires careful consideration of differences in the surface-to-volume ratio, background tissue fluorescence and other technical factors. We used three different approaches to evaluate relative QNa.
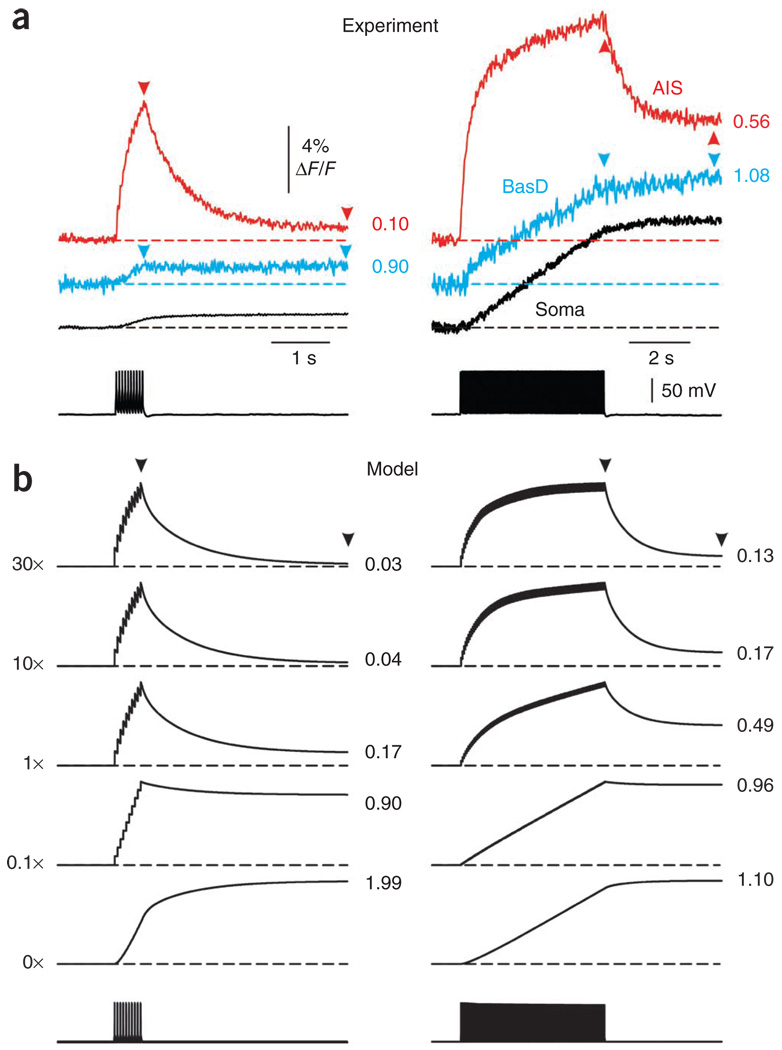
Our first approach focused on the shapes of the Na+ transients in the AIS, the soma and the basal dendrites. We elicited Na+ transients with trains of 10 and 100 action potentials (Fig. 4a). Under both protocols, [Na+]i grew steadily throughout the train in all neuronal compartments. In the AIS, [Na+]i declined rapidly after reaching its peak, achieving a steady level at about 2 s. In the soma and basal dendrites, the [Na+]i also stopped rising at the end of the train, but did not fall over the next few seconds. The steady level in all three compartments was about the same, indicating that diffusional equilibrium was achieved.
Figure 4.
The shape of spike-evoked Na+ transients constrains the ratio of Na+ channel densities in different compartments. (a) Na+ transients elicited by 10 (left) and 100 (right) action potentials at the soma (black) in a basal dendrite (20 µm from the edge of the soma, blue) and in the AIS (20 µm from the hillock, red) of a representative neuron. Arrowheads indicate the points where the steady state and the end-of-train fluorescence values were measured and the numbers are the ratios of the steady-state to end-of-train ΔF/F values. (b) Na+ transients in models with different process-to-soma ratios of Na+ channel density. Somatic Na+ channel density was kept constant and Na+ channel density in the process was varied over the range of 0–30-fold of the somatic density (numbers along the left side of the figure). The numbers to the right of the traces are the calculated ratios of the steady-state to end-of-train signal. The model required the basal dendrite Na+ channel density to be 0.1–0.3-fold larger than the somatic density and the AIS density to be one- to threefold larger than the somatic density to match the experimentally determined signals.
The rapid recovery of the AIS signal shows that the peak [Na+]i change in that compartment was much higher than the end-of-train [Na+]i in the soma. But it is not immediately clear whether this results from a higher QNa across the membrane or because the axon is thinner than the soma. That the dendritic signal did not decline after the train indicates that the [Na+]i change in the dendrite was close to the [Na+]i change in the soma. As the surface-to-volume ratio of the dendrite is much greater than that of the soma, this closeness suggests that QNa into the basal dendrite must be less than QNa into the soma.
To obtain a more quantitative estimate of the QNa ratio among the three compartments, we measured the peak and steady-state ΔF/F values and then constructed a simple compartmental model to reproduce these values. The ΔF/F steady state to end-of-train ratio of the AIS Na+ transients was 0.22 ± 0.07 (mean ± s.d., n = 7) following a train of ten action potentials and 0.47 ± 0.08 following a train of 100 action potentials; in the basal dendrites, the ratios were 1.29 ± 0.22 and 0.92 ± 0.13, respectively.
We next performed computer simulations to find the ratio of channel densities consistent with the measured ΔF/F time courses and relative magnitudes. The somatic Na+ channel density was kept constant, whereas Na+ channel density in the process (either axon, consisting of a 40-µm-long AIS followed by a myelinated internode, or basal dendrite) was systematically varied. To match the experimentally determined steady state to end-of-train ΔF/F ratios, the Na+ channel density in the AIS has to be one- to threefold larger than the somatic density and the Na+ channel density in the basal dendrite has to be 0.1–0.3-fold larger than the somatic density (Fig. 4b). The presence of a myelinated segment made only a small difference in the predicted recovery curves (data not shown).
A second approach to estimating relative QNa from the imaging data relies on the following considerations. First, Δ[Na+]i ≈ ΔF/F, where Δ[Na+]i is the Na+ concentration change per spike and ΔF/F is the fractional change in SBFI fluorescence. This relationship holds if the change in fluorescence is linear, which should be true because the KD for the Na+-SBFI interaction is high (26 mM)15 and ΔF/F values are ≪ 1. Second, , where QNa is the transmembrane Na+ influx per spike and V is the volume of the region of interest, assuming the removal rate of Na+ is slow compared with the rate of entry. Assuming that the indicator concentration is uniform throughout all compartments, which is reasonable for the peri-somatic region after 15 min of dialysis, then V ≈ F and QNa ≈ ΔF, as the fluorescence of SBFI in a region should be proportional to the volume of the region. This relationship allows us to directly compare the charge entries in different regions. Additional technical considerations are described in the Online Methods.
We measured ΔF in the soma, AIS and basal dendrites following trains of five action potentials at 10-ms intervals (Fig. 5a) or ten action potentials at 30-ms intervals. We found that ΔFAIS/ΔFsoma = 2.05 ± 0.27 for the faster train (mean ± s.d., n = 11) and 1.54 ± 0.42 for the slower train (n = 19), measured in a region around the soma and a rectangle over the axon. The ratios were sensitive to changes in the areas of the circles or rectangles (Supplementary Fig. 2), but the resulting error was not greater than 20%. If we correct the amplitude determined from the slower train for the reduction resulting from axial diffusion (increase by ~40%) then that ratio becomes 2.16 ± 0.59, which is close to the ratio determined from the faster train. The average is 2.10 ± 0.43. Applying an additional correction factor of 0.78 for the difference in surface to cross section for the axon and soma (see Online Methods), we estimated that the ratio of QNa in the two compartments, QAIS/Qsoma, to be 2.69 ± 0.55.
Figure 5.
Relative spike-evoked Na+ flux in different neuronal compartments. (a) Top, ΔF/F and ΔF changes at the indicated locations (colored traces) elicited by a train of five action potentials. Bottom, pseudocolor maps of the changes between the times marked by the arrowheads. (b) Action potential–evoked Na+ charge transfer derived from the amplitude of Na+ transients and morphological data. Dots represent individual measurements of calculated for soma (n = 14), AIS (n = 14) and basal dendrites (n = 9). Dashed lines are the mean values. (c) AIS to soma and AIS to basal dendrite Na+ charge transfer ratios calculated from ΔF (n = 11) and (n = 9–14) values.
We made a similar comparison between the axon and basal dendrites (Fig. 5a). We found that ΔFaxon/ΔFbasal = 8.64 ± 0.86 for spikes at 10-ms intervals (mean ± s.d., n = 11) and 6.01 ± 2.69 for spikes at 30-ms intervals (n = 9). We still had to apply a correction for diffusion for the slower train, but there was no need to correct for differences in the surface to cross section, as both compartments were assumed to be cylinders. After correcting and averaging, we found that QAIS/ΔQbasal = 8.4 ± 3.6.
The third approach for estimating QNa starts from the same equations. As there is almost no buffering of entering Na+ ions (see Online Methods), , where k is the change in [Na+]i that causes ΔF/F = 1%, CFaraday is Faraday’s constant, ΔF is the action potential–evoked change in fluorescence and F is the resting fluorescence of SBFI in the volume (equal to the total measured fluorescence minus Fa, the tissue autofluorescence). Typically, Fa was about 5% of F in the soma and 35% of F in the axon and basal dendrites. The volume-to–surface area ratio was assumed to be diameter divided by 6 for a quasispherical soma and diameter divided by 4 for the cylindrical AIS and basal dendrites. The diameters of the soma, axon and basal dendrites for each neuron were estimated from two-photon reconstructions.
In the soma, AIS and basal dendrites, the mean values of were 0.30 ± 0.06 (n = 14), 0.78 ± 0.14 (n = 14) and 0.08 ± 0.01% µm (n = 9), respectively (Fig. 5b). QNa in soma, AIS and in basal dendrites, assuming k = 0.4 mM per 1% ΔF/F (ref. 15) was 11.8 ± 2.1, 30.1 ± 5.7 and 3.3 ± 0.5 fC µm−2, respectively. Using these numbers, we found that QAIS/Qsoma = 2.95 ± 0.45 (n = 14) and QAIS/Qbasal = 8.88 ± 1.74 (n = 9), close to the estimates obtained by the first two approaches (Fig. 5c).
In these experiments, the [Na+]i changes were elicited by trains of action potentials. We also evaluated the SBFI fluorescence changes associated with single action potentials, although the small signal size and the inability to completely eliminate vibrations made the analysis more difficult. In two neurons in which the single spike changes could be clearly measured in both the soma and the axon, the QAIS/Qsoma ratio for one action potential was similar to that determined with spike trains (Supplementary Fig. 3). Because the rise time of the single spike signals was much faster than the fall time there was no need to correct for diffusion.
Sodium dynamics at nodes of Ranvier
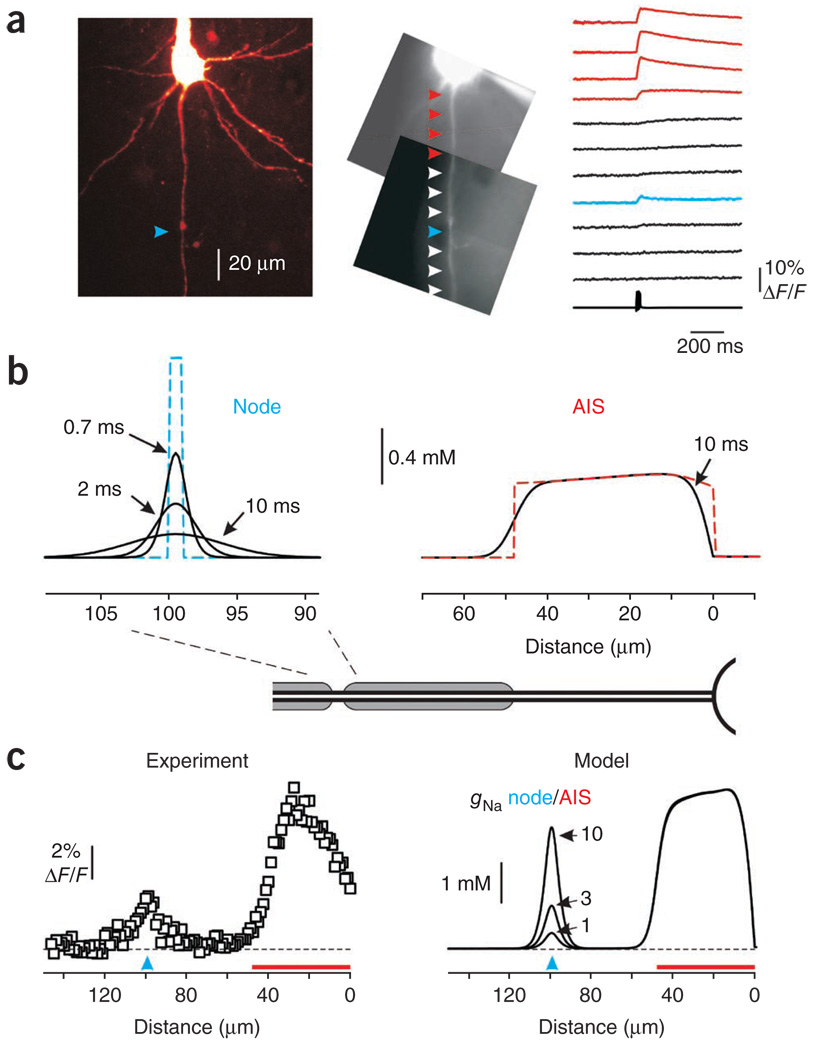
In eight cortical neurons, nodes were identified by the characteristics of their Na+ transients (see below). In four of these neurons, subsequent two-photon reconstructions revealed thin collateral axonal braches that originated from the axonal trunk at the presumed node location (Fig. 6a). An additional node was found in the axon of a cerebellar Purkinje cell (data not shown). In both cell types, the first nodes were found within 95–115 µm of the soma. In the two axons in which second nodes of Ranvier were identified (both in Na+-imaging experiments and in two-photon reconstructions), they were located 22 and 28 µm from the first node. Physiologically, nodes were identified as isolated locations where the time course of the Na+ transient was in synchrony with the spike train (Fig. 6a). When these Na+ elevations occurred, they were evident for distances of 10–20 µm along the axonal length, obviously longer than the dimensions of a central node of Ranvier16. The maximal amplitudes of nodal Na+ transients elicited by five action potentials at 10-ms intervals (ΔF/F = 0.5 to 2%) were <10% of those in the AIS of the same neuron (Fig. 6a). The amplitude was maximal in the center of a segment and decayed in both directions (Supplementary Fig. 4).
Figure 6.
Action potential–evoked Na+ charge transfer in nodes of Ranvier is comparable to the transfer in the AIS. (a) Left, reconstruction of a stack of 61 optical sections through part of a layer 5 pyramidal neuron filled with 2 mM SBFI. The first axonal branching point is ~100 µm from the hillock (blue arrowhead). Middle, the same neuron as seen during the fluorescence imaging experiment with a NeuroCCD-SMQ camera. Right, averaged Na+ transients (n = 20) elicited by a train of five action potentials (200 Hz). See also Supplementary Figure 4. (b) Simulated changes in [Na+]i elicited by a single action potential plotted against distance from the axon hillock. The node was assumed to be 1 µm long and the AIS to be 45 µm. Dashed lines are [Na+]i changes in a model with no Na+ diffusion. Black continuous lines are [Na+]i values 10 ms following the peak of the spike in the AIS and 0.7, 2 and 10 ms following the peak in the region around the node of Ranvier. (c) Experimentally observed (left) and simulated (right) changes in [Na+]i elicited by a train of five action potentials plotted against distance from the axon hillock. Left, dots are averaged ΔF/F values from each 1.15-µm-long pixel along the axon during the time interval 2–10 ms following the peak of the last spike in train. Red line, AIS; blue arrowhead, the first node of Ranvier. Right, changes in [Na+]i in models with different node-to-AIS ratios of Na+ channel density.
Because the nodes are small, Na+ ions diffused laterally almost as quickly as they enter across the nodal membrane. Computer simulations showed that diffusion-mediated [Na+]i equilibration between the nodal and internodal volumes should be nearly complete in milliseconds (Fig. 6b) and that more than 90% of Na+ ions that enter a 1-µm-long node were already in the internodal region within 10 ms of the peak of the action potential. In contrast, 10 ms after an action potential in the 48-µm-long AIS, dissipation of the Na+ gradient was minimal.
We cannot measure the peak [Na+]i in the node even if we record the fluorescence change at 500 frames per s, as Na+ ions diffuse away from the node too quickly. However, because diffusion is the primary clearance mechanism, we expect that all of the Na+ that entered at the node will be contained for a short time in a region extending 20 µm to either side. Thus, integration of the [Na+]i change over this region 10 ms after the spike train estimates the total charge transfer via nodal Na+ channels. This measure was similar to the charge transfer density in the AIS (Fig. 6c).
This conclusion was supported by computer simulations in which the AIS Na+ channel density was held constant while Na+ channel density in the first node was varied (Fig. 6c). Comparison of the experimentally determined ΔF/F changes in this cell with those predicted by the model indicates a nodal Na+ channel density of the same order of magnitude as in the AIS (Supplementary Fig. 4).
[Na+]i changes associated with persistent Na+ conductance
To examine [Na+]i changes resulting from activation of INaP, we applied subthreshold pulses of varying durations. These depolarizations evoked a large change in SBFI fluorescence in the axon that persisted throughout the pulse and were completely and reversibly blocked by tetrodotoxin (data not shown, n = 3), indicating that they reflect Na+ influx through voltage-sensitive Na+ channels. In all of the neurons studied, the amplitude of the axonal Na+ transients elicited by 1-s subthreshold depolarizations was comparable to or larger than the transients elicited by single action potentials, even though the signal associated with the prolonged subthreshold pulse must have included Na+ diffusion out of the AIS (Fig. 7a). Even with subthreshold depolarizations as long as 3 s, the signal was maintained throughout the duration of the pulse, revealing the truly persistent nature of the underlying Na+ current (Fig. 7b). INaP undergoes some slow inactivation17, but this was not evident in the imaging data.
Figure 7.
At subthreshold voltages, persistent Na+ current is generated predominately in the AIS. (a) A 1-s, 70-pA current step to just subthreshold voltage elicited a large [Na+]i increase in the AIS (red trace). A brief (5 ms) pulse that generated a single action potential generated a Na+ transient that rose sharply. (b) Subthreshold pulses of 0.3, 1.0 and 3.0 s each generated a [Na+]i increase that lasted the duration of the pulse. The rapid recovery at the end of the pulses indicates that the current was active throughout. A sharply rising Na+ transient elicited by a train of five action potentials at 50 Hz is shown for comparison. (c) A just subthreshold, 1-s stimulus elicited a large [Na+]i increase in the AIS (red trace), whereas the increase in the soma (black trace) was much smaller. The train of ten action potentials (40 Hz) caused sizable Na+ transients in both locations. (d) A 2-s voltage ramp from −70 to −40 mV elicited Na+ transients only in the axon. Interpolating along the ramp indicates that INaP and axonal [Na+]i both began to change at the same voltage (−57 ± 6 mV, n = 5). (e) A 2-s-long voltage ramp from −70 to 0 mV elicited Na+ signals that were clearly detectable in soma, basal and proximal apical dendrites. With this larger ramp, the membrane current and AIS optical signals still began to change at −57 ± 5 mV (n = 21), but the signals in the soma and basal dendrites began to change at −41 ± 5 mV (n = 21).
In most experiments, there was no detectable persistent Na+ entry in the adjacent basal dendrite (Fig. 7a) and soma (Fig. 7c) following subthreshold depolarization. Extensive signal averaging revealed a small somatic signal in only 3 of the 12 cells. We determined the AIS-to-soma ratio of the amplitudes of the signal evoked by a 1-s subthreshold depolarization and found that it was much larger than the ratio of amplitudes evoked by a train of ten action potentials (Fig. 7c). This result was found even though Na+ diffusion out of the AIS should have a much greater effect on peak axonal ΔF/F during the prolonged pulse than on the rapid, spike-evoked signal.
To evaluate the contribution of INaP at suprathreshold potentials, we examined the Na+ transients generated during ramps under voltage clamp. Slow voltage ramps from −70 mV to −40 mV or to 0 mV (Fig. 7d,e) were applied to somata with K+ currents blocked using Cs+ as the main intracellular cation and Ca2+ currents blocked by adding 200 µM Cd2+ to the bath6,18. Analysis of ramp-induced Na+ transients in 21 neurons showed that voltage onset of INaP generation (−57 ± 5 mV) was accompanied by a parallel onset of the change in AIS SBFI fluorescence (−57 ± 6 mV), whereas somatic and basal dendritic Na+ signals were first detected at more depolarized potentials (−41 ± 6 mV). The signals in the soma and dendrites correspond primarily to Na+ entry into those regions and not diffusion from the axon, as the signals began to decline as soon as the ramp ended. This difference in onset voltages suggests that INaP has shifted voltage dependence in the axon compared with the other compartments.
Implications of Na+ flux distribution for spike generation
In axonal recordings, action potential maximal rates of rise are greater than 1,000 V s−1 (ref. 3) and the preferred location for spike initiation is near the distal end of the AIS11,19. Some studies3,7 suggest that a high axon-to-soma ratio of Na+ conductances is required to account for these observations. As our imaging experiments found that the conductance ratio between these compartments was not high, we asked whether a model with a reasonable set of parameters could be constructed that would match our observations and still produce action potentials with these characteristics. We found that we could simulate the fast upstroke despite the much lower AIS Na+ channel density by assigning faster, more realistic values to the kinetic parameters of the Na+ channels (Fig. 8a).
Figure 8.
Compartmental model of an action potential that matches the fast maximal rates of rise of recorded spikes and initiates in the axon without requiring a high AIS Na+ channel density. (a) Top left, blue line indicates τm versus Vm in a model based on published recordings2. Light blue and red lines represent the same relationships using scaling factors of 0.2 and 0.05. Top right, single compartment simulations of Na+ currents produced by voltage steps from −100 mV to 0 mV as if recorded with an ‘open bandwidth’ amplifier (continuous line) or filtered at 2 kHz. Bottom left, axonal action potentials (left) and the first derivative of action potential voltage (right) in the models with τm curves as indicated. The AIS Na+ conductance was 500 pS µm−2. Bottom right, maximal rate of rise versus axonal Na+ channel density (gNa) if τm is 0 (black), using scaling factors of 0.05, 0.2 and 1 relative to the model2. Dashed line indicates 1,130 V s−1, the maximal measured rate of rise3. (b) Effect of AIS channel density and properties on action potential initiation. Top left, with AIS gNa as in the soma, the action potential initiated simultaneously in the soma and in the AIS. Top right, with threefold higher AIS gNa and with shifted voltage dependence, initiation shifted to the axon. Bottom left, scaling Na+ channel τm by 0.2 in all compartments increases the rate of rise, shifts the threshold and enhances the axo-somatic delays and voltage gradients. Bottom right, with GNaP being 5% of the total AIS Na+ conductance, the shifts in threshold and the axo-somatic delays and voltage gradients are greater.
The velocity of the action potential upstroke is given by where INa is the current flowing through the Na+ channels and Clocal is the membrane capacitance (assuming that INa is substantially larger than other currents). Thus, the smallest INa that could underlie a of 1,000 V s−1 is ~10 pA µm−2 (assuming C = 1 µF cm−2), which corresponds to a gNa of 200 pS µm−2 (assuming that at the point at which is maximal the driving force for Na+ is 50 mV). This is the value if Na+ channel activation is instantaneous; it will be larger if the time constant of Na+ channel activation (τm in the Hodgkin-Huxley formalism) is slow. The value of τm at physiological temperature is not known and is difficult to measure. Generally, the bandwidth used for Na+ channel recordings in CNS neurons is 2 kHz (−3 dB, 8-pole Bessel filter) (for example, refs. 2,3), which optimizes the signal-to-noise ratio and permits accurate capacitive transient subtraction. However, recording at 2 kHz at 23 °C distorts and slows the onset kinetics of currents if τm is faster than about 120 µs (Fig. 8a).
The most accurate estimate of Na+ channel activation kinetics in central neurons was made from mossy fiber boutons20. Recording at 23 °C, with a 10-kHz low-pass filter, the authors reported τm of 38 µs and 14 µs for 0 mV and +40 mV, respectively. At 32 °C, if Q10 = 3 and the rate is nonsaturating, τm will be13 µs and 5 µs, respectively. When τm is in this range, very fast action potentials are possible with considerably lower current density (Fig. 8a). The action potentials are shown at the distal end of the AIS, where they are most isolated from the soma by the thin AIS.
Using simulations, we examined the effects of density and properties of the Na+ channels in the AIS at the site of action potential initiation (Fig. 8b). In all models, the voltage dependence of axonal gNa was shifted by −6 mV compared with somatodendritic channels2–4,21. When Na+ conductance was equal in the soma and AIS (250 pS µm−2), somatic current injection (2 ms, 1 nA) produced a relatively slow and uniform regenerative response across soma, proximal axon and dendrites (Fig. 8b; see also Supplementary Movie 2). A threefold increase in the AIS conductance, however, was sufficient to change this pattern toward axonal spike initiation (Fig. 8b). The action potential voltage threshold was shifted to more negative potentials and axo-somatic delays and voltage gradients were greater in models with a faster τm (τm × 0.2; Fig. 8b) and the axo-somatic difference was even more evident when persistent Na+ conductance was added to the AIS (Fig. 8b; see also Supplementary Movie 3). In all of these models, action potentials backpropagated over the dendrites following initiation in the AIS.
DISCUSSION
Na+ imaging allowed us to quantitatively evaluate the characteristics and density of sodium channels in thin neuronal processes, providing a complementary approach to electrophysiological and immunocytochemical techniques, which have sometimes been controversial. For example, immunocytochemical measurements3 might overestimate channel density by labeling channel proteins that are not functional and may not be relevant to excitability. We concluded that Na+ channel density (or more precisely, Na+ current density per action potential) in the soma is about threefold lower than in the AIS and threefold greater than in basal dendrites. In nodes of Ranvier, action potential–induced Na+ influx was of the same order of magnitude as in the AIS. At a functionally critical subthreshold range of voltages, INaP was primarily generated by the AIS. Passive diffusion, not active pumping, was responsible for rapid clearance of Na+ from beneath the membrane of the AIS and node of Ranvier during repetitive action potential generation.
Na+ channel density in different neuronal compartments
The finding that action potential–induced Na+ flux density is about threefold greater in the AIS than in the soma would indicate the same ratio of channel densities if the amplitude and shape of the action potential and the temporal kinetics of the channels were the same in the two regions. In the proximal AIS, the maximal rate of rise of the action potential is about twice that of the somatic action potential, whereas spike amplitudes are similar in both compartments3. Because the time to peak is about 20–30% briefer than in the soma, we would underestimate the ratio of peak gNa by about that amount if we use the axon-to-soma flux ratio that we measured to estimate the conductances. On the other hand, the Na+ current is entirely inactivated by the time of the peak of the action potential in the isolated pyramidal cell soma22. Because the peak is reached earlier in the axon, there may be some Na+ current during repolarization. As this is an additional current going through the same channels that are open on the rising phase, the axon-to-soma flux ratio of 3:1 that we recorded probably reflects an even smaller ratio of peak gNa, and channel densities. In fact, several studies2,3,8 have estimated channel densities on the basis of electrophysiological recordings from cell-attached or excised patches in the AIS and arrived at estimates in the AIS that are less than threefold higher than their estimates for the soma.
One suggestion3 is that the ratios determined from patch recordings do not reflect the effective physiological channel density because Na+ channels in the AIS are anchored to the cytoskeleton and do not completely reveal themselves in patch recordings. This analysis is based on experiments that disrupted the connection of Na+ channels to ankyrin G and thereby increased the apparent channel density. However, even if that interpretation is correct, there was only a threefold increase in density, which is still closer to the range of cell-attached measurements and our determination than to the factor of ~40–50 that has been proposed on the basis of other considerations3.
The relative action potential–evoked [Na+]i changes that we recorded in different regions were qualitatively similar to those reported in pyramidal neurons3 and in other cell types23,24. All experiments agree that the fractional fluorescence change (ΔF/F) following a depolarizing stimulus is much lower in the soma than in the AIS. However, ΔF/F reflects concentration change and not QNa, which is the parameter closest to channel density. When we used ΔF, which is more closely related to current density, the fluorescence changes in the two compartments were similar. In our other approach, where we compared [Na+]i changes, but corrected for the difference in the surface-to-volume ratio, we came to the same conclusion.
Another experimental observation that suggested3 a high density of channels in the AIS is the extremely fast rise time of the action potential in AIS. The model used in that analysis3 probably required a high channel density to compensate for a high estimate of the Na+ channel activation time constant τm. We were able to simulate the same fast rise time with a much lower Na+ conductance in the AIS, provided we assigned faster values (based on other experiments20,25) to the kinetic parameters of the Na+ channels (Fig. 8). Another argument3 for a high Na+ channel density in the AIS is that it is required to support the experimental finding that action potentials initiated at the distal portion of the AIS and not in the first node of Ranvier. This argument, however, depends critically on the relative Na+ channel density in the nodes and in the AIS. The model referred to in that argument3 used estimated values based on recordings in rat peripheral nerve20 (21,000 channels per node). Our results (Fig. 6) indicate that, in the axon of the pyramidal neuron, where nodes are smaller and closer together, the current density is smaller. There is therefore no need to postulate high Na+ channel density in the AIS.
Our findings about the relative basal dendrite/axon current densities are consistent with previously reported imaging data3. As with the axonal data, the interpretation of these results in terms of channel densities depends on the relative spike amplitudes in the different compartments. There is still some disagreement about action potential properties in the basal dendrites. Direct patch recordings from the basal dendrites suggest attenuated back-propagation of action potentials26, whereas experiments using voltage-sensitive dyes found less attenuation27. If the spikes are attenuated, we would expect a slightly higher relative channel density than that estimated by QNa. As our measurements were made less than 30 µm from the soma, where attenuation is minimal, we expect the correction to be small.
Persistent Na+ current
Our imaging data confirm that persistent Na+ conductance in layer 5 pyramidal cells was generated predominately in the axon in the functionally critical subthreshold voltage range, as previously reported6. The voltage-clamp experiments indicate that persistent Na+ conductance was also activated in the somatic and dendritic membranes at more depolarized potentials.
It remains unclear whether different voltage dependence of the persistent conductance in these compartments parallels the reported difference in voltage dependence for the transient Na+ current2–4,21. It is generally accepted that the activation voltage of INaP is more negative than that of the transient current, although the extent of the leftward shift is not known (for review, see ref. 28). Thus, the axonal INaP undoubtedly contributes to the lower threshold of the AIS1,8.
Diffusion and clearance of Na+ ions
Many types of central neurons fire high-frequency bursts that propagate via myelinated axons to target cells with a timing precision that is critical both for ongoing communication between neurons29 and for synaptic plasticity30. The structural relationships between the axonal membrane and the myelin sheath are optimized for initiation and rapid, saltatory propagation of action potentials. Our results suggest an additional function for this organization, that the geometrical dimensions of myelinated and nonmyelinated segments of the axon permit rapid, energy efficient restoration of transmembrane Na+ gradients beneath the excitable membrane.
We found both experimentally and using numerical simulations that, in myelinated axons, action potentials are associated with a marked heterogeneity in [Na+]i over a short length of AIS-soma and node-internode assemblies. We did find small voltage-dependent Na+ and Ca2+ entry in the myelinated region (Supplementary Fig. 5), consistent with previous observations31,32, but the spike-evoked Na+ entry in this region was much smaller than in the AIS or first node. The resultant sharp Na+ gradients facilitated rapid recovery of [Na+]i by diffusion. Of course, Na+ ions that enter the axoplasm during activity will eventually have to be extruded by the Na+/K+-ATPase, which uses about a half of the brain energy budget for this purpose33. However, in the time frame of milliseconds, it is Na+ clearance mediated by lateral diffusion that prevents Na+ accumulation in the functionally critical, nonmyelinated segments. Moreover, as Na+ influx is predominately restricted to the initial segment and to the periodic nodes of Ranvier, the Na+ extrusion mechanism utilizes the whole neuronal membrane, including the myelinated internodes and soma where the pump is present34.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Manita for excellent help with the preparation of slices. This work was supported by a US-Israel Binational Science Foundation grant (2003082), a grant from the Israel Science Foundation (1376-06), a Grass Faculty grant from the Marine Biological Laboratory, a US National Institutes of Health grant (NS16295), a Multiple Sclerosis Society grant (PP1367) and a fellowship from the Gruss Lipper Foundation.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
I.A.F., N.L.-R., M.J.G. and W.N.R. designed the study, performed the cortical experiments and wrote the paper. N.L.-R. and W.N.R. performed the cerebellar experiments. I.A.F. constructed the computational models.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Kole MH, Stuart GJ. Is action potential threshold lowest in the axon? Nat. Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- 2.Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat. Neurosci. 2002;5:533–538. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- 3.Kole MH, et al. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, et al. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 5.Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 6.Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J. Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mainen ZF, Joerges J, Huguenard JR, Sejnowski TJ. A model of spike initiation in neocortical pyramidal neurons. Neuron. 1995;15:1427–1439. doi: 10.1016/0896-6273(95)90020-9. [DOI] [PubMed] [Google Scholar]
- 8.Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J. Neurosci. 1996;16:6676–6686. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minta A, Tsien RY. Fluorescent indicators for cytosolic sodium. J. Biol. Chem. 1989;264:19449–19457. [PubMed] [Google Scholar]
- 10.Callaway JC, Ross WN. Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J. Neurophysiol. 1997;77:145–152. doi: 10.1152/jn.1997.77.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J. Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lelievre L, Zachowski A, Charlemagne D, Laget P, Paraf A. Inhibition of (Na+ + K+)-ATPase by ouabain: involvement of calcium and membrane proteins. Biochim. Biophys. Acta. 1979;557:399–408. doi: 10.1016/0005-2736(79)90338-9. [DOI] [PubMed] [Google Scholar]
- 13.Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- 14.Clark BA, Monsivais P, Branco T, London M, Hausser M. The site of action potential initiation in cerebellar Purkinje neurons. Nat. Neurosci. 2005;8:137–139. doi: 10.1038/nn1390. [DOI] [PubMed] [Google Scholar]
- 15.Rose CR, Kovalchuk Y, Eilers J, Konnerth A. Two-photon Na+ imaging in spines and fine dendrites of central neurons. Pflugers Arch. 1999;439:201–207. doi: 10.1007/s004249900123. [DOI] [PubMed] [Google Scholar]
- 16.Peters A. The node of Ranvier in the central nervous system. Q. J. Exp. Physiol. Cogn. Med. Sci. 1966;51:229–236. doi: 10.1113/expphysiol.1966.sp001852. [DOI] [PubMed] [Google Scholar]
- 17.Fleidervish IA, Gutnick MJ. Kinetics of slow inactivation of persistent sodium current in layer V neurons of mouse neocortical slices. J. Neurophysiol. 1996;76:2125–2130. doi: 10.1152/jn.1996.76.3.2125. [DOI] [PubMed] [Google Scholar]
- 18.Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J. Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Royeck M, et al. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J. Neurophysiol. 2008;100:2361–2380. doi: 10.1152/jn.90332.2008. [DOI] [PubMed] [Google Scholar]
- 22.Carter BC, Bean BP. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron. 2009;64:898–909. doi: 10.1016/j.neuron.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–271. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lasser-Ross N, Ross WN. Imaging voltage and synaptically activated sodium transients in cerebellar Purkinje cells. Proc. Biol. Sci. 1992;247:35–39. doi: 10.1098/rspb.1992.0006. [DOI] [PubMed] [Google Scholar]
- 25.Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- 26.Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 2007;10:206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- 27.Acker CD, Antic SD. Quantitative assessment of the distributions of membrane conductances involved in action potential backpropagation along basal dendrites. J. Neurophysiol. 2009;101:1524–1541. doi: 10.1152/jn.00651.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bean BP. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 29.Sugihara I, Lang EJ, Llinas R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J. Physiol. (Lond.) 1993;470:243–271. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 31.Shrager P. The distribution of sodium and potassium channels in single demyelinated axons of the frog. J. Physiol. (Lond.) 1987;392:587–602. doi: 10.1113/jphysiol.1987.sp016798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CL, Wilson JA, Williams J, Chiu SY. Action potentials induce uniform calcium influx in mammalian myelinated optic nerves. J. Neurophysiol. 2006;96:695–709. doi: 10.1152/jn.00083.2006. [DOI] [PubMed] [Google Scholar]
- 33.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 34.Mata M, Fink DJ, Ernst SA, Siegel GJ. Immunocytochemical demonstration of Na+,K+-ATPase in internodal axolemma of myelinated fibers of rat sciatic and optic nerves. J. Neurochem. 1991;57:184–192. doi: 10.1111/j.1471-4159.1991.tb02114.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.