Abstract
Estrogen has well-documented neuroprotective effects in a variety of clinical and experimental disorders of the CNS, including autoimmune inflammation, traumatic injury, stroke, and neurodegenerative diseases. The beneficial effects of estrogens in CNS disorders include mitigation of clinical symptoms, as well as attenuation of histopathological signs of neurodegeneration and inflammation. The cellular mechanisms that underlie these CNS effects of estrogens are uncertain, because a number of different cell types express estrogen receptors in the peripheral immune system and the CNS. Here, we investigated the potential roles of two endogenous CNS cell types in estrogen-mediated neuroprotection. We selectively deleted estrogen receptor-α (ERα) from either neurons or astrocytes using well-characterized Cre-loxP systems for conditional gene knockout in mice, and studied the effects of these conditional gene deletions on ERα ligand-mediated neuroprotective effects in a well-characterized model of adoptive experimental autoimmune encephalomyelitis (EAE). We found that the pronounced and significant neuroprotective effects of systemic treatment with ERα ligand on clinical function, CNS inflammation, and axonal loss during EAE were completely prevented by conditional deletion of ERα from astrocytes, whereas conditional deletion of ERα from neurons had no significant effect. These findings show that signaling through ERα in astrocytes, but not through ERα in neurons, is essential for the beneficial effects of ERα ligand in EAE. Our findings reveal a unique cellular mechanism for estrogen-mediated CNS neuroprotective effects by signaling through astrocytes, and have implications for understanding the pathophysiology of sex hormone effects in diverse CNS disorders.
Keywords: multiple sclerosis, astrogliosis, conditional knockout
The female sex hormone, estrogen, is neuroprotective in many clinical and experimental CNS disorders, including autoimmune conditions such as multiple sclerosis (MS), neurodegenerative conditions such as Alzheimer's and Parkinson diseases, and traumatic injury and stroke (1–4). Estrogen treatment has been shown to ameliorate clinical disease and decrease neuropathology in these disease models (1–4). Pharmacological studies have suggested roles for different estrogen receptors, but the cell types that mediate neuroprotective effects of estrogen are not known for any experimental or clinical condition. Identifying cells that bear specific estrogen receptor subtypes and are essential for specific estrogen-mediated effects is fundamental to elucidating and therapeutically exploiting the mechanisms that underlie estrogen-mediated neuroprotection. Toward this end, we used a genetic loss-of-function strategy. We selectively deleted estrogen receptor-α (ERα) from two different CNS cell types, neurons and astrocytes, and then determined the effects of these conditional gene deletions on the ability of ERα-ligand treatment to ameliorate disease severity of experimental autoimmune encephalomyelitis (EAE) in mice.
EAE is the most widely used mouse model of MS and, like MS, is a CNS autoimmune disease characterized by demyelination and axonal degeneration (5). Estrogen exerts a beneficial effect on the clinical course and neuropathology of EAE that is mediated at least in part by ERα, as shown by studies using ERα-selective ligands or global gene deletion (6, 7). ERα ligand treatment in EAE significantly ameliorates clinical symptoms and reduces both inflammation and axonal loss in the spinal cord (8). The cell types mediating the effects of ERα in EAE are not known because ERα is expressed by various immune cells, as well as CNS neurons and glia (9–12). Bone marrow chimera studies revealed that ERα expression in the peripheral immune system is not required for estradiol-mediated protection during EAE (9), suggesting that ERα expression on a CNS cell type is critical for ERα-mediated neuroprotection. Although neurons are an obvious potential CNS target of estradiol and ERα ligand-mediated protection, astrocytes, a type of CNS glia with many complex functions in health and disease (13, 14), represent an alternative candidate cell that has been implicated in regulation of CNS inflammation and neuroprotection in vivo in various models of CNS diseases, including EAE (15, 16).
In this study we tested the hypothesis that CNS neuroprotective effects of estrogen mediated through ERα in vivo are effectuated via expression of ERα in either neurons or astrocytes. We selectively deleted ERα in either neurons or astrocytes using well-characterized Cre-loxP systems for conditional gene knockout (CKO) in mice. We then studied the effects of adoptive EAE (4) in neuronal-ERα-CKO, astrocyte-ERα-CKO, and WT mice that were gonadectomized and treated with ERα ligand or vehicle. Gonadectomized mice were used to avoid the potential confound of various circulating sex hormones. Our findings show that signaling through ERα in astrocytes, but not in neurons, is essential for the neuroprotective effects of systemic treatment with ERα ligand on clinical function, CNS inflammation, and axonal loss during EAE.
Results
ERα Is Specifically Deleted from Either Neurons or Astrocytes in the Respective Neuronal-ERα-CKO or Astrocyte-ERα-CKO Models.
To target ERα-CKO to neurons, we used a rat neuronal specific enolase (rNSE)-Cre line previously shown at the single-cell level to reliably target Cre activity selectively to essentially all neuronal cells throughout the brain and spinal cord, with no targeting of astrocytes, microglia, or oligodendrocytes (17, 18). To target ERα-CKO to astrocytes, we used a mouse glial fibrillary acid protein (mGFAP)-Cre line previously shown at the single-cell level to reliably target Cre activity selectively to essentially all astrocytes throughout the brain and spinal cord (19). Previous evaluation of reporter protein expression of over 2,000 cells at the single-cell level, quantitatively demonstrated that this mGFAP-Cre line reliably targets Cre activity to over 98% of reactive astrocytes, with no targeting of neurons, microglia, or oligodendrocytes in traumatically injured spinal cord. In addition, no cortical or brainstem neurons that project into the spinal cord are targeted by Cre-activity, as shown by retrograde tract-tracing (19). These neuronal or astrocyte Cre mice were crossed with previously well-characterized ERα-loxP mice (20).
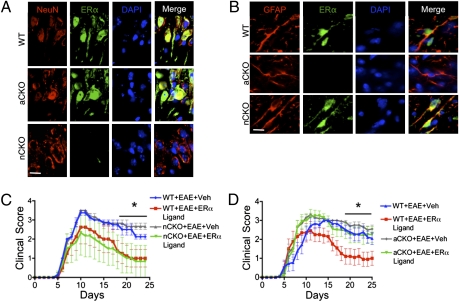
To determine the efficacy and selectivity of ERα deletion in either neurons or astrocytes, we assessed levels of immunohistochemically detectable ERα using a well-characterized antibody (21). WT mice with EAE exhibited readily detectable immunoreactive ERα in CNS neurons (Fig. 1A) and astrocytes (Fig. 1B). Neuronal-ERα-CKO mice with EAE did not express ERα at detectable levels in neurons (Fig. 1A) but did express ERα in astrocytes at levels that were indistinguishable from those in WT mice (Fig. 1B). Astrocyte-ERα-CKO mice with EAE did not express ERα at detectable levels in astrocytes (Fig. 1B) but did express ERα in neurons at levels that were indistinguishable from those in WT mice (Fig. 1A). In addition, ERα mRNA and protein, as detected by RT-PCR (Fig. S1 A and C) or immunoblot (Fig. S1 B and C), respectively, were essentially absent in astrocyte cultures from astrocyte-ERα-CKO mice, although present in astrocytes from WT mice. Together, these findings demonstrate both the efficacy and specificity of our models for conditional ERα deletion from either neurons or astrocytes. Untreated neuronal-ERα-CKO mice and astrocyte-ERα-CKO mice were behaviorally and histologically indistinguishable from WT mice.
Fig. 1.
(A and B) Verification of gene deletion specificity in astrocyte-ERα-CKO (aCKO) and neuronal-ERα-CKO (nCKO) mouse models. (C and D) EAE clinical disease severity scores showing that protective effects of ERα ligand require ERα in astrocytes, but not neurons. (A) Immunohistochemistry shows ERα colocalized with NeuN and DAPI in WT and aCKO mice with EAE, but not in nCKO mice with EAE. (B) ERα is colocalized with GFAP and DAPI in WT and nCKO mice with EAE, but not in aCKO mice with EAE. (Scale bars, 15 μm.) (C) WT and nCKO mice with EAE and given ERα ligand both had significantly better clinical scores compared with WT and nCKO mice with EAE and given vehicle. n = 6 per group. (D) Only WT mice, but not aCKO mice, with EAE and given ERα ligand had significantly better clinical scores compared with WT or aCKO mice with EAE and given vehicle. n = 12 per group. *P < 0.05 (repeated-measures ANOVA with post hoc Bonferroni pairwise analysis).
ERα Expression Is Necessary in Astrocytes, but Not Neurons, for Clinical Disease Protection.
We first determined the clinical effect of ERα ligand treatment starting 7 d before adoptive EAE induction (4) in separate experiments that compared neuronal-ERα-CKO mice or astrocyte-ERα-CKO with their respective controls (Fig. 1 C and D). Neuronal-ERα-CKO mice (Fig. 1C) and astrocyte-ERα-CKO mice (Fig. 1D) treated with vehicle exhibited EAE courses that were indistinguishable in clinical severity from those of vehicle-treated WT mice, whereas ERα ligand-treated WT mice exhibited significantly less severe clinical disease (Fig. 1 C and D), demonstrating a protective effect of ERα ligand when administered in the effector phase of adoptive EAE. Neuronal-ERα-CKO mice treated with ERα ligand also exhibited significant amelioration of clinical disease that was of a level comparable to that seen in WT EAE mice treated with ERα ligand (Fig. 1C). In striking contrast, astrocyte-ERα-CKO mice treated with ERα ligand exhibited severe clinical disease that was indistinguishable in severity from that of vehicle-treated WT EAE mice (Fig. 1D). Taken together, these observations demonstrate that ERα expression in astrocytes, but not in neurons, is required for the protective effects of ERα ligand on EAE clinical scores.
ERα Expression Is Necessary in Astrocytes, but Not Neurons, to Prevent Macrophage and T-Cell Inflammation in the CNS.
Following final assessments of clinical scores on day 25 after initiation of EAE, all mice were either fixed by cardiac perfusion for histopathological evaluation or processed for flow cytometry. CNS inflammation during EAE includes both T cells and cells of the monocyte lineage (8, 15). We first used immunohistochemical identification and a stereological procedure (StereoInvestigator) to quantify these cell types in the spinal cord dorsal column (Fig. 2).
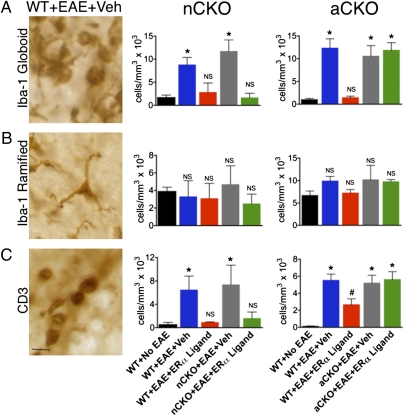
Fig. 2.
Immunohistochemical evidence that ERα is required in astrocytes, but not neurons, to reduce numbers of Iba-1 globoid macrophages and CD3 T cells in dorsal column white matter. (A) Iba-1 globoid macrophages were significantly reduced in WT and nCKO mice with EAE treated with ERα ligand, but not in aCKO mice with EAE treated with ERα ligand. (B) Iba-1 ramified microglia exhibited no significant difference in number across all experimental groups. (C) CD3 T cells were reduced in WT and nCKO mice with EAE treated with ERα ligand, but not in aCKO mice with EAE treated with ERα ligand. (Scale bar, 15 μm.) n = 6 per group. *P < 0.05; NS, not significant vs. WT+No EAE; #P < 0.05 vs. WT+EAE+Veh, aCKO+EAE+Veh, or aCKO+EAE+ERα ligand (ANOVA with post hoc Bonferroni pairwise analysis).
To identify cells of the monocyte lineage, we used immunohistochemistry for Iba-1 and divided positive cells into two phenotypes: those with a globoid shape associated with monocytes and phagocytic macrophages (Fig. 2A), and those with a ramified shape associated with CNS resident microglia (Fig. 2B), as previously described (15). Vehicle-treated WT, neuron-ERα-CKO, and astrocyte-ERα-CKO mice with EAE all exhibited significantly greater numbers of globoid Iba-1–stained cells compared with WT mice without EAE (Fig. 2A), in a manner consistent with macrophage infiltration in the CNS of EAE mice. ERα ligand treatment significantly reduced the numbers of globoid Iba-1 cells in WT and neuron-ERα-CKO mice with EAE to levels indistinguishable from WT mice without EAE, but had no effect in astrocyte-ERα-CKO mice with EAE, which had numbers indistinguishable from vehicle-treated EAE mice (Fig. 2A and Fig. S2 A and C). There were no differences in the number of ramified Iba-1 microglia among the four experimental groups (Fig. 2B).
To identify T cells, we used immunohistochemistry for CD3 (Fig. 2C and Fig. S2 B and D). Vehicle-treated WT, neuron-ERα-CKO, and astrocyte-ERα-CKO mice with EAE all exhibited significantly greater numbers of CD3-stained T cells in the spinal cord dorsal columns compared with WT mice without EAE (Fig. 2C). ERα ligand treatment significantly reduced the numbers of CD3 T cells in WT and neuron-ERα-CKO mice with EAE to levels indistinguishable from WT mice without EAE, but had no effect in astrocyte-ERα-CKO mice with EAE, which had numbers of CD3 T cells indistinguishable from vehicle-treated EAE mice (Fig. 2C and Fig. S2 B and D).
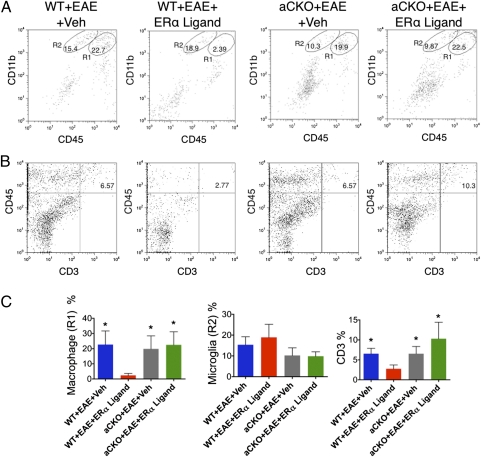
To compliment and extend the immunohistochemical data, we used flow cytometry, focusing on the astrocyte-ERα-CKO mice in which the effects of ERα ligand treatment examined thus far had been significantly abrogated. ERα ligand treatment significantly reduced the numbers of CD11bhi/CD45hi macrophages and of CD45hi/CD3hi T cells in the CNS of WT mice with EAE compared with vehicle-treated WT mice with EAE, but had no effect on the numbers of these cells in astrocyte-ERα-CKO mice with EAE (Fig. 3). As with immunohistochemistry, there were no differences in the number of microglia (CD11bhi/CD45int) among the four experimental groups (Fig. 3).
Fig. 3.
Flow cytometry evidence that ERα is required in astrocytes, but not neurons, to reduce macrophage and T-cell inflammation. (A and C) Macrophages (R1) (CD11bhi/CD45hi) and (B and C) T-cells (CD45hi/CD3hi) were significantly reduced in WT, but not aCKO mice, treated with ERα ligand via flow cytometry from the CNS. (A and C) There were no significant differences among all groups in numbers of microglia (R2) (CD11bhi/CD45int). n = 5 per group. *P < 0.05. NS, not significant vs. WT+EAE+ERα ligand (ANOVA with post hoc Bonferroni pairwise analysis).
Taken together, these immunohistochemical and flow cytometry data demonstrate that ERα expression in astrocytes, but not in neurons, is required for the ability of ERα ligand treatment to significantly ameliorate macrophage and T-cell infiltration into CNS parenchyma, the hallmarks of CNS inflammation in EAE.
ERα Expression Is Necessary in Astrocytes, but Not Neurons, to Attenuate Axonal Loss and Gliosis.
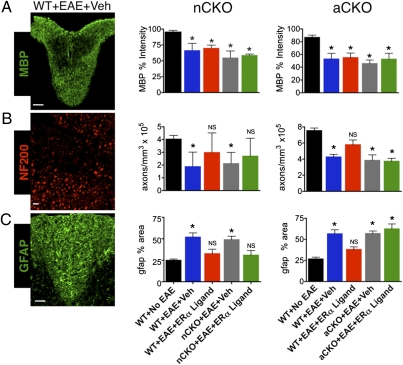
We next evaluated EAE neuropathology, which is characterized by patchy demyelination and axonal loss in spinal cord white matter (8, 15, 22). Axonal loss correlates with clinical disease severity (22). To assess demyelination, we quantified the area occupied by immunofluorescence staining of myelin basic protein (MBP) in the spinal cord dorsal column. Compared with WT mice without EAE, mice from all EAE experimental groups exhibited many patchy areas of severe loss of MBP staining (Fig. S3 A and D) and average levels of MBP staining were significantly lower (Fig. 4A), but there were no significant differences among any of the EAE treatment groups (Fig. 4A), suggesting that ERα ligand treatment did not act to protect against myelin loss. To determine axon numbers in the spinal cord dorsal column we used immunofluorescence staining for neurofilament 200 (NF200) and semiautomated counting software (Image J) (15). Vehicle-treated WT, neuron-ERα-CKO, and astrocyte-ERα-CKO mice with EAE all exhibited patchy areas of axon loss (Fig. S3 B and E) and average numbers of axons were significantly lower compared with WT mice without EAE (Fig. 4B). EAE WT and neuron-ERα-CKO mice treated with ERα ligand exhibited axon numbers not significantly different from WT mice without EAE (Fig. 4B). In contrast, astrocyte-ERα-CKO mice with EAE treated with ERα ligand had axon numbers not significantly different from vehicle-treated EAE mice (Fig. 4B), demonstrating that ERα expression in astrocytes, but not in neurons, is required for the protective effects of ERα ligand treatment on axon number in EAE.
Fig. 4.
Immunohistochemical evidence that ERα is required in astrocytes, but not neurons, to protect against axonal loss and reactive astrogliosis. (A) MBP stained intensity and area were significantly reduced in all EAE groups, but were not significantly altered by ERα ligand treatment in any group. (Scale bar, 120 μm.) (B) Numbers of NF200+ axons were significantly reduced in WT mice with EAE; and treatment with ERα ligand ameliorated axonal loss in WT and nCKO mice, but not aCKO mice, with EAE. (Scale bar, 20 μm.) (C) GFAP stained area was significantly increased in WT mice with EAE; and treatment with ERα ligand ameliorated this increase in WT and nCKO mice, but not aCKO mice, with EAE. (Scale bar, 40 μm.) n = 6 per group. *P < 0.05; NS, not significant vs. WT+No EAE (ANOVA with post hoc Bonferroni pairwise analysis).
Astrogliosis is prominent in areas of tissue pathology in EAE (15). We quantified the area occupied by immunofluorescence staining for GFAP, the canonical marker for reactive astrocytes (16). ERα ligand treatment significantly reduced the areas of GFAP staining in dorsal column white matter of both WT and neuron-ERα-CKO mice with EAE to levels indistinguishable from WT mice without EAE, but had no effect on GFAP staining area in astrocyte-ERα-CKO mice with EAE (Fig. 4C and Fig. S3 C and F), demonstrating that ERα expression in astrocytes, but not in neurons, is required for the reduction in reactive astrogliosis mediated by ERα ligand treatment.
Discussion
Taken together, these data demonstrate that neuroprotective effects of ERα ligand treatment in EAE, including improved clinical function, reduced white matter inflammatory cell infiltrate, and axonal sparing, are dependent on signaling through ERα in astrocytes, but not neurons. The findings do not exclude additional effects of estrogens or related steroids that might be mediated by ERβ, or nonclassical effects, on astrocytes or other CNS cell types, including neurons (8, 20, 21). Sexual dimorphism is increasingly recognized in CNS molecular mechanisms (23), and circulating levels of endogenous or administered estrogens influence disease severity in a wide variety of CNS disorders, including autoimmune inflammation (4), traumatic injury (24, 25), stroke (3), and neurodegenerative disease (1, 3, 26), all of which have postulated inflammatory involvement. Our findings show that astrocytes are the principal cells required for mediating the neuroprotective effects of ERα signaling in an autoimmune CNS inflammatory condition.
Astrocytes are complex cells that are increasingly implicated as playing essential roles in normal CNS function and the response to CNS disease (13, 14). Astrocytes in vitro can produce a wide variety of molecules with pro- or anti-inflammatory effects and astrocytes in vivo can regulate CNS inflammation through both pro- and anti-inflammatory mechanisms (16). Although it was known that astrocytes express estrogen receptors (21) and that estrogen treatment decreases expression of various proinflammatory molecules made by astrocytes in vitro (27), until now a direct effect of estrogen on ERα-bearing astrocytes in vivo had not been addressed. Our data identify astrocytes as unique and critical effector cells of estrogen-mediated neuroprotective effects during CNS inflammation, and have implications for understanding the pathophysiology of sex hormone effects in diverse CNS disorders. Rather than by acting directly through neurons, our findings show that estrogen signaling via ERα can significantly attenuate an inflammatory neurodegenerative process by acting through astrocytes, pointing toward novel cell-nonautonomous mechanisms of neuroprotection in the CNS.
Materials and Methods
Neuron-ERα-CKO were generated by crossing rNSE-Cre mice (17, 18) with mice carrying an ERα gene in which exon 3 was flanked by loxP sites (ERαflox/flox) (20), as detailed in SI Materials and Methods. This process created WT (rNSE Cre−/ ERαflox/flox) mice and nCKO (rNSE Cre+/ ERαflox/flox) mice. Astrocyte-ERα-CKO mice were generated by crossing mice of mGFAP-Cre line 73.12 (19), with mice carrying an ERα gene in which exon 3 was flanked by loxP sites (ERαflox/flox) (20). This process created WT (mGFAP Cre−/ ERαflox/flox) mice and aCKO (mGFAP Cre+/ ERαflox/flox) mice. Purified primary astrocyte cultures were prepared from individual postnatal day 1 to 3 male and female mouse pups, as described in SI Materials and Methods. Ovary tissue and primary hypothalamic astrocyte cultures were prepared from two female and two male 40-d-old WT and CKO mice, as described in SI Materials and Methods. For adoptive EAE, recipient female C57BL/6 WT and CKO mice had been gonadectomized at 4 wk of age, and had EAE induced by adoptive transfer at 8 wk of age. Recipient mice were either treated every other day with the ERα ligand, 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (Tocris) at the dose of 10 mg/kg per day or vehicle diluted with 10% molecular-grade ethanol (EM Sciences) and 90% Miglylol 812N liquid oil (Sasol North America), beginning 7 d before adoptive transfer. EAE mice were killed and after 25 d of disease, as described in SI Materials and Methods. Immunohistochemisty was performed on the dorsal column of thoracic spinal cords using CD3, Iba-1, GFAP, NF200, and MBP, as detailed in SI Materials and Methods. Flow cytometry was performed on day 25, as well using CD45, CD3, and CD11b cell surface markers, as detailed in SI Materials and Methods. Differences in EAE clinical scores were determined by repeated measures one-way ANOVA. All data shown are mean ± SEM. Immunohistochemical data were analyzed by one-way ANOVA. For these analyses, one-way ANOVA, Bonferroni post hoc analysis was performed on F-stat values and significance was determined at the 95% confidence interval (Prism).
Supplementary Material
Acknowledgments
We thank members of the R.R.V. and M.V.S. laboratories, as well as Dr. Tiwari-Woodruff, for discussion and assistance, and Dr. Pierre Chambon for his generosity in sharing the estrogen receptor-α floxed mice. This work was supported by Grants RG4033, RG4364, and CA1028 from the National Multiple Sclerosis Society (to R.R.V.), the Adelson Medical Research Foundation (R.R.V. and M.V.S.), National Institutes of Health and National Institute of Neurological Disorders and Stroke Grants NS 062117 (to R.R.V.) and NS057624 (to M.V.S.), and the Skirball Foundation, Hilton Foundation, and Sherak Family Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103833108/-/DCSupplemental.
References
- 1.Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- 3.Wise PM. Estrogens and neuroprotection. Trends Endocrinol Metab. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: Implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 5.Trapp BD, Nave K-A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 6.Morales LBJ, et al. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polanczyk M, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari-Woodruff S, Morales LBJ, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci USA. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garidou L, et al. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2004;173:2435–2442. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- 10.Pérez SE, Chen E-Y, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 11.García-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 12.Mitra SW, et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 13.Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voskuhl RR, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon C-H, et al. Neuron-specific enolase-cre mouse line with cre activity in specific neuronal populations. Genesis. 2006;44:130–135. doi: 10.1002/gene.20197. [DOI] [PubMed] [Google Scholar]
- 18.Forss-Petter S, et al. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann JE, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 21.Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc Natl Acad Sci USA. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wujek JR, et al. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Jazin E, Cahill L. Sex differences in molecular neuroscience: From fruit flies to humans. Nat Rev Neurosci. 2010;11:9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- 24.Chaovipoch P, et al. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- 25.Dubal DB, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J Steroid Biochem Mol Biol. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2010;58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.