Abstract
Ca2+-activated Cl− channels (CaCCs) are exceptionally well adapted to subserve diverse physiological roles, from epithelial fluid transport to sensory transduction, because their gating is cooperatively controlled by the interplay between ionotropic and metabotropic signals. A molecular understanding of the dual regulation of CaCCs by voltage and Ca2+ has recently become possible with the discovery that Ano1 (TMEM16a) is an essential subunit of CaCCs. Ano1 can be gated by Ca2+ or by voltage in the absence of Ca2+, but Ca2+- and voltage-dependent gating are very closely coupled. Here we identify a region in the first intracellular loop that is crucial for both Ca2+ and voltage sensing. Deleting 448EAVK in the first intracellular loop dramatically decreases apparent Ca2+ affinity. In contrast, mutating the adjacent amino acids 444EEEE abolishes intrinsic voltage dependence without altering the apparent Ca2+affinity. Voltage-dependent gating of Ano1 measured in the presence of intracellular Ca2+ was facilitated by anions with high permeability or by an increase in [Cl−]e. Our data show that the transition between closed and open states is governed by Ca2+ in a voltage-dependent manner and suggest that anions allosterically modulate Ca2+-binding affinity. This mechanism provides a unified explanation of CaCC channel gating by voltage and ligand that has long been enigmatic.
Keywords: allosteric mechanism, chloride channel, patch clamp
Ca2+-activated Cl− channels (CaCCs) play manifold roles in cell physiology (1, 2), including epithelial secretion (3, 4), sensory transduction and adaptation (5–8), regulation of smooth muscle contraction (9), control of neuronal and cardiac excitability (10), and nociception (11). This myriad of functions has attracted attention for more than 25 years (12, 13), but a lack of consensus regarding their molecular composition has stymied a mechanistic understanding of their gating. Recently, two members of the TMEM16/anoctamin family (Ano1 and Ano2) were identified as CaCC channels (14–16) and shown to be essential for salivary exocrine secretion (14, 17, 18), gut slow-wave activity (18, 19), tracheal secretion (18, 20, 21), and olfactory transduction (5–7).
Ano1 and Ano2 are well suited for their diverse roles because they are dually gated by voltage (Vm) and intracellular Ca2+ concentration ([Ca2+]i), so that their activity is tuned by the interplay between metabotropic and ionotropic inputs (14, 16, 22–24). The molecular mechanisms underlying Vm and Ca2+ gating are unknown, however. Unlike typical Vm-gated channels or ligand-gated channels, CaCCs exhibit both Vm dependence and ligand gating that are strongly coupled and apparently reciprocally related. Ano1 does not contain obvious Vm-sensing or Ca2+-sensing domains (14). Because Ca2+ is often stabilized in proteins by oxygen atoms (25), it seemed reasonable to hypothesize that acidic amino acids contribute to the Ca2+-binding site. Ca2+ sensors in two other Ca2+-activated channels, BK and Best1, are associated with sequences rich in acidic amino acids (26, 27). The Ano1 sequence has a similar domain in the first intracellular loop (amino acids 430–480), composed of residues 444EEEEEAVK451 (Fig. S1). Here we show that this region plays a key role in transducing both Vm and Ca2+ signals.
Results
Voltage and Ca2+ Synergistically Gate Ano1.
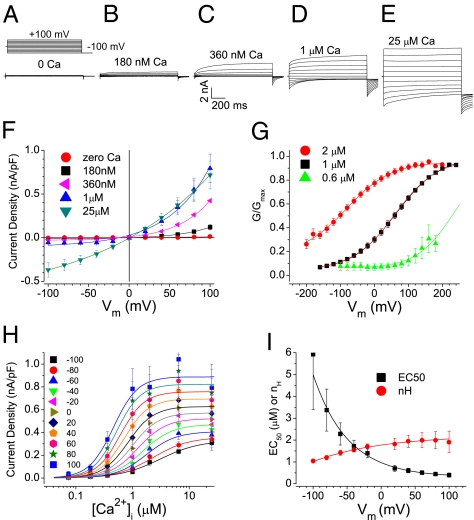
At low [Ca2+]i (<1 μM), WT Ano1 (splice variant a,c) was activated synergistically by Ca2+ and depolarization. In the absence of Ca2+, no current was evident at Vm between −100 mV and +100 mV, but as [Ca2+]i was increased, an outward current was activated by depolarization and deactivated by hyperpolarization (Fig. 1 A–D and F). As [Ca2+]i was increased, outward rectification (Fig. 1F) and the fraction of total current exhibiting time-dependence (Fig. 1E) were reduced. Vm-dependent activation of Ano1 was evaluated by plotting normalized conductance versus Vm (G/Gmax vs. Vm curves; Fig. 1G). The data were well fit by the Boltzmann equation,
Fig. 1.
WT Ano1 gating by voltage and Ca2+. (A–E) Representative ICl,Ano1 in transfected HEK293 cells at the indicated free [Ca2+]. Voltage protocol is shown above A. (F) Steady-state current–voltage relationships (n = 5–9). (G) G/Gmax vs. Vm curves for Ano1 in excised patches activated by 0.6 μM (▲), 1 μM (▪), and 2 μM (●) Ca2+. (H) Instantaneous tail current density at −100 mV after prepulses to Vm plotted vs. free [Ca2+] and fitted to the Hill equation. (I) EC50 (▪) and nH (●) values from fits in H plotted vs. Vm.
where G/Gmax is normalized conductance; z is the equivalent gating charge associated with voltage-dependent channel opening; V0.5 is the membrane potential (Vm) where G/Gmax is half-maximal and is related to the conformational energy associated with voltage-independent channel opening; and F/RT = 0.039 mV−1. At 1 μM Ca2+, V0.5 was 64 ± 0.9 mV (Fig. 1G, black squares); doubling [Ca2+] to 2 μM shifted the G/Gmax vs. Vm curve to the left by −145 mV (Fig. 1G, red circles) with no significant effect on z. Because Ca2+ shifts the G/Gmax vs. Vm curves so dramatically, a complete G/Gmax vs. Vm curve could be recorded for only a narrow range of [Ca2+]i. For these [Ca2+], z was not obviously Ca2+-dependent (z = 0.40–0.46). This indicates that Ca2+ does not change the Vm sensitivity (z) of the Ano1 channel, but rather shifts V0.5, the energy associated with Vm-independent gating.
Although these data may imply that Ano1 is a simple ligand-gated channel, Ano1 is actually more complicated, because Ca2+ gating is strongly influenced by Vm. The EC50 of Ano1 for Ca2+ decreases ∼15-fold from 5.9 ± 2.5 μM at −100 mV to 0.4 ± 0.1 μM at +100 mV while the Hill coefficient, nH, indicative of the cooperativity of Ca2+ binding, increases from 1.0 ± 0.1 to 2.0 ± 0.4 (Fig. 1 H and I). These data support a mechanism of Ano1 gating with Vm and Ca2+ converging on the switch between closed and open conformations.
Glutamic Acids in the First Intracellular Loop Contribute to Channel Gating.
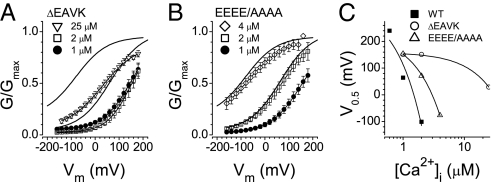
The first intracellular loop of Ano1 contains five consecutive glutamic acids (444EEEEE448), resembling the “Ca2+ bowl” of BK channels (26) and the acidic cluster in hBest1 (27) that are linked to Ca2+ sensing (Fig. S1). The last glutamic acid of this cluster, 448E, is the first residue of a naturally occurring alternatively spliced segment, 448EAVK451 (28). To investigate this region in detail, we mutated the first four glutamic acids to alanines (444EEEE/AAAA447) and deleted 448EAVK451 (ΔEAVK) (current traces; Fig. S2). The ΔEAVK deletion and, to a lesser extent, the 444EEEE/AAAA447 mutation, shifted G/Gmax vs. Vm curves at each [Ca2+]i to the right without altering the slope (Fig. 2). For ΔEAVK, the position of the G/Gmax vs. Vm curve on the Vm axis was relatively insensitive to Ca2+: 2 μM Ca2+ had no effect, and 25 μM Ca2+ shifted the curve only to a position comparable to the WT curve at 1 μM Ca2+. The slope of the relationship between V0.5 and [Ca2+]i was reduced ∼50-fold for ΔEAVK and ∼3-fold for 444EEEE/AAAA447 compared with WT (Fig. 2C). Because V0.5 represents the conformational energy associated with Vm-independent channel opening, these mutations decrease the ability of Ca2+ to reduce the activation energy required for the channel to open. This could be caused by either lower Ca2+-binding affinity or decreased stability of the Ca2+-bound open state.
Fig. 2.
G/Gmax vs. Vm curves for mutant Ano1 in excised patches at the indicated [Ca2+]i. (A and B) Solid lines without symbols: WT from Fig. 1G at 1 μM and 2 μM Ca2+. (A) ΔEAVK with 25 μM (▽), 2 μM (□), and 1 μM (●) Ca2+. (B) 444EEEE/AAAA447 with 25 μM (◇), 2 μM (□), and 1 μM (●) Ca2+. (C) V0.5 vs. [Ca2+] for WT (▪), ΔEAVK ( ), and 444EEEE/AAAA447 (△).
), and 444EEEE/AAAA447 (△).
Ca2+ Dissociation Is Vm-Dependent and Slow.
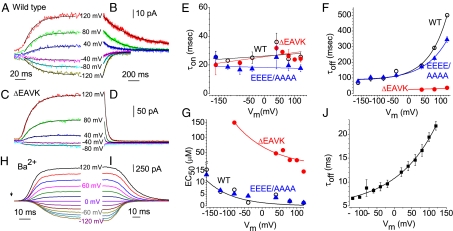
To test whether these mutations alter Ca2+ affinity, we performed fast perfusion experiments in which excised patches were switched between zero and high [Ca2+] within several ms (Fig. S3). Fig. 3 displays examples of the time course of Ano1 activation at the indicated Vm when Ca2+ was increased rapidly from 0 μM to 20 μM (Fig. 3A) and deactivation when Ca2+ was withdrawn (Fig. 3B). The time course of activation was sigmoid and displayed a lag period consistent with multistep channel opening. For simplicity, current activation after the initial lag period was fit to a monoexponential equation to calculate the time constant of activation, τon (Fig. 3A). τon was decreased when [Ca2+] was increased, as expected if Ca2+ binding is a rate-limiting step in channel opening. τon was not strongly Vm-dependent (Fig. 3E). On removal of Ca2+, the currents decayed monoexponentially (Fig. 3B), and τoff was strongly Vm-dependent, with an e-fold slowing per 70.3-mV depolarization for WT (Fig. 3F). 444EEEE/AAAA447 and ΔEAVK mutations had no significant effect on τon (Fig. 3E). In contrast, τoff was greatly accelerated by ΔEAVK and slightly accelerated by the 444EEEE/AAAA447 mutation at positive Vm (Fig. 3F). At +120 mV, τoff was 501.5 ± 10.2 ms for WT, 37.4 ± 1.1 ms for ΔEAVK, and 345.0 ± 18.8 ms for 444EEEE/AAAA447. Because τoff for WT was surprisingly slow, for comparison, we performed an experiment using patches containing the BK Ca2+-activated K+ channel (Fig. S3). τoff for the BK channel was 28 ms, ∼20-times faster than for WT Ano1.
Fig. 3.
Activation and deactivation kinetics of Ano1 with rapid Ca2+ and Ba2+ perfusion. (A–D) Representative traces of ICl,Ano1 in response to application (A and C) and washout (B and D) of 20 μM Ca2+ at the indicated holding potentials. (A and B) WT Ano1. (C and D) ΔEAVK. (E–G) Vm dependence of τon, τoff, and EC50 for WT Ano1 (○), 444EEEE/AAAA447 (▲), and ΔEAVK (●). (H and I) Representative traces of increase in current on application (H, arrowhead) and washout (I) of 1 mM Ba2+ (1 mM Ba2+, 100 μM EGTA) for WT Ano1. (J) τoff for Ba2+.
τoff is equal to the reciprocal of the rate constant(s) of the rate-limiting step(s) leading to channel closure, which could be Ca2+ dissociation or channel closure itself. As a test of the idea that τoff reflects ligand dissociation, we measured τoff for Ba2+-activated Ano1. Because the free energy of hydration of Ba2+ is smaller than that of Ca2+, Ba2+ binding to sites composed of coordinating oxygen ligands is significantly less stable than that of Ca2+ (25). For example, Ba2+ is almost inactive in activating CaM-dependent phosphodiesterase or displacing Ca2+ from CaM (29). The apparent EC50 for Ano1 activation is >10-fold larger for Ba2+ than for Ca2+, reflected as a 20-fold faster turnoff of the Ano1 current on Ba2+ washout (Fig. 3 I and J). These observations support the conclusion that τoff reflects ligand dissociation and, therefore, ΔEAVK dramatically increases the rate of Ca2+ dissociation from the channel at all voltages.
Assuming that the rate-limiting steps are Ca2+ binding and unbinding, the apparent EC50 for Ca2+ can be calculated as
Deletion of EAVK produces a large increase in EC50 at all voltages (Fig. 3G), suggesting an important role in Ca2+ sensing. In contrast, 444EEEE/AAAA447 is similar to WT. The Vm-dependence of EC50 is well described by the equation
where EC50(0mV) is the EC50 value at 0 mV, z = 2, and δ is the electric field fraction (Fig. 3G). The EC50(0mV) values were 1.39 μM for WT and 71 μM for ΔEAVK. Values of δ were similar, 0.16 for WT and 0.12 for ΔEAVK. The Vm dependence of the EC50 values agree with other published data for both Ano1 and endogenous CaCCs (14, 22, 23, 28).
Both the Ca2+-activated and the Ba2+-activated currents exhibit a sigmoid onset (Fig. 3 A and H), indicating that current activation is a highly cooperative multistep process. The sigmoid onset is more pronounced with Ba2+, and the activation, especially the lag period after switching to Ba2+ before the current begins to increase, is distinctly Vm-dependent. This Vm dependence is not as obvious with Ca2+, but does appear to be present (Fig. 3A). The Vm-dependent sigmoid onset of the current supports a multistep model of Ano1 activation with Vm altering the affinity of the ligand-binding site.
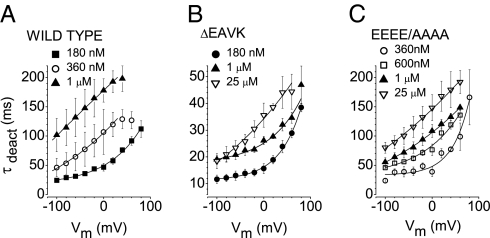
The Vm dependence of τoff supports the idea that Ca2+ binding is Vm-dependent. If this were the case, then we would expect current deactivation produced by hyperpolarization to reflect Ca2+ dissociation as a result of a Vm-dependent decrease in Ca2+ affinity. Consequently, we predict that the time constant of current deactivation (τdeact) would become faster with hyperpolarization but slower with increased [Ca2+]. This is indeed what we observe (Fig. 4A). Consistent with the rapid perfusion, ΔEAVK displays a faster τdeact at each [Ca2+] tested (Fig. 4B). A smaller effect is observed with 444EEEE/AAAA447 (Fig. 4C).
Fig. 4.
Deactivation of ICl,Ano1. (A–C) Vm and [Ca2+] dependence of tail current deactivation. Currents were activated using a +100-mV pulse, and tail currents were measured at the indicated potentials on the x-axis. (A) WT Ano1. (B) ΔEAVK. (C) 444EEEE/AAAA447. n = 4–6.
Ano1 Exhibits Intrinsic Voltage Dependence.
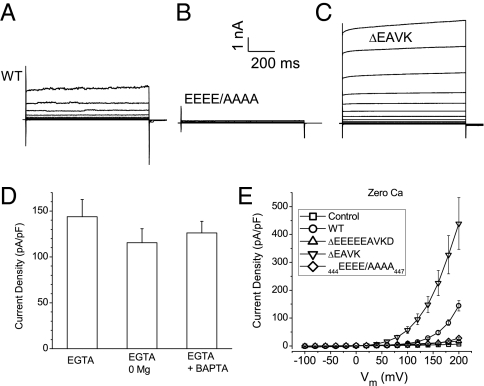
Although there are no obvious classical Vm-sensor domains in the predicted transmembrane segments of Ano1 (Fig. S1), Ano1 can be opened by Vm in the absence of Ca2+ or other divalent cations by strong depolarization (Fig. 5). Under several conditions with zero [Ca2+]i, depolarizations >100 mV evoke outward currents (Fig. 5 A, D, and E). The magnitude of the Ca2+-independent current was ∼100 pA/pF at +200 mV which is <10% that activated by maximum [Ca2+]i (Fig. 1H). The Ca2+-independent currents are mediated by Ano1 because they are not present in HEK cells transfected with GFP alone.
Fig. 5.
Activation of Ano1 in zero intracellular Ca2+. (A–C) Representative traces of WT Ano1 (A), 444EEEE/AAAA447 (B), and ΔEAVK (C) activated by Vm in nominally zero Ca2+. (D) Mean current density at +200 mV with zero intracellular Ca2+ plus 5 mM EGTA, zero intracellular Ca2+ and Mg2+ plus 5 mM EGTA (EGTA + 0 Mg), or zero Ca2+ plus 5 mM EGTA plus 1 mM BAPTA in both intracellular and extracellular solutions (EGTA + BAPTA). n = 5–11. (E) Steady-state I-V curve from cells dialyzed with zero Ca2+: nontransfected cells (control; □), WT Ano1 ( ), Δ444EEEEEAVKD452 (△), ΔEAVK (▽), and 444EEEE/AAAA447 (♢). (n = 4–11).
), Δ444EEEEEAVKD452 (△), ΔEAVK (▽), and 444EEEE/AAAA447 (♢). (n = 4–11).
To investigate the role of acidic residues in the Ca2+-independent current, 444EEEEEAVKD452 was deleted. These channels were not activated by depolarizations to +200 mV (Fig. 5E), but were activated by Ca2+ with an EC50 of 45 μM. 444EEEE/AAAA channels were not activated by Vm either (Fig. 5 B and E), despite the fact that their Ca2+ sensitivity was similar to that of WT. In contrast, ΔEAVK channels were activated more readily by depolarization compared with WT (Fig. 5 C and E). Taken together, Figs. 2–5 argue that 444EEEE447 and 448EAVK451 are critical in both Ca2+-dependent and Vm-dependent gating of Ano1. The reciprocal relationship of Ca2+ and Vm is illustrated by the disruption of 448EAVK451, which decreases apparent Ca2+ affinity while stabilizing the Vm-gated open state. The 444EEEE447 mutation, on the other hand, seems to stabilize the closed states of both ligand-gated and voltage-gated pathways.
Voltage Gating Is Affected by Permeant Anions.
The observation that neutralization of 444EEEE447 does not change the slope of the G/Gmax vs. Vm curve (Fig. 2) argues that these residues are not themselves the voltage sensor. Because previous observations showing that the gating of CaCCs in Xenopus oocytes and salivary glands is affected by permeant anions (30, 31), we tested the idea that Ano1 voltage sensitivity is related to occupancy of the pore by anions. Replacement of external Cl− with NO3− greatly reduced Vm-dependent gating of Ano 1, as evidenced by a reduction in outward rectification and an increase in the fraction of current activating instantaneously with depolarization (Fig. 6 A–D). Furthermore, G/Gmax vs. Vm curves were shifted to negative potentials when Cl− was replaced by NO3− or SCN− (Fig. 6 E and F). These ions would be expected to exhibit a higher occupancy in the pore than Cl−, because they have lower hydration energies and higher relative permeabilities than Cl− (31).
Fig. 6.
WT Ano1 gating is dependent on permeant anions. (A and B) Current traces from a cell dialyzed with 180 nM Ca2+ and bathed in symmetrical 150 mM Cl− (A) or 150 mM NO3− (B). (C) Fraction of instantaneous current relative to total current at the end of a 700-ms pulse to +100 mV. (D) Normalized (at +100 mV) I-V curves with Cl− (●) or NO3− (▪) as permeant ions. (E) G/Gmax vs. Vm curves for WT Ano1 activated at different [Ca2+]i with Cl− (open symbols) or SCN− (filled symbols) as permeant anions. Circles represent 360 nM Ca2+; squares, 600 nM Ca2+; triangles, 1 μM; diamonds, 2 μM. (F) V0.5 determined from G/Gmax vs. Vm curves for Cl−, NO3−, and SCN−. Red bars represent 0.6 μM Ca2+; green bars, 1 μM Ca2+. n = 4–11. (G) Cl− dependence of G/Gmax vs. Vm curves. [Cl−]e: 1 mM (green), 10 mM (red), 72 mM (blue), and 136 mM (black). (H) Anomalous mole fraction behavior of WT Ano1. The change in Erev is plotted as a function of the mole fraction of iodide (with the balance Cl−). The red line represents the prediction from the Goldman–Hodgkin–Katz equation.
If channel gating is dependent on pore occupancy by permeant anions, Vm dependence would be expected to depend on [Cl−]. As expected, increasing [Cl−]e shifts the G/Gmax vs. Vm curves to more negative potentials (Fig. 6G). Furthermore, the reversal potential of the current carried by mixtures of Cl− and I− cannot be described by the Goldman–Hodgkin–Katz equation (Fig. 6H), supporting the idea that channel gating is dependent on ion permeation.
Discussion
The gating of Ano1 is controlled by a complex interplay among [Ca2+]i, Vm, and permeant anions. The most important contribution to gating is provided by an increase in [Ca2+]i under our conditions. At high concentrations, Ca2+ alone is able to open the channel even at very hyperpolarized potentials; however, at lower [Ca2+], voltage has a significant effect that is likely physiologically relevant (see below).
Although Ano1 resembles ligand-gated channels, its Vm sensitivity is unique. For example, Ano1 differs from the ligand-gated nAChR because although nAChR is weakly Vm-sensitive, its affinity for ACh is not obviously Vm-sensitive (32), whereas the Ca2+ sensitivity of Ano1 is clearly Vm-sensitive. Furthermore, unlike nAChRs, Ano1 can be completely closed by hyperpolarization in the presence of <1 μM Ca2+ and can be opened by strong depolarization itself in the absence of Ca2+. The observation that the channel can be opened in the absence of Ca2+ supports the conclusion that the “Vm sensor” is unlikely to be Ca2+ itself. Ano1 also differs significantly from the large-conductance Ca2+-activated K+ channel (BK), in that Ano1 has much higher sensitivity to Ca2+ and does not have an obvious protein-based Vm sensor.
Ca2+ and Vm Dependence Are Coupled by the First Intracellular Loop.
Residues 444EEEEEAVK451 are involved in the transduction of both Ca2+ and Vm signals. Deletion of 448EAVK451 severely decreases Ca2+ sensitivity and shifts V0.5 values to positive voltages. Because V0.5 represents the energy of Vm-independent channel opening, ΔEAVK apparently increases the activation energy required for Ca2+ to gate the channel open. This could be caused by decreased Ca2+-binding affinity or diminished stability of the Ca2+-bound open state. Colquhoun (33) discussed the difficulty in separating the effects of mutations on channel gating and on ligand binding; however, our fast perfusion experiments provide strong support for a major effect of ΔEAVK on Ca2+ binding. Nonetheless, there are several reasons why 444EEEEEAVK451 is unlikely to form part of the Ca2+-binding site. At very positive voltages, the EC50 values of WT and ΔEAVK appear to be convergent (Fig. 3G), which would not be expected if ΔEAVK disrupted the Ca2+-binding site. Second, neutralizing 444EEEE448 has little effect on apparent Ca2+ sensitivity, but renders the channels insensitive to Vm activation in the absence of Ca2+ and shifts the G/Gmax vs. Vm curve to positive potentials in the presence of Ca2+. This suggests that 444EEEE447 is involved in transducing depolarization to channel opening, but is unlikely to contribute to Ca2+ binding.
The Vm dependence of the EC50 values can result if depolarization drives Ca2+ into a binding site within the electrical field or alters binding site conformation and Ca2+ affinity. The hypothesis that Vm alters binding affinity is supported by the observation that the Vm dependence of current activation and deactivation in rapid perfusion experiments is different for Ca2+ and Ba2+ (e.g., a 70 mV/e-fold change in τoff for Ca2+, compared with 115 mV for Ba2+; Fig. 3), whereas electrophoretic effects should be similar, because these ions have similar ionic mobilities (119 cm2.Ω-1·M−1 for Ca2+ vs. 127 cm2.Ω-1·M−1 for Ba2+).
Permeant anions control Vm-dependent gating of native CaCCs in Xenopus oocytes and parotid acinar cells (30, 31), as well as other Cl− channels like ClC-2 (34). Permeant anions also affect Ano1 gating, and we surmise that permeant anion occupancy of the pore stabilizes the open state and thereby allosterically modulates Ca2+-dependent gating. Channel rectification and Vm-dependent gating can be explained by asymmetric access of permeant anions to a critical site in the pore.
We hypothesize that Vm-dependent Ca2+ binding may confer a majority of Vm dependence. Simple models with a Vm-dependent Ca2+ affinity can reproduce the Ano1 current (22, 23), but allosteric models, such as those used to explain the gating of BK channels (35, 36), are likely to be more successful in explaining the intricate gating of Ano1. However, more structural information is needed about the number of Ca2+-binding sites, the oligomerization state (37, 38), and the involvement of CaM (39). The first intracellular loop of Ano1 is very hydrophilic and unlikely to be located within the transmembrane voltage field (Fig. S1) (40). The physical relationship of 444EEEEEAVK451 to the Ca2+-binding site(s) and the pore, proposed to be in the reentrant loop between transmembrane domains 5 and 6 (14, 15), remains to be determined.
Ano1 Splice Variants and the Role of Calmodulin.
Human Ano1 has four different alternatively spliced segments, a, b, c, and d, corresponding to an alternative initiation site, exon-6b, exon-13, and exon-15 (28). Segments a and b are located in the N terminus, and segments c and d are in the first intracellular loop. Ferrera et al. (28) concluded that deleting segment c (ΔEAVK) in human Ano1 affects Vm dependence, but not Ca2+ sensitivity. This discrepancy with our conclusion is explained by our observation that ΔEAVK channels are strongly activated by Vm in the absence of Ca2+ (Fig. 5). Thus, the current amplitudes reported by Ferrera et al. (28) include both Ca2+-dependent and Ca2+-independent components. The EC50 of ΔEAVK channels measured by voltage steps in the presence of steady [Ca2+] will be contaminated by a Ca2+-independent component that will make the EC50 appear smaller than it actually is. In contrast, our fast perfusion experiments determine only the Ca2+-dependent component. This difference in methodology likely explains the discrepancy, but differences between human Ano1(a,b) and mouse Ano1(a) also could play a part.
A recent paper reported that CaM binds to a 22-aa region, CaM-BD1, that overlaps with the b segment and is essential for gating Ano1(a,b,c) (39). However, Tian et al. (39) showed that, unlike Ano1(a,b,c), Ano1(a,c), which lacks CaM-BD1, does not require CaM. Also, our finding that Ano1(a,c) is activated by Ba2+ supports a direct effect of Ca2+ on Ano1(a,c), because CaM is not significantly activated by Ba2+ (29). Some endogenous CaCCs are regulated directly by Ca2+, whereas others are regulated by CaM-dependent pathways (2, 41, 42), which might be explained by the expression of different splice variants.
Physiological Significance of Synergistic Gating of Ano1.
In cells in which resting Vm is typically between −90 and −30 mV and resting Ca2+ is ∼100 nM, Ano1 channel activation requires both depolarization and increased Ca2+. Elevation of Ca2+ to the low μM range at hyperpolarized potentials will be ineffective in opening Ano1, as will modest depolarization without a rise in intracellular Ca2+. This dual regulation by Ca2+ and Vm makes Ano1 particularly well suited to serve as a feedback regulator of Vm and [Ca2+]i. Because ECl is dynamically regulated both temporally and spatially in many cells (1), Cl− fluxes may be inward or outward, depending on local values of ECl, [Ca2+]i, and Vm. Thus, Ano1 might have a negative or a positive feedback function. This might have important physiological and pathological implications in, for example, the genesis of cardiac arrhythmias, where an outward CaCC current normally participates in repolarization of the cardiac action potential in some species (2). However, under conditions of Ca2+ overload, this channel produces transient inward currents, which are arrhythmogenic. Similarly, because Ano1 rectification is regulated by [Ca2+], Ano1 in epithelial cells could mediate secretion or absorption, depending on the interplay of Vm, ECl, and [Ca2+]i. Thus, the exquisite Vm and Ca2+ sensitivity of CaCC channels places these channels in a pivotal position for regulation of cellular excitability.
Experimental Procedures
Electrophysiology.
HEK-293 cells were transiently transfected with 0.1–1 μg of mouse Ano1(a,c) tagged on the C terminus with EGFP (provided by Dr. Uhtaek Oh, Seoul National University) per 35-mm dish using Fugene-6 (Roche) and patch-clamped 24–72 h later at room temperature (27). Mutations were made by PCR-based mutagenesis (Quickchanger; Agilent) and confirmed by sequencing. Transfected cells were identified by EGFP fluorescence. For whole-cell recording, zero Ca2+ pipette solution contained 146 mM CsCl, 2 mM MgCl2, 5 mM EGTA, 10 mM sucrose, and 8 mM Hepes (pH 7.3), adjusted with N-methyl-D-glucamine. High Ca2+ pipette solution contained 5 mM Ca2+-EGTA, instead of EGTA (free Ca2+ ∼25 μM). Different free [Ca2+] solutions were made by mixing zero- Ca2+ and high-Ca2+ solutions. The 126 μM Ca2+ was made by adding 0.2 mM CaCl2 to high-Ca2+ solution. Br2-BAPTA [5,5′-dibromo-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, 3.5 mM; Invitrogen] was used to make 1-μM to 4-μM free [Ca2+] solutions in some experiments. Standard external solution contained: 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM Hepes (pH 7.4). The study of the effects of [Cl−]e or [I−]e on Ano1 gating used a pipette solution with 2.241 mM CaCl2, 25.241 mM EGTA-TEA, and 50 mM Hepes (pH 7.3) (TEA-OH), and 85 mM d-mannitol (calculated [Ca2+] = 75 nM). High-Cl− external solution contained 135 mM TEA-Cl, 0.5 mM CaCl2, 20 mM Hepes, and 75 mM d-mannitol. Low-Cl− external solution contained 0.5 mM CaCl2, 200 mM Hepes, and 45 mM d-mannitol. External solutions with different [Cl−] concentrations were made by mixing high- and low-Cl− solutions. Iodide mixtures were made by mixing high-Cl− external solution with a solution in which Cl− was replaced by I−.
Rapid Perfusion.
The fast application of Ca2+ to excised inside-out patches was performed using a double-barreled theta tubing (1.5 mm o.d.; Sutter Instruments) with a tip diameter of ∼50 μm attached to a piezobimorph on a micromanipulator (43). One barrel was filled with standard zero-[Ca2+]i solution, and the other barrel was with intracellular solution containing Ca2+. Excised patches were switched between streams by applying ∼100 V to the piezobimorph. The time course of solution exchange across the laminar flow interface was estimated by liquid junction potential measurements to be 0.5 ms (10–90% rise time) for a 10-fold difference in ionic strength (Fig. S3). For BK channel recordings, CsCl was replaced with equimolar KCl.
Data Analysis.
Traces were analyzed with Clampfit 9 (Molecular Devices). Conductance (G) from tail currents measured 200 μs after repolarization to −100 mV from various test potentials was normalized to maximum conductance, Gmax. G/Gmax vs.Vm curves were fitted to a Boltzmann function with Origin 7 (OriginLab). Results are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Uhtaek Oh for helpful discussions and Steve Traynelis and Katie Vance for discussions and technical assistance with fast perfusion. This work was supported by National Institutes of Health Grants GM60448 and EY014852 (to H.C.H.), CONACyT Grants 79897 (to J.A.) and 105457 (to P.P.C.), and an American Heart Association postdoctoral fellowship (to Q.X.).
Footnotes
The authors declare no conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102147108/-/DCSupplemental.
References
- 1.Duran C, Thompson CH, Xiao Q, Hartzell HC. Chloride channels: Often enigmatic, rarely predictable. Annu Rev Physiol. 2010;72:95–121. doi: 10.1146/annurev-physiol-021909-135811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 3.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004;1:42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 4.Tarran R, et al. Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl− conductances. J Gen Physiol. 2002;120:407–418. doi: 10.1085/jgp.20028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephan AB, et al. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci USA. 2009;106:11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengl T, et al. Molecular components of signal amplification in olfactory sensory cilia. Proc Natl Acad Sci USA. 2010;107:6052–6057. doi: 10.1073/pnas.0909032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasche S, et al. Tmem16b is specifically expressed in the cilia of olfactory sensory neurons. Chem Senses. 2010;35:239–245. doi: 10.1093/chemse/bjq007. [DOI] [PubMed] [Google Scholar]
- 8.Stöhr H, et al. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29:6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Large WA, Wang Q. Characteristics and physiological role of the Ca(2+)-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- 10.Duan D. Phenomics of cardiac chloride channels: The systematic study of chloride channel function in the heart. J Physiol. 2009;587:2163–2177. doi: 10.1113/jphysiol.2008.165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B, et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 13.Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 15.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanenko VG, et al. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285:12990–13001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F, et al. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci USA. 2009;106:21413–21418. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang SJ, et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Rock JR, et al. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J Biol Chem. 2009;284:14875–14880. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuruma A, Hartzell HC. Bimodal control of a Ca(2+)-activated Cl(−) channel by different Ca(2+) signals. J Gen Physiol. 2000;115:59–80. doi: 10.1085/jgp.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pifferi S, Dibattista M, Menini A. TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch. 2009;458:1023–1038. doi: 10.1007/s00424-009-0684-9. [DOI] [PubMed] [Google Scholar]
- 25.Falke JJ, Drake SK, Hazard AL, Peersen OB. Molecular tuning of ion binding to calcium signaling proteins. Q Rev Biophys. 1994;27:219–290. doi: 10.1017/s0033583500003012. [DOI] [PubMed] [Google Scholar]
- 26.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Q, Prussia A, Yu K, Cui YY, Hartzell HC. Regulation of bestrophin Cl channels by calcium: Role of the C terminus. J Gen Physiol. 2008;132:681–692. doi: 10.1085/jgp.200810056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrera L, et al. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem. 2009;284:33360–33368. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao SH, Suzuki Y, Zysk JR, Cheung WY. Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol. 1984;26:75–82. [PubMed] [Google Scholar]
- 30.Perez-Cornejo P, De Santiago JA, Arreola J. Permeant anions control gating of calcium-dependent chloride channels. J Membr Biol. 2004;198:125–133. doi: 10.1007/s00232-004-0659-x. [DOI] [PubMed] [Google Scholar]
- 31.Qu Z, Hartzell HC. Anion permeation in Ca(2+)-activated Cl(−) channels. J Gen Physiol. 2000;116:825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach A, Sigurdson W, Chen J, Akk G. Voltage dependence of mouse acetylcholine receptor gating: Different charge movements in di-, mono- and unliganded receptors. J Physiol. 1996;494:155–170. doi: 10.1113/jphysiol.1996.sp021482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Rodríguez JE, De Santiago-Castillo JA, Arreola J. Permeant anions contribute to voltage dependence of ClC-2 chloride channel by interacting with the protopore gate. J Physiol. 2010;588:2545–2556. doi: 10.1113/jphysiol.2010.189175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels, I: Mslo ionic currents in the absence of Ca(2+) J Gen Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheridan JT, et al. Characterization of the oligomeric structure of the Ca(2+)-activated Cl− channel Ano1/TMEM16A. J Biol Chem. 2011;286:1381–1388. doi: 10.1074/jbc.M110.174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fallah G, et al. TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol Cell Proteom. 2011;10(2):1–10. doi: 10.1074/mcp.M110.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian Y, et al. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011;25:1058–1068. doi: 10.1096/fj.10-166884. [DOI] [PubMed] [Google Scholar]
- 40.Das S, et al. Topology of NGEP, a prostate-specific cell:cell junction protein widely expressed in many cancers of different grade level. Cancer Res. 2008;68:6306–6312. doi: 10.1158/0008-5472.CAN-08-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arreola J, Melvin JE, Begenisich T. Differences in regulation of Ca2+-activated Cl- channels in colonic and parotid secretory cells. Am J Physiol Cell Physiol. 1998;274:C161–C166. doi: 10.1152/ajpcell.1998.274.1.C161. [DOI] [PubMed] [Google Scholar]
- 42.Kaneko H, Möhrlen F, Frings S. Calmodulin contributes to gating control in olfactory calcium-activated chloride channels. J Gen Physiol. 2006;127:737–748. doi: 10.1085/jgp.200609497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.