Abstract
Many salmon populations in both the Pacific and Atlantic Oceans have experienced sharply decreasing returns and high ocean mortality in the past two decades, with some populations facing extirpation if current marine survival trends continue. Our inability to monitor the movements of marine fish or to directly measure their survival precludes experimental tests of theories concerning the factors regulating fish populations, and thus limits scientific advance in many aspects of fisheries management and conservation. Here we report a large-scale synthesis of survival and movement rates of free-ranging juvenile salmon across four species, 13 river watersheds, and 44 release groups of salmon smolts (>3,500 fish tagged in total) in rivers and coastal ocean waters, including an assessment of where mortality predominantly occurs during the juvenile migration. Of particular importance, our data indicate that, over the size range of smolts tagged, (i) smolt survival was not strongly related to size at release, (ii) tag burden did not appear to strongly reduce the survival of smaller animals, and (iii) for at least some populations, substantial mortality occurred much later in the migration and more distant from the river of origin than generally expected. Our findings thus have implications for determining where effort should be invested to improve the accuracy of salmon forecasting, to understand the mechanisms driving salmon declines, and to predict the impact of climate change on salmon stocks.
Keywords: acoustic arrays, Pacific Ocean Shelf Tracking, mark-recapture
Existing knowledge about the marine movements of salmon is primarily based on analysis of fish marked with simple mechanical tags (1–7). Such tags are recovered at very low rates (typically <1%) and usually in fishing gear, so the species of interest must generally be the target of a substantial fishery, and many fish must be tagged to generate useful information. This dependence greatly limits the range and size of species studied and introduces bias as a result of the movements, techniques, gear, and reporting behavior of the fishermen. Further, only release and recovery locations are known, precluding detailed movement information of individuals. Electronic tags that transmit archived data to satellites or cell phones transcend some of these limitations but are limited to use on large animals (8–10). Of equal importance, survival cannot be measured on fine temporal or geographic scales by using archival tags.
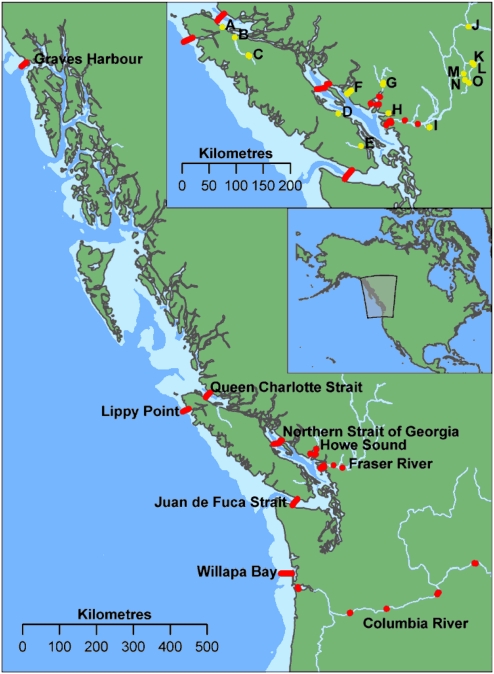
Here we report direct in situ survival and movement estimates for greater than 3,500 juvenile Pacific salmon (“smolts”) that were tagged and released in British Columbia (BC), Canada, during their migration downriver and a substantial portion of their journey north along the continental shelf, for the years 2004 through 2007 (Table S1). These fish represent four of the six North American species of Pacific salmon: coho (Oncorhynchus kisutch), chinook (Oncorhynchus tshawytscha), sockeye (Oncorhynchus nerka), and steelhead (Oncorhynchus mykiss). Each fish was surgically implanted with an individually identifiable acoustic transmitter and the tags were detected with a large-scale acoustic telemetry system, the Pacific Ocean Shelf Tracking (POST) array (Fig. 1), which currently extends more than 1,500 km along the North American continental shelf.
Fig. 1.
The POST array, showing the main continental shelf and river system subarrays in the Pacific Northwest. We collectively refer to the internal water bodies bounded by the JDF Strait and QCS subarrays (Puget Sound, Strait of Georgia, Johnstone Strait, and QCS) as the Salish Sea. Letters beside yellow dots refer to locations where tagged fish were released (Table S1). Receivers are shown with red dots; marine lines extend across straits, or from near shore to the edge of the continental shelf (shaded; ≤200 m depth).
The overall POST array is formed from a series of regional subarrays, with each subarray consisting of several acoustic receivers (“nodes”) independently positioned above the sea (or river) bed in a 3D spatial geometry that provided a high probability of detecting tagged animals passing over each subarray. By successfully recovering the stored detection data on the majority of these receivers, it is possible to describe both the early marine movements of individuals and the survival of groups as they exit rivers and migrate out along the continental shelf.
The POST array is part of a new generation of acoustic technologies that are beginning to yield an understanding of fish movement and behavior on a very large scale (11, 12). Telemetry arrays sited on the continental shelf-slope are particularly well suited for the study of juvenile salmon, as this period of their life history is thought to be essentially shelf-limited (3, 5, 6, 13–15). Continental shelves are also of key ecological importance: although they cover less than 8% of the global ocean by area (16), they contribute 69% of the world fish catch [89% if upwelling zones are included (17)], and support high biodiversity and large populations of marine mammals (18) and seabirds (19).
Results
Migration Routes.
The unprecedented detail stemming from extensive telemetry arrays can most effectively be demonstrated by animating the movements of the tagged animals (SI Materials and Methods); from this, a general picture of the direction and speed of movement for the salmon smolts immediately emerges. Our data demonstrate two distinct species-specific patterns of marine migration, with sockeye and steelhead undertaking sustained long-distance migration and coho and chinook apparently remaining resident in the Salish Sea for the duration of the tags’ batteries (>4 mo). Emigration from the Salish Sea occurred almost exclusively via the northern route through Queen Charlotte Strait [QCS; Fig. 1; sockeye, 91%; steelhead, 81%; calculated as a proportion of the total tags detected on either QCS or Juan de Fuca (JDF) subarrays], a finding consistent with earlier studies (2). However, some individual steelhead populations exited only or predominantly via the southern JDF route whereas other nearby populations exited primarily via the northern route, with some variability in the proportion of fish going north versus south evident between years for some populations (Movies S1–S3) (20). The reasons for these differences among and within populations are unclear, but suggest that genetic and environmental factors affecting migration direction may operate at relatively fine scales.
Only one juvenile BC salmonid was detected on the Alaskan subarray, located some 1,000 km north of QCS, although tagged smolts from the Columbia River and other nonsalmonids were detected there more commonly (21, 22).*
Steelhead, unlike other species of Pacific salmonids, are thought to move directly offshore (15), so the failure to detect BC steelhead in Alaska may reflect this migration pattern. In contrast, genetic analysis of juvenile sockeye collected in research surveys during the past decade provides ample evidence that BC sockeye remain on the shelf and are caught beyond the location of the Alaskan subarray (3, 5, 15). This suggests that the smolts swam off the shelf around the Alaska receiver line, stopped their shelf migration before reaching it, or died before reaching it.
Migration Speeds.
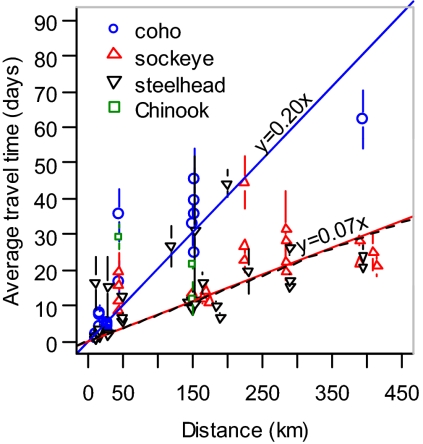
Despite their small size (13–25 cm), juvenile sockeye and steelhead undertook strongly directed migrations (Fig. 2), swimming as much as 400 km downriver and 400 km through the Salish Sea in an average of 22 d after ocean entry. These migration rates correspond to average straight-line speeds of approximately 0.95 and 0.86 body lengths (BL/s) per second, respectively (Table 1), consistent with the theoretically optimal migration speeds of 0.8 to 2 BL/s calculated for small sockeye (23–26). Juveniles of both species had virtually identical rapid rates of travel over the marine components of the array. Similar marine travel speeds were observed for steelhead across a number of Puget Sound populations as well (27–29).
Fig. 2.
Average travel time (±1 SE) for individual populations from river mouth to the marine subarrays [Northern Strait of Georgia (NSOG), Howe Sound inner and outer lines, QCS, and JDF Strait] compared with minimum migration distance to the lines. Population estimates for each of the years 2004 to 2007 are separated by species (see Fig. 1 for locations). Lines show regression fits of travel time on distance, constrained to go through the origin, for coho (upper line) and sockeye and steelhead juveniles (lower lines); a regression line is not plotted for chinook because of sparse data, but point estimates are shown. The reciprocal of the slope represents speed, estimated at 5 km/d for coho and 14 km/d for sockeye and steelhead. More than one data point may be presented for a given population, representing different ocean lines encountered. Population estimates are not presented if only a single fish was detected.
Table 1.
Marine migration speeds for four species of Pacific salmon
| Species | N | Mean | Median | SD |
| Chinook | 5 | 0.33 | 0.15 | 0.44 |
| Coho | 87 | 0.96 | 0.29 | 4.93 |
| Sockeye | 128 | 0.95 | 0.89 | 0.38 |
| Steelhead | 189 | 0.86 | 0.79 | 0.54 |
Marine migration speeds are in BL•s−1. Sockeye estimates do not include kokanee. Speeds were estimated for individual smolts as the time between departure from the river mouth until arrival at QCS or JDF, divided by the minimum migration distance between those two locations in BL/s at time of tagging. Individual speeds were then summarized for each species.
In contrast, juvenile coho and chinook showed outmigration speeds similar to steelhead and sockeye while in freshwater, but much slower and more variable rates of migration after reaching the ocean. The few tagged coho and chinook detected on distant marine subarrays took much longer to reach the subarrays than sockeye and steelhead (Fig. 2), and had a strongly skewed distribution of migration speeds relative to sockeye and steelhead, suggesting that an initially rapid marine migration to nearby subarrays was replaced by a much less directed movement pattern, such that these species did not immediately migrate out of the Salish Sea (Table 1). Supporting this, coho were often detected on multiple nearby receivers over a period of several days, indicating considerable milling. This was also consistent with recent evidence that juvenile coho tagged in the marine waters of the Salish Sea in late summer did not begin migrating out of the Strait until October (30, 31), well beyond the rated lifespan of our tags.
Survival.
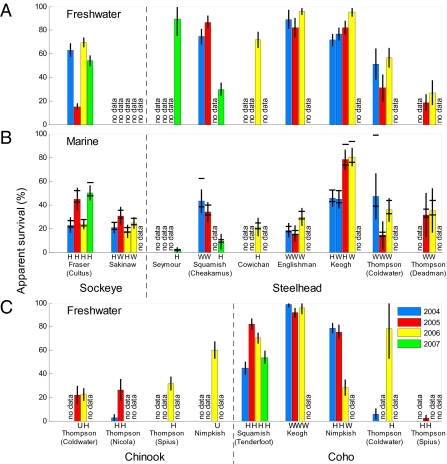
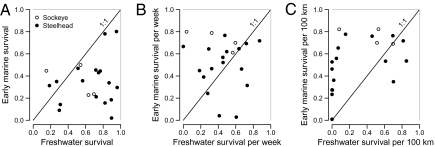
Previous research on salmon survival has had limited ability to partition the mortality occurring between the downstream migration of smolts and the return of adults, leading to uncertainty as to when the major factors controlling salmon populations exert their effects. Our results demonstrate substantial interannual and interpopulation variation in survival during both the freshwater and the early marine migration (Fig. 3). Year effects varied from population to population; none of the 4 y showed consistently higher or lower survival across all populations, and a population's freshwater survival during the downriver migration did not appear to be related to its survival during the early coastal ocean migration (Fig. 4A). Further, the variation in survival within these environments appeared roughly similar.
Fig. 3.
Survival estimates for southern BC salmon populations. (A and B) Freshwater and early marine survival for sockeye and steelhead populations. (C) Freshwater estimates for Chinook and coho. Freshwater survival is from the release site to the river mouth subarray and early marine survival is from the river mouth to exit from the Salish Sea (operationally defined as the QCS and JDF marine lines). Error bars indicate ±1 SE on survival estimates. Horizontal bands in B show bracketed estimates of early marine survival arising from uncertainty in the fixed value of p assumed for the QCS and JDF lines (SI Materials and Methods). Rearing origin (H, hatchery; W, wild; U, unknown) is indicated below the bar for each population. “No data” indicates tagging did not occur in that year.
Fig. 4.
Comparisons of survival of steelhead and sockeye during downriver migration and early marine migration: (A) survival, (B) survival scaled by time, and (C) survival scaled by distance. Freshwater travel times were estimated for each population as the median time between release and arrival on the river mouth line. Early marine travel time was estimated as the median time between departure from the river mouth line and arrival at the final (QCS/JDF) marine lines. The solid line shows the 1:1 line where early marine survival and downriver survival are equal.
Some differences between species were apparent. In general, survival during the marine coastal migration was roughly comparable for sockeye and steelhead (Figs. 3B and 4A). The extremely low early marine survival estimates of coho and chinook are confounded with patterns of residency, as noted earlier, preventing direct comparison. There were no consistent differences in survival among wild and hatchery-reared groups, although only two populations (Keogh and Sakinaw) had fish tagged from both rearing histories in the same year. In those two cases, survival was similar for hatchery and wild groups.
Comparison of marine and freshwater survival rates is complicated because survival may be measured relative to either the time over which fish were observed or the distance they traveled (22, 28). We estimated lower overall survival for sockeye and steelhead during their early marine migration than during their down-river migration (Fig. 4A; all but three estimates lie below the 1:1 line). However, when scaled by time, survival rates of Cultus Lake sockeye were consistently higher during the marine migration than during the down-river migration (above the 1:1 line), whereas steelhead survival rates were roughly equivalent (Fig. 4B). When scaled by distance traveled (Fig. 4C), survival rates of both species were higher during the marine migration.
Estimates of the uncertainty in the survival values shown in Fig. 3 are relatively small compared with other methods for estimating survival, despite the modest numbers of juveniles tagged (Table S1). This primarily results from relatively high detection probabilities, particularly of the larger V9 tags, achieved at the marine subarrays (Fig. S2). The variation in detection probability on subarrays that did occur was mainly accounted for by loss of receivers to commercial fishing activity and secondarily by year-to-year variation in the spacing between the receivers on a subarray. In freshwater, detection probability was more variable than at marine subarrays, and lower overall in the Fraser River but higher on average in other rivers. We also observed a seasonal degradation of detection probability on some freshwater subarrays (likely caused by faster migration speeds past the receivers sited in freshwater during high flow events) that was not seen on ocean subarrays (32) (SI Materials and Methods).
Smolt Size and Survival.
The relatively simplistic architecture forming the pilot phase (POST) array was developed in 2001 to 2003 when only the larger V9 acoustic tag was commercially available. The smaller (and acoustically quieter) V7 tag only became available in 2005, after the pilot array was deployed; the prototype array design detected approximately 90% of V9 tagged smolts but only approximately 70% of V7 tagged smolts (Fig. S2). This is a large performance difference because the fraction of V9 tagged smolts detected on the subarrays needs to be increased by only approximately 10% (i.e., 0.9−1) to account for detection inefficiencies in the coastal ocean, a relatively trivial correction. However, this correction is more important and error-prone for V7 tags because their lower acoustic power means that the correction factor is larger (0.7−1 = 43%), and small estimation errors in detection efficiency cause larger errors in estimated survival.
The full size spectrum of wild Pacific salmon smolts other than steelhead cannot be surgically implanted by using the V7 or V9 tag, so the applicability of our current survival measurements to animals smaller than our minimum size limits is unclear. (These size limits were set at 130 and 140 mm fork length in 2006 to 2007, although some individuals smaller than these limits were tagged in 2004 and 2005.) As acoustic tags appropriate for implantation in smaller animals transmit signals that are more difficult to detect, a redesigned array that can effectively be used with smaller-sized animals will require some combination of increased numbers of receivers and tags to compensate for the reduced detection rates. Is there evidence that (i) smaller animals have lower survival because the relatively larger tag burden reduces their fitness after release, or that (ii) within the size range of smolts tagged, larger smolts survive better because they are less vulnerable to predators? Both questions raise important issues concerning the applicability of baseline survival estimates to the full size spectrum of wild populations; they also have substantial cost implications if more sophisticated array designs are contemplated.
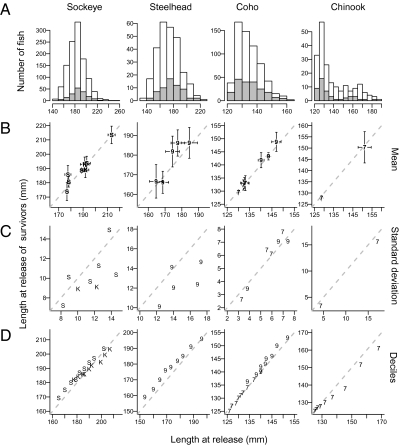
To evaluate the effect of smolt and tag size on survival, we compared the change in the frequency distribution of smolt sizes at the time of tagging with the size distribution of survivors detected at outer reaches of the array (Fig. 5). We defined sockeye and steelhead survivors as smolts detected at the JDF Strait or QCS subarrays, as these species consistently migrated out of the Salish Sea. We defined coho and chinook survivors as smolts detected at the mouth of the Fraser River, as these two species ceased migration in the Strait of Georgia but had long freshwater migrations down the Fraser River (Fig. 1) (22, 33); for Cheakamus coho, which enter Howe Sound, we defined survivors as fish reaching the outer Howe Sound subarray, because outmigration occurred consistently to this point.
Fig. 5.
Evaluation of possible size-dependent effects of downstream and early marine survival. Plots show summaries of length differences between the tagged smolts at release and the subset of animals surviving to reach distant parts of the array, separated by species. (A) Frequency distribution of fork length at time of tagging for released animals (white) and survivors (gray), with all release groups combined. (B) Mean fork length for individual release groups (±2 SE). (C) SD for individual release groups. (D) Quantile-quantile (qq) plots of the deciles of the empirical length distributions of released fish and survivors (years and stocks are pooled within species and tag size categories). The 1:1 lines are indicated. Individual release groups are identified by an asterisk in Table S1, and consist of all species, stock, hatchery/wild provenance, acoustic tag type, and release year combinations consisting of at least 25 individuals released and at least 10 individuals detected. The smaller V7 and larger V9 acoustic tag types implanted into smolts from these individual release groups are distinguished in the plots by 7 and 9; sockeye (S) and kokanee (K) were all tagged with V9 tags. Individual panels consisted almost entirely of hatchery or wild origin smolts, so these are not distinguished.
The overall shape of size–frequency distributions changed little between the released animals and the survivors detected after substantial freshwater and early marine mortality occurred (Fig. 5A), and their mean size was generally indistinguishable (Fig. 5B). The SD of the two normally distributed distributions (sockeye and steelhead) showed some evidence that the size of survivors was more tightly distributed around the mean than at release (Fig. 5C), but this could be a result of the small number of smolts in the largest and smallest size categories (Fig. 5A) rather than size-related mortality. The SD was unchanged for coho and chinook.
Quantile-quantile plots (34) are effective at detecting changes in distributional shape (i.e., skew or kurtosis), which would occur if mortality was preferentially acting to remove the smallest or largest tagged animals after release. The results (Fig. 5D) indicate that the shape of the size–frequency distribution remained similar between the release groups and survivors; there is some slight evidence that larger chinook smolts were slightly underrepresented among survivors, whereas smaller steelhead were slightly overrepresented. A very slight difference is also evident for Sakinaw Lake kokanee (a genetically distinct life history type of sockeye generally considered to remain exclusively in freshwater), with the largest animals possibly underrepresented among outmigrating animals. In each species, the effect is, at most, a few millimeters. Although morphologically indistinguishable from sockeye at the time of tagging, additional data show that some Sakinaw kokanee and sockeye remained resident in the Strait of Georgia after release off their river mouth, and 12% of Sakinaw kokanee (but no sockeye) then migrated back into their natal lake within a few weeks of release (35); size dependence in residency behavior may therefore confound these apparent size–survival relationships for kokanee. This pattern is not evident for Sakinaw or Cultus Lake sockeye, which showed an essentially identical size distribution between released animals and survivors.
Discussion
Many salmon populations in both the Pacific and Atlantic oceans have experienced sharply decreasing returns and high ocean mortality in the past two decades, with some populations facing extirpation if current marine survival trends continue (36, 37). The need for a better understanding of the spatial and temporal patterns of salmon mortality in the sea is frequently cited (37, 38), particularly in the period just after ocean entry when most mortality is thought to occur (15, 39). Our findings provide a new perspective on where and when this mortality occurs. Historically (40), fisheries biologists argued that mortality rates were highest early in the life history (when the fish were smallest), so this was the time period likely to determine the number of adult fish that survived.
The median survival of the steelhead and sockeye populations estimated in this study was 16.5% (i.e., one in six juveniles surviving to exit from the Salish Sea), with population-specific marine travel times as long as 28 d depending on the distance traveled. In contrast, survival over the entire juvenile-to-adult lifespan of many Salish Sea salmon populations has decreased to only 1% to 4% in the past two decades (28, 41–45), with concerns raised about the relative role of salmon aquaculture, hatcheries, climate change, and ecosystem changes in causing the decline (46–48). Our measurements of survival within the first weeks of the migration (i.e., one of six smolts surviving) can thus be compared with total survival over the period of approximately 2.5 y until adult return generated by other methods (approximately one in 25–100 of outmigrating juveniles); the implication is that the cumulative total mortality beyond the Salish Sea is approximately four to 17 times larger than what is experienced within the geographic limits of the Salish Sea array in roughly the first month of life in the sea, making it unlikely that year-class strength is primarily determined very early in the marine life history.
The 2007 outmigrating Cultus Lake (Fraser River) sockeye smolts, whose adults returned in 2009, provided a particularly clear example. A catastrophically low return of adult sockeye occurred in the summer of 2009, with nearly all Fraser River populations experiencing survival rates of only 1%, including the Cultus Lake hatchery population reported here (49, 50). The smolts were implanted with specially programmed tags that transmitted during both the outbound smolt and subsequent 2009 inbound adult migration phases, with an intervening 25-mo quiet period to conserve battery power (49). Although the 2007 smolts experienced 28% survival after migrating downriver and out of the Salish Sea (which was equal to or higher than the survival in the previous 3 y) (44), only 1% of the released smolts (two of 200) returned as adults, consistent with the smolt-to-adult survival of both the untagged Cultus Lake hatchery smolts (0.5%) and wild-origin smolts (1.4%) (49). Both adults were detected returning to BC within 1 d of each other via the JDF Strait in 2009, even though they had emigrated via QCS as smolts in 2007 1 wk apart (Movies S2 and S3).
Survival experienced in the freshwater and early marine period (28% in the first 6 wk until the smolts migrated out of the Salish Sea) relative to what must have occurred in the following approximately 2 y can be expressed as:
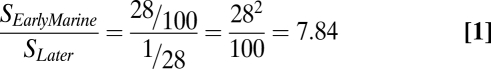 |
Our survival measurements for Cultus Lake sockeye, which encompass the whole marine life history, therefore indicate that mortality within the Salish Sea is only approximately one eighth the mortality still to come, or that SEarly is approximately 8·SLater, consistent with the general calculation developed earlier. Thus, much mortality (and thus the chances for major changes in that value) likely occurs later in the life history of Pacific salmon, after the first month of life in the sea has passed.
Our results support the longstanding assumption that the rate of mortality is highest in the first month of the migration, but also show that much of the (cumulative) juvenile-to-adult mortality occurs after juvenile salmon leave the waters of the Salish Sea. The results further suggest that the conditions that caused the costly collapse in 2009 of Fraser River sockeye probably occurred outside the Salish Sea. A common concern in tagging studies is that the tagging process may reduce survival. If it were true that survival of untagged fish is greater than survival of tagged fish (and our size–survival results show little evidence for this), then there would necessarily be even greater mortality beyond the Salish Sea than what we have estimated for tagged fish.
The extent to which significant mortality occurs later in the oceanic life phase of other salmon populations is unknown, given that we are only now beginning to develop a baseline of early marine survival estimates. The size of available tags has until recently limited current studies on salmon survival to the use of larger smolts representing only part of the naturally occurring size spectrum, range of species, and diversity of life histories. Expanding the size range of juveniles tagged, as well as broadening the range of release dates, will be important steps toward increasing the utility and relevance of the survival measurements, although our analysis suggests that mortality seems to be rather uniform across the size range of salmon smolts tagged to date. Telemetry arrays may also be useful in combination with other methods for testing hypotheses concerning the relative fitness of hatchery and wild fish (51), the need for water releases from dams to support juvenile migrations to the ocean (52), the impact of transfer of sea lice and other pathogens from farmed to wild salmon (47, 53, 54), and determining the linkage between the physiological state and health of individual fish and their subsequent survival (55). Given the projected major changes in climate (56), more broadly based survival baselines should be developed and used to explicitly test the many theories that will be put forward to explain the large-scale changes likely in marine fish populations.
Materials and Methods
Analysis of movement speeds for individuals and population aggregates was obtained by calculating the elapsed time for individuals to travel between subarrays (calculated as the time difference between last and first detection on successive arrays) and dividing this value into the minimum straight-line distance in water between arrays. Survival was estimated using standard Cormack–Jolly–Seber (CJS) statistical models (SI Materials and Methods).
Supplementary Material
Acknowledgments
This work is a contribution to the Census of Marine Life and was supported by funding from the Census of Marine Life, Gordon and Betty Moore Foundation, Pacific Salmon Commission, Pacific Salmon Foundation, Canadian National Railway, the British Columbia Pacific Salmon Forum, the US Department of Energy (Bonneville Power Administration), and the Northwest Power Planning and Conservation Council. All work involving live fish was annually reviewed and approved as meeting or exceeding the standards laid out by the Canadian Council on Animal Care. Protocols were approved by the Pacific Region Animal Care Committee (Fisheries and Oceans Canada) in 2004 (04-026) and 2005 (05-004), and by the Animal Care Committee of Malaspina University-College (now Vancouver Island University) in 2006 (2006-08), 2007 (2006-08-R1), and 2008 (2006-08-R2).
Footnotes
Conflict of interest statement: D.W.W. is architect of the Pacific Ocean Shelf Tracking (POST) acoustic array concept and President of Kintama Research Services, an environmental consultancy and the company that developed and operates substantial elements of the overall POST telemetry array.
All data used in this paper have been deposited in the publicly accessible Pacific Ocean Shelf Tracking (POST) database, www.postcoml.org.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014044108/-/DCSupplemental.
*At migration speeds of 20 km/d, the juvenile salmon should have covered the 1,000 km distance from the QCS to Alaska in approximately 50 d, well within the remaining lifespan of the tags.
References
- 1.Groot C, Cooke K. Are the migrations of juvenile and adult Fraser River sockeye salmon (Oncorhynchus nerka) in near-shore waters related? Can Spec Pub Fish Aquat Sci. 1987;96:53–60. [Google Scholar]
- 2.Groot C, Margolis L, Bailey R. Does the route of seaward migration of Fraser River sockeye salmon (Oncorhynchus nerka) smolts determine the route of return migration of the adults? In: McCleave JD, Arnold GP, Dodson JJ, Neill WH, editors. Mechanisms of Migration in Fishes. New York: Plenum; 1984. pp. 283–292. [Google Scholar]
- 3.Trudel M, et al. Distribution and migration of juvenile Chinook salmon derived from coded-wire tag recoveries along the continental shelf of western North America. Trans Am Fish Soc. 2009;138:1369–1391. [Google Scholar]
- 4.Trudel M, et al. Regional variation in the marine growth and energy accumulation of juvenile Chinook salmon and coho salmon along the West coast of North America. In: Grimes CB, Brodeur RD, Haldorson LJ, McKinnell SM, editors. The Ecology of Juvenile Salmon in Northeast Pacific Ocean: Regional Comparisons. Symposium 57. Bethesda, MD: American Fisheries Society; 2007. pp. 205–232. [Google Scholar]
- 5.Tucker S, et al. Seasonal stock-specific migrations of juvenile sockeye salmon along the west coast of North America: Implications for growth. Trans Am Fish Soc. 2009;138:1458–1480. [Google Scholar]
- 6.Peterson WT, Morgan CA, Fisher JP, Casillas E. Ocean distribution and habitat associations of yearling coho (Oncorhynchus kisutch) and Chinook (O. tshawytscha) salmon in the northern California Current. Fish Oceanog. 2010;19:508–525. [Google Scholar]
- 7.Weitkamp LA. Marine distributions of chinook salmon from the west coast of North America determined by coded wire tag recoveries. Trans Am Fish Soc. 2009;139:147–170. [Google Scholar]
- 8.Block BA. Physiological ecology in the 21st Century: Advancements in biologging. Integr Comp Biol. 2005;45:305–320. doi: 10.1093/icb/45.2.305. [DOI] [PubMed] [Google Scholar]
- 9.Block BA, et al. Electronic tagging and population structure of Atlantic bluefin tuna. Nature. 2005;434:1121–1127. doi: 10.1038/nature03463. [DOI] [PubMed] [Google Scholar]
- 10.Bonfil R, et al. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science. 2005;310:100–103. doi: 10.1126/science.1114898. [DOI] [PubMed] [Google Scholar]
- 11.Heupel M, Semmens J, Hobday A. Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshwater Res. 2006;57:1–13. [Google Scholar]
- 12.Makris NC, et al. Fish population and behavior revealed by instantaneous continental shelf-scale imaging. Science. 2006;311:660–663. doi: 10.1126/science.1121756. [DOI] [PubMed] [Google Scholar]
- 13.Bi H, Ruppel RE, Peterson WT. Modeling the pelagic habitat of salmon off the Pacific Northwest (USA) coast using logistic regression. Mar Ecol Prog Ser. 2007;336:249–265. [Google Scholar]
- 14.Fisher J, et al. Comparisons of the coastal distributions and abundances of juvenile Pacific salmon from central California to the northern Gulf of Alaska. Am Fish Soc Symp. 2007;57:31–80. [Google Scholar]
- 15.Hartt AC, Dell MB. Early oceanic migrations and growth of juvenile Pacific salmon and steelhead trout. Int North Pacific Fish Comm. 1986;46:1–105. [Google Scholar]
- 16.Menard HW, Smith SM. Hypsometry of Ocean Basin Provinces. J Geophys Res. 1966;71:4305–4325. [Google Scholar]
- 17.Pauly D, Christensen V. Primary production required to sustain global fisheries. Nature. 1995;374:255–257. [Google Scholar]
- 18.Keiper K, Ainley DG, Allen SG, Harvey JT. Marine mammal occurrence and ocean climate off central California, 1986 to 1994 and 1997 to 1999. Mar Ecol Prog Ser. 2005;289:285–306. [Google Scholar]
- 19.Acha EM, Mianzan HW, Guerrero RA, Favero M, Bava J. Marine fronts at the continental shelves of austral South America: Physical and ecological processes. J Mar Syst. 2004;44:83–105. [Google Scholar]
- 20.Melnychuk MC, Welch DW, Walters CJ. Spatio-temporal migration patterns of Pacific salmon smolts in rivers and coastal marine waters. PLoS ONE. 2010;5:e12916. doi: 10.1371/journal.pone.0012916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindley ST, et al. Marine migration of north American green sturgeon. Trans Am Fish Soc. 2008;137:182–194. [Google Scholar]
- 22.Welch DW, et al. Survival of migrating salmon smolts in large rivers with and without dams. PLoS Biol. 2008;6:e265. doi: 10.1371/journal.pbio.0060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brett JR. Energetics. In: Groot C, Margolis L, Clarke WC, editors. Physiological Ecology of Pacific Salmon. Vancouver: UBC Press; 1995. pp. 1–68. [Google Scholar]
- 24.Hinch SG, Cooke SJ, Healey MC, Farrell AP. Behavioural physiology of fish migrations: salmon as a model approach. In: Sloman KA, Wilson RW, Balshine S, editors. Fish physiology. Vol 24. New York: Elsevier; 2006. pp. 239–295. [Google Scholar]
- 25.Trudel M, Tucker S, Morris JFT, Higgs DA, Welch DW. Indicators of energetic status in juvenile coho salmon and Chinook salmon. N Am J Fish Manage. 2005;25:374–390. [Google Scholar]
- 26.Weihs D. Optimal fish cruising speed. Nature. 1973;245:48–50. [Google Scholar]
- 27.Moore ME, et al. Early marine migration patterns of wild coastal cutthroat trout (Oncorhynchus clarki clarki), steelhead trout (Oncorhynchus mykiss), and their hybrids. PLoS ONE. 2010;5:e12881. doi: 10.1371/journal.pone.0012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore ME, Berejikian BA, Tezak EP. Early marine survival and behavior of steelhead smolts through Hood canal and the strait of Juan de Fuca. T Am Fish Soc. 2010;139:49–61. [Google Scholar]
- 29.Payne JC, et al. Tracking fish movements and survival on the Northeast Pacific Shelf. In: McIntyre A, editor. Marine Life: Diversity, Distribution and Abundance. London: Wiley Blackwell; 2010. [Google Scholar]
- 30.Chittenden CM, Beamish RJ, Neville CM, Sweeting RM, McKinley RS. The use of acoustic tags to determine the timing and location of the juvenile coho salmon migration out of the Strait of Georgia, Canada. Trans Am Fish Soc. 2009;138:1220–1225. [Google Scholar]
- 31.Healey MC. The ecology of juvenile salmon in Georgia Strait, British Columbia. In: McNeil C, Himsworth WJ, editors. Salmonid Ecosystems of the North Pacific. Corvallis, OR: Oregon State Univ Press; 1980. pp. 203–230. [Google Scholar]
- 32.Melnychuk MC. Estimation of survival and detection probabilities for multiple tagged salmon stocks with nested migration routes, using a large-scale telemetry array. Mar Freshw Res. 2009;60:1231–1243. [Google Scholar]
- 33.Chittenden CM, Melnychuk MC, Welch DW, McKinley RS. An investigation into the poor survival of an endangered Coho salmon population. PLoS ONE. 2010;5:e10869. doi: 10.1371/journal.pone.0010869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleveland WS. Visualizing Data. Summit, NJ: Hobart Press; 1993. [Google Scholar]
- 35.Wood CC, Welch DW, Godbout L, Cameron J. Marine migratory behaviour of hatchery-reared anadromous and wild non-anadromous sockeye salmon revealed by acoustic tags. In: McKenzie J, Phelps Q, Kopf R, Mesa M, Parsons B, Seitz A, editors. Advances in Fish Tagging and Marking Technology. Symposium 76. Bethesda, MD: American Fisheries Society; 2011. [Google Scholar]
- 36.Gustafson RG, et al. Pacific salmon extinctions: Quantifying lost and remaining diversity. Conserv Biol. 2007;21:1009–1020. doi: 10.1111/j.1523-1739.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 37.Mills D. The Ocean Life of Atlantic Salmon. Environmental and Biological Factors Influencing Survival. Oxford, UK: Blackwell; 2000. [Google Scholar]
- 38.National Research Council . Upstream: Salmon and Society in the Pacific Northwest. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 39.Pearcy WG. Ocean Ecology of North Pacific Salmonids. Seattle: Univ of Washington; 1992. [Google Scholar]
- 40.Hjort J. Fluctuations in the year classes of important food fishes. J Conseil. 1926;1:5–35. [Google Scholar]
- 41.Beamish RJ, et al. Trends in coho marine survival in relation to the regime concept. Fish Oceanogr. 2000;9:114–119. [Google Scholar]
- 42.Coronado C, Hilborn R. Spatial and temporal factors affecting survival in coho salmon (Oncorhynchus kisutch) in the Pacific Northwest. Can J Fish Aquat Sci. 1998;55:2067–2077. [Google Scholar]
- 43.English KK, Glova GJ, Blakley AC. An Upstream Battle: Declines in 10 Pacific Salmon Stocks and Solutions for Their Survival. Vancouver: David Suzuki Foundation; 2008. [Google Scholar]
- 44.Welch DW, et al. Freshwater and marine migration and survival of endangered Cultus Lake sockeye salmon smolts using POST, a large-scale acoustic telemetry array. Can J Fish Aquat Sci. 2009;66:736–750. [Google Scholar]
- 45.Welch DW, Ward BR, Smith BD, Eveson JP. Temporal and spatial responses of British Columbia steelhead (Oncorhynchus mykiss) populations to ocean climate shifts. Fish Oceanogr. 2000;9:17–32. [Google Scholar]
- 46.Cooke SJ, et al. Developing a mechanistic understanding of fish migrations by linking telemetry with physiology, behaviour, genomics and experimental biology: An interdisciplinary case study on adult Fraser River sockeye salmon. Fisheries. 2008;33:321–338. [Google Scholar]
- 47.Krkosek M, et al. Declining wild salmon populations in relation to parasites from farm salmon. Science. 2007;318:1772–1775. doi: 10.1126/science.1148744. [DOI] [PubMed] [Google Scholar]
- 48.Myers RA, et al. Hatcheries and endangered salmon. Science. 2004;303:1980. doi: 10.1126/science.1095410. [DOI] [PubMed] [Google Scholar]
- 49.Welch DW. POST Array-Based Measurements of Coastal Ocean Survival of Juvenile Salmon, 2006-2009. In: Crawford WR, Irving JR, editors. State of Physical, Biological, and Selected Fishery Resources of Pacific Canadian Marine Ecosystems in 2009 (DFO Canadian Science Advisory Secretariat Research Document–2010/053) Ottawa: Oceans and Fisheries Canada; 2010. pp. 121–127. [Google Scholar]
- 50.Peterman RM, et al. Synthesis of Evidence from a Workshop on the Decline of Fraser River Sockeye. June 15–17, 2010. A Report to the Pacific Salmon Commission. Vancouver: Pacific Salmon Commission; 2010. [Google Scholar]
- 51.Araki H, Cooper B, Blouin MS. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science. 2007;318:100–103. doi: 10.1126/science.1145621. [DOI] [PubMed] [Google Scholar]
- 52.Williams JG. Mitigating the effects of high-head dams on the Columbia River, USA: Experience from the trenches. Hydrobiologia. 2008;609:241–251. [Google Scholar]
- 53.Marty GD, Saksida SM, Quinn TJ., II Relationship of farm salmon, sea lice, and wild salmon populations. Proc Nat Acad Sci USA. 2010 doi: 10.1073/pnas.1009573108. 10.1073/pnas.1009573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price MHH, et al. Sea louse infection of juvenile sockeye salmon in relation to marine salmon farms on Canada's west coast. PLoS ONE. 2011;6:e16851. doi: 10.1371/journal.pone.0016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crossin GT. Exposure to high temperature influences in the behaviour, physiology, and survival of sockeye salmon during spawning migration. Can J Zool. 2008;86:127–140. [Google Scholar]
- 56.Intergovernmental Panel on Climate Change . In: Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment. Pachauri RK, Reisinger A, editors. Geneva: Intergovernmental Panel on Climate Change; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.