Abstract
Retinoic acid receptor (RAR) signaling is required for morphogenesis of the ventral optic cup and closure of the choroid fissure, but the mechanisms by which this pathway regulates ventral eye development remain controversial and poorly understood. Although previous studies have implicated neural crest–derived periocular mesenchyme (POM) as the critical target of RA action in the eye, we show here that RAR signaling regulates choroid fissure closure in zebrafish by acting on both the ventral optic cup and the POM. We describe RAR-dependent regulation of eight genes in the neuroepithelial cells of the ventral retina and optic stalk and of six genes in the POM and show that these ventral retina/optic stalk and POM genes function independently of each other. Consequently, RAR signaling regulates ventral eye development through two independent, nonredundant mechanisms in different ocular tissues. Furthermore, the identification of two cohorts of genes implicated in ventral eye morphogenesis may help to elucidate the genetic basis of ocular coloboma in humans.
Coloboma is a common visual system disorder in humans characterized by the failure of the choroid fissure in the ventral optic cup to close (1). Gestational vitamin A deficiency causes disruption of the anterior eye segment, microphthalmia, and coloboma (2–4). These defects are phenocopied in mice lacking various combinations of the Aldh1A1–3 retinoic acid (RA)-producing enzymes, or retinoic acid receptors (RARs), indicating that vitamin A is required for RA synthesis and RAR activation during choroid fissure closure (2, 3, 5).
Aldh1A1/3 are expressed in restricted domains along the retinal dorsoventral (DV) axis (2, 5). However, DV polarity is normally established in Aldh1A or RAR mutant eyes (2, 3, 5). Furthermore, retinal development proceeds normally after abrogation of RARα, the only RAR expressed in the mouse retina at embryonic day 10.5 (E10.5) (3), suggesting that RAR signaling within the retina does not have a major role in eye morphogenesis (3).
A major RA target is the neural crest–derived periocular mesenchyme (POM) surrounding the developing eye (3, 5), where RAR signaling controls expression of the transcriptional regulators FoxC1a, Eya2, and Pitx2 (2, 5). RAR-dependent regulation of POM development is instrumental in anterior segment formation (2, 3, 5) and, in addition, neural crest–specific knockout of all RAR genes in mice causes coloboma and eversion of the ventral optic cup (3), suggesting that RA acts via the POM to guide the morphogenetic movements of the optic cup. It remains unclear how POM influences ventral eye morphogenesis and what role, if any, RA exerts directly on optic cup cells.
In this study, we elucidate the actions of RA on choroid fissure closure through analysis of 14 genes that we show depend on RAR signaling for their expression in and around the developing zebrafish eye. Eight of these genes are expressed in the ventral retina (VR)/optic stalk (OS), demonstrating a robust transcriptional response to RAR signaling within the optic cup, and six genes are expressed in POM. Both of these genetic programs are required for choroid fissure closure, but they function independently of each other. Thus, RAR signaling controls ventral eye morphogenesis through two distinct, nonredundant mechanisms operating, respectively, in the ventral optic cup and in the surrounding POM.
Results
Inhibition of RAR Signaling During Eye Morphogenesis Causes Coloboma Without Affecting Eye Polarity.
In zebrafish, aldh1A3 expression is initiated in the evaginating optic vesicles at the 3- to 6-somite stage (3–6s), at 11–12 h postfertilization (hpf) (Fig. S1), followed by transcription of aldh1A2 at around 20s (19 hpf) (6). RA synthesis is thus likely to be initiated during optic vesicle evagination and maintained during optic cup formation. Given this timing, to block RAR signaling in and around the forming eye, we treated zebrafish embryos with AGN194310 (AGN), a small-molecule inhibitor of all RARs (7), from 3 to 6 s up to 60 hpf.
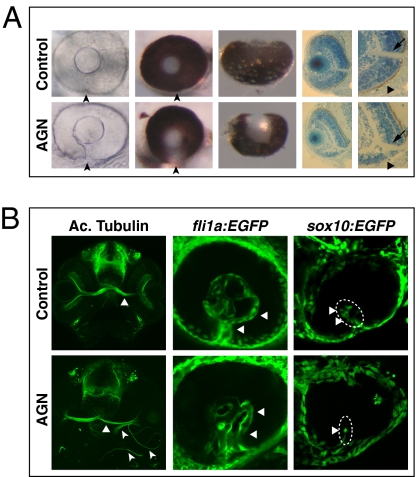
At least 75% of AGN-treated embryos displayed coloboma at 60 hpf (n = 87). The most affected eyes showed a marked gap in pigmentation in the ventral optic cup (Fig. 1A), and histological sections highlighted both the lack of retinal pigmented epithelium and a shortening of the retina ventral to the optic nerve head (Fig. 1A). Defective closure of the choroid fissure was already detectable by differential interference contrast microscopy at 48 hpf (Fig. 1A). At 60 hpf, embryos treated with AGN until 25–26 hpf or treated from 18s (18 hpf) showed coloboma (at least 60%, n > 40 in each case). Temporally restricted AGN treatments between 17–18s and 25–26 hpf also caused coloboma at 64 hpf (at least 37%, n = 106). Thus, the developmental period during which AGN treatments interfere with choroid fissure closure includes the time window between 17–18s and 25–26 hpf, although stages flanking this window contribute to the penetrance of the phenotype.
Fig. 1.
Inhibition of RAR signaling causes coloboma in zebrafish. (A) Coloboma (arrowheads) in embryos treated with 2.5–5 μM AGN from 3 to 6 s. (Left) Lateral view of 48-hpf phenylthiourea-treated embryos. (Center) Lateral (Center Left) and ventral (Center Right) views of 60-hpf eyes. (Right) Sections of 60-hpf eyes showing the lack of retinal pigmented epithelium (triangles) and a shortening of the retina ventral to the optic nerve head (arrows) in the AGN-treated embryo. (B, Left) Immunostaining with an anti-acetylated (Ac.) tubulin antibody at 48–50 hpf, showing abnormal retinal axon trajectories in the AGN-treated embryo (arrowheads), besides the normal contralateral projection (triangles). (Center) fli1a:EGFP transgenic embryos at 48 hpf. The AGN-treated embryo exhibits disrupted vascularization (triangles). (Right) sox10:EGFP transgenic embryos at 32–36 hpf. The AGN-treated embryo shows less POM cells within the choroid fissure (triangles).
The choroid fissure serves as a route for retinal axons and blood vessels out of and into the eye, respectively. In control eyes, retinal axons form a compact bundle at the optic nerve head and project contralaterally at the midline (Fig. 1B). After AGN treatments, we observed axons projecting ipsilaterally and aberrantly around the eye or leaving the retina from ectopic locations (Fig. 1B). Vascularization of the retina through the choroid fissure, as detected by using the endothelial cell reporter fli1a:EGFP (8), was also disorganized, with both the patterning and diameter of the hyaloid vessels affected (Fig. 1B). The eye vascular system also contains pericytes originating from neural crest–derived POM cells migrating inside the choroid fissure (9). Using a sox10:EGFP reporter labeling neural crest–derived POM (10), we found that AGN treatments caused a significant decrease in the number of EGFP-positive cells within the choroid fissure at 26–28 hpf (DMSO: n = 6.3 ± 1.7, 12 eyes; AGN: n = 2.8 ± 1.9, 12 eyes; P < 0.001) (Fig. 1B).
Since coloboma phenotypes can arise because of impaired DV patterning of the eye (1), we examined the effects of AGN treatments on the expression of markers of eye polarity but found no changes at 24–25 hpf and 31 hpf (Fig. S2 A–C). Ventral patterning of the eye depends on Shh and Fgf8 signals from the ventral forebrain and/or OS (11, 12), and AGN-treated embryos showed no appreciable effects on the expression of Shh and Fgf8 pathway genes or ventral forebrain markers (Fig. S2D).
RAR Signaling Regulates Gene Expression in Optic Cup Cells Flanking the Choroid Fissure and in the POM.
To identify genes misregulated in the developing eye region upon RAR inhibition, we selected ∼40 genes that were candidates for being regulated by RAR signaling based on restricted expression near sites of ocular RA production and/or previous studies implicating them in ventral optic cup morphogenesis. Expression analyses of these genes in DMSO- and AGN-treated embryos yielded 14 genes showing robust transcriptional changes in or around the developing eye upon AGN treatments (see below and Table S1).
These 14 genes could be grouped into 8 primarily expressed in VR/OS cells flanking the choroid fissure (vax1, net1a, cyb5a, itgA5, dact1, nlz2, dhrs3a, and rdh10a) and 6 primarily expressed in POM within the fissure and/or around the optic cup (foxC1a, nlz1, twist1a, eya2, pitx2, and lmx1b1) (Fig. S3). The VR/OS gene itgA5 was also expressed in extraocular tissue ventral and medial to the eye, corresponding to neural crest and/or endodermal derivatives (Fig. S3) (13). The POM gene pitx2 is expressed as two different isoforms (pitx2a and pitx2c) generated by the use of two promoters and alternative splicing (14), and we found that pitx2a is the predominant isoform in the POM (Fig. S3B).
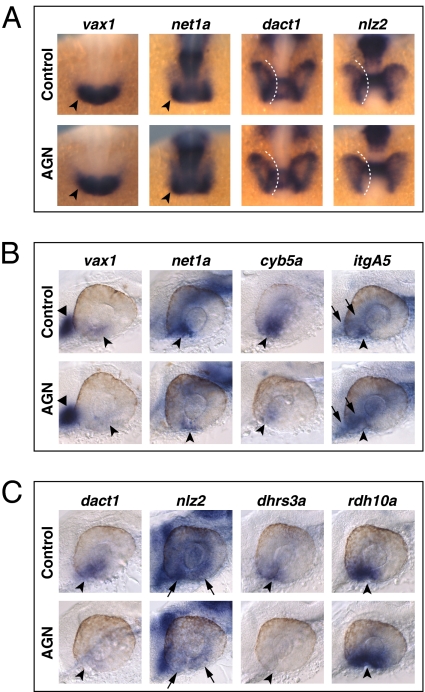
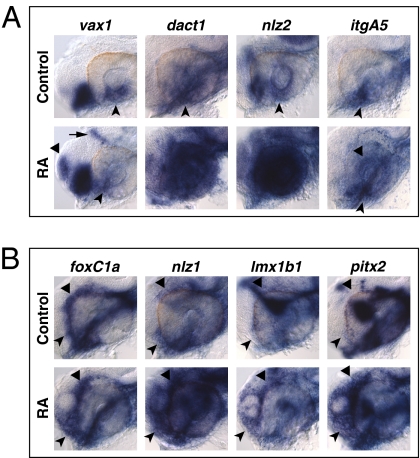
We determined the effects of RAR signaling on the VR/OS and POM genes by treating embryos with AGN from 3s and analyzing their expression at different time points (18s, 24–25 hpf and 31 hpf). AGN treatments from 3s did not cause major changes in the early expression patterns of the VR/OS genes vax1, net1a, dact1, and nlz2 in the optic vesicle at 18s (Fig. 2A). cyb5a and dhrs3a were barely detectable in the eye at this stage. By contrast, 24- to 25-hpf embryos showed down-regulation of vax1, net1a, cyb5a, itgA5, dact1, and nlz2 in the VR/OS, whereas other expression sites of these genes were unaffected (Fig. 2 B and C, Fig. S4 A and B, and Table S1). Dhrs3a and Rdh10a are enzymes involved in RA metabolism and, in other contexts, feedback regulation by RA activates dhrs3a and represses rdh10a expression (15). As expected, RAR inhibition eliminated dhrs3a and increased rdh10a expression in the VR/OS (Fig. 2C, Fig. S4B, and Table S1). Similar changes for most of these genes were also observed at 31 hpf (Figs. S5 A and B and S6 A and B). Abrogation of RAR signaling did not generally compromise ventral eye development, as other VR/OS-expressed genes were not obviously affected (Fig. S2).
Fig. 2.
RAR signaling regulates gene expression in the VR/OS. (A–C) Embryos treated with DMSO or 10 μM AGN from 3s and hybridized at 18s (A) or 24–25 hpf (B and C) with the indicated probes. (A) Dorsal/anterior views of heads. AGN treatment has no major effects on the expression of vax1 and net1a at the optic vesicle/forebrain junction (arrowheads) and of dact1 and nlz2 in the optic vesicle. Dashed lines highlight the proximal edge of the optic vesicle. (B and C) Lateral views of eyes. AGN treatment causes down-regulation of vax1, net1a, cyb5a, itgA5, dact1, nlz2, and dhrs3a and up-regulation of rdh10a in the VR/OS (arrowheads). Triangles point to vax1 expression in the ventral forebrain (B). Arrows point to itgA5 expression in extraocular tissue at the back of the eye (B) and nlz2 expression on each side of the choroid fissure (C). Panels are representative images of the following numbers (n) of independent experiments: vax1, net1a, n = 4; itgA5, n = 3; and cyb5a, dact1, nlz2, dhrs3a, rdh10a, n = 2. Approximately 15–30 embryos were assayed for each condition and probe in each experiment.
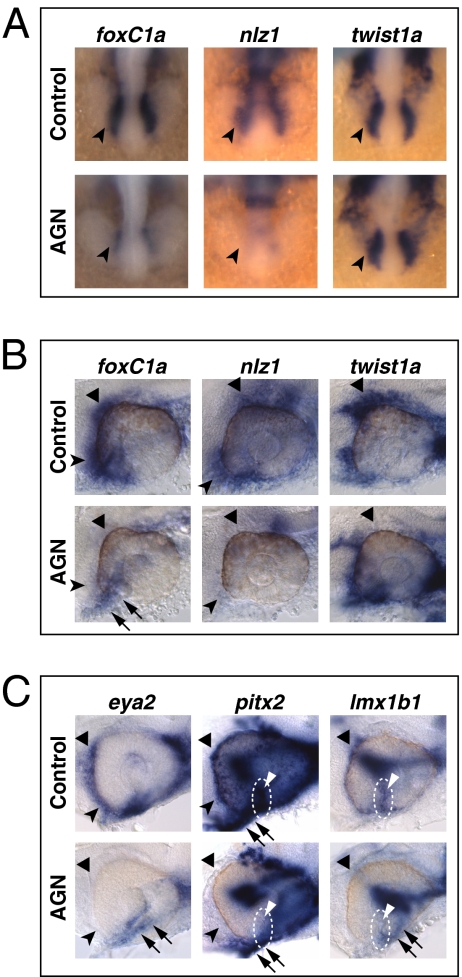
POM genes also showed severely reduced expression upon AGN treatments. foxC1a and nlz1, but not twist1a, were already down-regulated at 18s (Fig. 3A). At 24–25 hpf and 31 hpf, both foxC1a and nlz1 were down-regulated in POM surrounding the dorsal, anterior, and ventral eye, whereas twist1a expression was decreased in the dorsal POM (Fig. 3C, Figs. S4C, S5B, and S6B, and Table S1). eya2, pitx2, and lmx1b1 expression in POM within the choroid fissure and/or in anterior POM became clearly detectable only by 31 hpf and was reduced in AGN-treated embryos (Fig. 3C, Fig. S6C, and Table S1).
Fig. 3.
RAR signaling regulates gene expression in POM. (A–C) Embryos treated with DMSO or 10 μM AGN from 3s and hybridized at 18s (A), 24–25 hpf (B), or 31 hpf (C) with the indicated probes. (A) Dorsal/anterior views. In the AGN-treated embryos, foxC1a and nlz1, but not twist1a, are down-regulated in migrating POM (arrowheads). (B and C) Lateral views of eyes. AGN treatment decreases foxC1a, nlz1, eya2, pitx2, and lmx1b1 expression in both anterior-ventral (arrowheads) and anterior-dorsal (black triangles) POM. AGN treatment decreases twist1a expression in dorsal POM (black triangles). Expression of pitx2 and lmx1b1 within the choroid fissure is also diminished (white dashed circles and triangles). Residual foxC1a, eya2, and lmx1b1 staining is detectable in a ventro-medial location behind the eye (arrows). pitx2 is expressed in extraocular mesodermal tissue in both the control and the AGN-treated embryo (arrows). Panels are representative images of the following numbers of independent experiments: pitx2, n = 4; foxC1a, n = 3; and nlz1, twist1a, eya2, lmx1b1, n = 2. Approximately 10–30 embryos were assayed for each condition and probe in each experiment.
The results described above suggest that RAR signaling acts early to initiate POM gene expression and later to maintain expression of the VR/OS genes. AGN treatments started at 23 hpf lasting until 31 hpf caused down-regulation of the VR/OS expression of vax1 and dhrs3a, but not net1a or cyb5a (Fig. S5 C and D). AGN treatments from 18s did, however, lead to reduced net1a and cyb5a expression (Fig. S5D). AGN treatments from 23 hpf also caused down-regulation of foxC1a and nlz1 (Fig. S5C). Thus, RAR signaling is necessary beyond 18s for the maintenance of both POM and VR/OS gene expression.
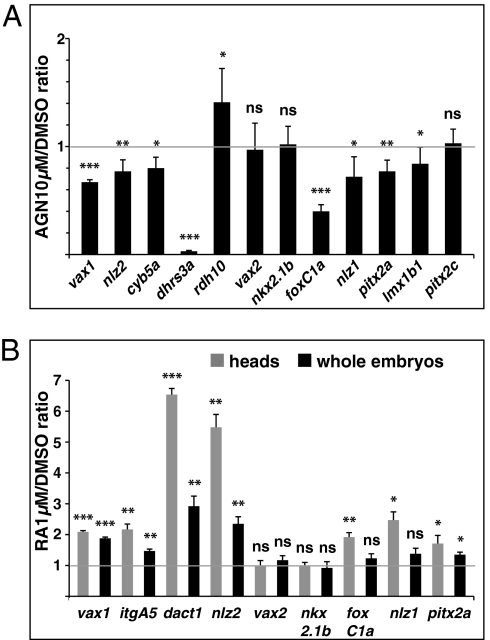
The relative transcriptional levels of some of the VR/OS and POM genes were quantified by real-time PCR on RNA purified from 31-hpf dissected heads of DMSO- or AGN-treated embryos. Supporting in situ hybridization (ISH) data, vax1, nlz2, and cyb5a were significantly down-regulated and dhrs3a expression was nearly abolished in AGN-treated heads, whereas rdh10a was up-regulated (Fig. 4A). Expression levels of the POM genes foxC1a, nlz1, and lmx1b1 were also significantly lower in AGN-treated heads (Fig. 4A). AGN treatments significantly down-regulated pitx2a, but not pitx2c, consistent with the differential expression of these isoforms in the POM (Fig. 4A). The relative levels of vax2 and nkx2.1b were not significantly changed, confirming that AGN treatments did not cause a global down-regulation of ventral eye and forebrain gene expression (Fig. 4A).
Fig. 4.
Quantification of gene expression changes after manipulations of RAR signaling. (A and B) Real-time PCR quantification of gene expression in 31- to 33-hpf dissected heads (A) or 25- to 28-hpf dissected heads and whole embryos (B) after treatments with the indicated reagents from 3s, shown as the mean ratio between AGN or RA and DMSO conditions in six (A) or three (B) biological replicates. Error bars show SDs. *P < 0.05; **P < 0.01; ***P < 0.001; ns, nonsignificant (P ≥ 0.05) according to two-tailed Student's t test.
Increased RA Signaling Leads to Up-Regulation of Most VR/OS and POM Genes.
To assess whether exogenous RA could promote expression of the VR/OS and POM genes, embryos were treated from 3s with DMSO or 0.125–0.5 μM RA and analyzed by ISH at 25–29 hpf. Confirming previous observations (16), exogenous RA expanded pax2 expression in the eye (Fig. S7A), but other markers of DV eye polarity were not evidently affected (Fig. S7B). RA treatments led to expanded expression of the VR/OS genes nlz2, dhsr3a, dact1, and itgA5, but not vax1, net1a, and cyb5a, in the dorsal retina (Figs. S7A and S8A). Expression of the POM genes foxC1a, nlz1, pitx2, and lmx1b1 also showed dose-dependent increase after RA treatments (Fig. S8B).
RA treatments from early somitogenesis disrupted eye morphogenesis (Fig. S8). To address the role of RA in conditions where optic cup formation was preserved, we started RA treatments at 18s. RA (1 μM) caused ectopic activation of dact1, nlz2, and itgA5 in the dorsal retina (Fig. 5A) and expanded the domains of foxC1a, nlz1, lmx1b1, and pitx2 in the anterior POM (Fig. 5B). These higher RA doses slightly reduced vax1 expression in the VR/OS, while activating it ectopically in the dorsal telencephalon and diencephalon (Fig. 5A).
Fig. 5.
Exogenous RA activates genes expressed in the VR/OS and in POM. (A and B) Lateral views of eyes from 31-hpf hybridized embryos treated with DMSO or 1 μM RA from 18s. (A) Arrowheads point at the expression of vax1, dact1, nlz2, and itgA5 in the VR/OS. RA treatment reduces vax1 expression in the VR/OS while inducing it ectopically in the dorsal telencephalon (triangle) and diencephalon (arrow). The RA-treated embryos show dact1 and nlz2 expression throughout the retina and ectopic itgA5 expression in the dorsal retina (triangle). (B) RA treatment increases foxC1a, nlz1, lmx1b1, and pitx2 expression in anterior-ventral (arrowheads) and/or anterior-dorsal (triangles) POM. Approximately 20 embryos for each condition and probe were used for these analyses.
Real-time PCR analysis confirmed ISH results. vax1, dact1, and nlz2 showed dose-dependent up-regulation in embryos treated with 0.125–1 μM RA (Fig. 4B and Fig. S9A). Similar or stronger effects were detected in heads dissected from treated embryos than in whole embryos, confirming that RA regulates these genes in the eye and forebrain region (Fig. 4B). Dissected heads also showed significant up-regulation of the POM genes foxC1a, nlz1, and pitx2a (Fig. 4B). No significant changes in the expression levels of vax2 and nkx2.1b were observed (Fig. 4B and Fig. S9A).
To determine the time course of the response of the VR/OS and POM genes to RA, we treated embryos with 0.5 μM RA from 18s and harvested them between 0.5 and 6 h later. The shortest analyzed incubation time in RA (30–50 min) was sufficient to detect up-regulation of both VR/OS and POM genes by ISH and/or real-time PCR (Fig. S9 B and C), indicating rapid transcriptional responses to RA.
Abrogation of FoxC1a, Nlz1, or Pitx2 Causes Coloboma Without Affecting Expression of VR/OS Genes.
Roles in choroid fissure closure are well documented for genes expressed in VR/OS cells (11, 17, 18), but the requirements for POM genes are less well studied. Consequently, we abrogated the activity of selected RAR-regulated POM genes with morpholino antisense oligonucleotides (MOs) and assessed ventral eye morphogenesis and the expression of VR/OS and other POM genes. We focused on foxC1a and nlz1, the earliest RAR-regulated genes in POM, and pitx2 because it is a direct RAR target in mouse POM (19).
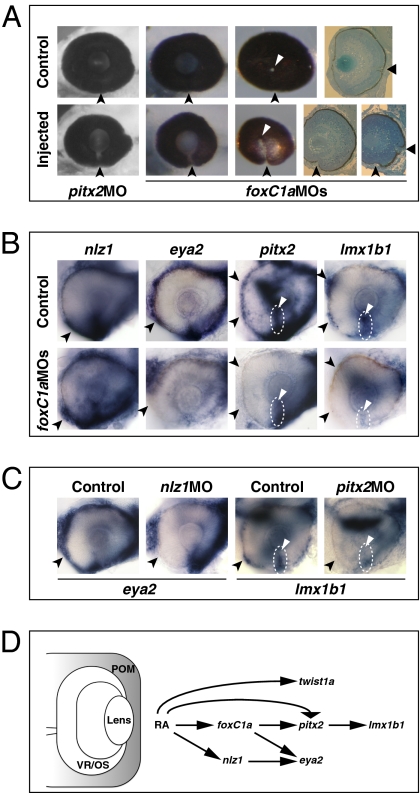
At 72 hpf, embryos injected with either foxC1a MOs or pitx2 MO showed a narrow coloboma (foxC1a MO: 48%, n = 63; pitx2 MO: 40%, n = 90) in which the choroid fissure had failed to fuse ventral to the optic disk (Fig. 6A). Abrogation of Nlz1 is also reported to cause coloboma (18). Despite defective choroid fissure morphogenesis, there were no evident effects on the expression of the VR/OS genes vax1, net1a, cyb5a, and itgA5 in foxC1a, nlz1, or pitx2 morphants (Fig. S10 A–C and Table S1), indicating that abrogation of POM gene activity probably leads to coloboma independently of the activity of VR/OS genes. FoxC1a was also not required for the expression of nlz1 and twist1a in POM (Fig. 6B and Fig. S11A). By contrast, expression of eya2, pitx2, and lmx1b1 was reduced in the choroid fissure POM and/or in the anterior POM of foxC1a morphants (Fig. 6B and Table S1). In nlz1 morphants, eya2 expression in the anterior POM was decreased (Fig. 6C and Table S1), whereas foxC1a, twist1a, pitx2, and lmx1b1 expression patterns were not obviously affected (Fig. S11 B and C). In pitx2 morphants, lmx1b1 expression was reduced in the anterior POM and within the choroid fissure (Fig. 6D and Table S1), whereas foxC1a, nlz1, and eya2 were largely unaffected (Fig. S11D).
Fig. 6.
Abrogation of FoxC1a, Nlz1, and Pitx2 function causes coloboma and affects gene expression in POM. (A) Eyes of embryos injected with pitx2 MO or foxC1a MOs showing coloboma at 72 hpf, as detected in lateral view (Left and Center Left), posterior view (Center Right), and histological sections (Right). The injected eyes exhibit an open choroid fissure (arrowheads) ventral to the optic disk (triangles). (B and C) Lateral views of 32-hpf eyes. Panels are representative images of two to three independent experiments. Approximately 10–20 embryos were used for each condition and probe in each experiment. (B) Decreased expression of eya2, pitx2, and lmx1b1, but not nlz1, in choroid fissure POM (dashed circles and triangles) and/or anterior POM (arrowheads) in the foxC1a MO-injected embryos. (C) Decreased expression of eya2 in anterior POM (arrowheads) of an nlz1 MO-injected embryo and of lmx1b1 in anterior POM (arrowheads) and within the choroid fissure (dashed circles and triangles) of a pitx2 MO-injected embryo. (D) Proposed model of the molecular mechanisms of RAR-dependent RA signaling during development of the zebrafish POM (highlighted in gray). See text for details.
Some VR/OS Genes Are Regulated in Parallel by RAR Signaling and Pax2.
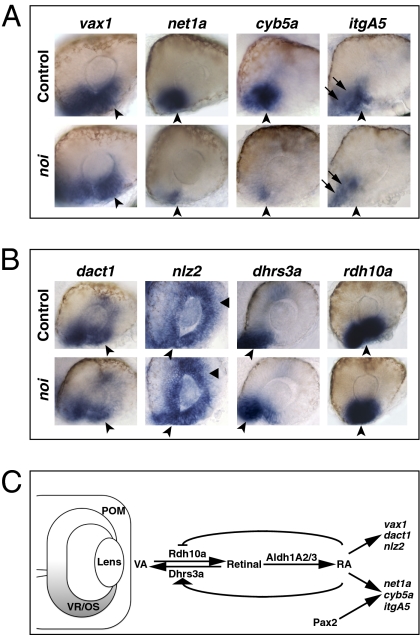
Pax2 is expressed in the VR/OS, and abrogation of Pax2 function leads to coloboma and reduced expression of net1a (17), prompting us to further explore the events downstream of RAR signaling and Pax2 during choroid fissure morphogenesis. RAR signaling can promote, but is not required for, pax2 expression in the ventral eye (Figs. S2A and S7A). Vice versa, pax2a (noi) mutants showed normal VR/OS expression of dhrs3a and rdh10a, indicating that ocular RA signaling is intact in this mutant (Fig. 7B). dact1 and nlz2 were also unaffected in noi mutant eyes (Fig. 7B), whereas expression of net1a, cyb5a, and itgA5 in the VR/OS was reduced (Fig. 7A). foxC1a, nlz1, pitx2, and lmx1b1 were not affected in noi mutants (Fig. S11E), indicating that any alterations to the VR/OS in noi mutants have no obvious effects on POM genes.
Fig. 7.
Pax2 regulates some VR/OS genes in parallel to RAR signaling. (A and B) Lateral views of 25- to 26-hpf eyes. Approximately 15–20 control or mutant embryos for each probe were used for these analyses. (A) The noi embryos show down-regulation of net1a, cyb5a, and itgA5, but not vax1, in the VR/OS (arrowheads). Arrows point to itgA5 expression in extraocular tissue at the back of the eye. (B) Expression of dact1, nlz2, dhrs3a, and rdh10a in ventral eye cells is not affected in the noi mutants (arrowheads). Triangles point to nlz2 dorsal retinal expression. (C) Proposed model of the molecular mechanisms of RAR signaling in the developing zebrafish VR/OS (highlighted in gray). See text for details.
Together with the data described above, these results show that RAR signaling and Pax2 can regulate the expression of some VR/OS genes in parallel and that, in both cases, this regulation is likely to be independent of the POM (Fig. 7C).
Discussion
In this study, we show that RAR signaling is required for choroid fissure closure through regulation of distinct genetic programs in the VR/OS and the POM. In both fish and mammals, RAR signaling is required for ventral eye morphogenesis and gene regulation in POM, but not for DV patterning of the retina. We show, however, that RAR signaling controls genes expressed in the ventral optic cup, and implicated in its morphogenesis, independently of POM. This finding indicates that not all RA actions in ventral eye development are mediated through POM cells.
We describe RAR-dependent expression of six transcription factor-encoding genes in POM. foxC1a, eya2, and pitx2 are known RA targets from mouse studies (2, 5), to which we add nlz1, twist1a, and lmx1b1. Neural crest–specific abrogation of either RARs or Pitx2 disrupts formation of the VR and/or OS in mice (3, 20), as does abrogation of Lmx1b in fish (21). In these cases, however, the morphogenetic movements driving the eyes away from the brain are compromised, and the developing VR/OS may be abnormally exposed to midline signals that influence eye development (11, 12).
Our functional analyses of FoxC1a and Pitx2 in zebrafish reveal more restricted roles for POM in the fusion of the lips of the ventral optic cup on each side of the choroid fissure. Thus, foxC1a and pitx2 morphants (in this study) or nlz1 morphants (18) show subtle colobomata, without overt effects on earlier eye morphogenesis or on the expression of VR/OS genes. Fewer POM cells enter the choroid fissure after inhibition of RAR function, and we speculate that POM cells invading the fissure facilitate the fusion of the ventral lips of the optic cup. Decreasing the supply of vitamin A in mouse embryos at the time of choroid fissure closure causes coloboma and persistence of the basal lamina within the fissure (4), suggesting that POM cells migrating inside the choroid fissure might have a role in basal lamina degradation before fissure closure.
The epistasis analyses of POM genes in this study and reported elsewhere (19) allow us to construct a tentative network of genetic interactions downstream of RAR signaling required for POM development (Fig. 6D). In this model, RAR signaling activates foxC1a and nlz1 expression in early neural crest cells migrating in the eye region. In turn, FoxC1a collaborates with active RARs and with Nlz1 to promote pitx2 and eya2 expression, respectively, whereas Pitx2 promotes lmx1b1 expression. Although there are caveats to making conclusions based on gene expression changes, this model provides a useful framework for future studies.
Our study challenges the hypothesis that RA signaling mediates eye morphogenesis solely through the POM (3). Neural crest–specific RAR knockout mice show similar eye phenotypes to ubiquitous knockout embryos, whereas abrogation of RAR function in the mouse retina at E10.5 causes no obvious retinal phenotype. However, neural crest–specific RAR mutant mice and Aldh1A1/A3 mutant mice show normal optic cup formation up to E10.5, but not later (2, 3, 5). Instead, Aldh1A1/A2/A3 mutants show clear defects in the ventral invagination of the optic cup by E10.5, possibly because of the lack of Aldh1A2-dependent RAR signaling in the optic vesicle before E10.5 (2). Altogether, current studies do not rule out the possibility of RAR signaling acting directly upon optic cup cells before E10.5 to regulate eye morphogenesis in mice.
We report on a cohort of genes regulated by RAR signaling in the developing VR/OS—vax1, net1a, cyb5a, itgA5, dact1, and nlz2—besides the RA pathway genes dhrs3a and rdh10a (Fig. 7C). RAR-dependent regulation of their expression is centered around a developmental window (between 18s and 23 hpf) coinciding with the invagination of the optic cup. Abrogation of Vax1 or Nlz2 function causes coloboma (11, 18). Dact1 functions in morphogenetic processes by regulating the planar cell polarity pathway (22). Netrins and integrins are implicated in cell migration in various contexts (e.g., refs. 23 and 24) and consequently may also be involved in the morphogenetic movements leading to choroid fissure closure. They could also be implicated in the defective retinal vascularization and optic nerve formation associated with RAR-dependent coloboma given their known roles in axon guidance and blood vessel formation (e.g., ref. 25).
It is unlikely that changes in expression of VR/OS genes upon RAR inhibition are an indirect consequence of abnormal development of POM or other nearby tissues because expression of VR/OS genes is not decreased in foxC1a, nlz1, or pitx2 morphants (in this study) or lmx1b1 morphants (21), and we see no obvious changes in midline tissues that could secondarily compromise ventral eye development (11, 12). Analysis of noi mutants reinforces the idea that regulation of net1a, cyb5a, and itgA5 expression is independent of POM. Finally, some VR/OS and POM genes show rapid transcriptional activation in response to RA, suggesting that their expression may be directly regulated by RAR signaling.
In conclusion, we show that functional RAR signaling is required not only for proper POM development but also for the correct regulation of a genetic program within the VR/OS that is important for choroid fissure closure.
Materials and Methods
Zebrafish.
Embryos were obtained and staged as previously described (26). Melanization was prevented with phenylthiourea when necessary. Embryos were treated in the dark with 2.5–10 μM AGN (7) or with 0.125–1 μM all-trans RA (Sigma), diluted from 25 mM stocks in DMSO. Controls received identical amounts of DMSO.
MOs.
MOs were obtained from Gene Tools or Open Biosystems and dissolved in water at a stock concentration of 1 mM. MO aliquots were heated for 5–10 min at 65 °C before use and injected at the 1- to 4-cell stage.
Real-Time PCR.
cDNA was prepared from total RNA extracted from 20 to 40 embryos or dissected embryonic heads and amplified on a Rotor-Gene 3000 (Corbett Life Science) using Qiagen kits.
Further details on transgenic and mutant lines, treatment protocols, MOs, and analyses can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Jeffrey Gross, Ying Liu, and members of our laboratories for discussions; Lynn Carter and John Bashford for help with histological sections and microscopy; and Adrian McNabb, Tomasz Dyl, and Carole Wilson and her team for fish care. This work was supported by funds from the Wellcome Trust (to W.A.H. and S.W.W.), the Medical Research Council (to G.G. and S.W.W.), and Telethon Italy (to G.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103802108/-/DCSupplemental.
References
- 1.Chang L, Blain D, Bertuzzi S, Brooks BP. Uveal coloboma: Clinical and basic science update. Curr Opin Ophthalmol. 2006;17:447–470. doi: 10.1097/01.icu.0000243020.82380.f6. [DOI] [PubMed] [Google Scholar]
- 2.Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matt N, Ghyselinck NB, Pellerin I, Dupé V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 4.See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: Mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009;325:94–105. doi: 10.1016/j.ydbio.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Matt N, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- 6.Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- 7.Hammond LA, et al. Antagonists of retinoic acid receptors (RARs) are potent growth inhibitors of prostate carcinoma cells. Br J Cancer. 2001;85:453–462. doi: 10.1054/bjoc.2001.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 9.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman TL, Javier AL, Campeau SA, Knight RD, Schilling TF. Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J Exp Zoolog B Mol Dev Evol. 2007;308:679–691. doi: 10.1002/jez.b.21189. [DOI] [PubMed] [Google Scholar]
- 11.Take-uchi M, Clarke JDW, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- 12.Lupo G, et al. Dorsoventral patterning of the Xenopus eye: A collaboration of Retinoid, Hedgehog and FGF receptor signaling. Development. 2005;132:1737–1748. doi: 10.1242/dev.01726. [DOI] [PubMed] [Google Scholar]
- 13.Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol. 2004;2:E244. doi: 10.1371/journal.pbio.0020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essner JJ, Branford WW, Zhang J, Yost HJ. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development. 2000;127:1081–1093. doi: 10.1242/dev.127.5.1081. [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Hernandez RE, Waxman JS, Yelon D, Moens CB. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol. 2010;338:1–14. doi: 10.1016/j.ydbio.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyatt GA, et al. Retinoic acid establishes ventral retinal characteristics. Development. 1996;122:195–204. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald R, et al. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development. 1997;124:2397–2408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- 18.Brown JD, et al. Expression profiling during ocular development identifies 2 Nlz genes with a critical role in optic fissure closure. Proc Natl Acad Sci USA. 2009;106:1462–1467. doi: 10.1073/pnas.0812017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Duester G. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol. 2010;340:67–74. doi: 10.1016/j.ydbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- 21.McMahon C, Gestri G, Wilson SW, Link BA. Lmx1b is essential for survival of periocular mesenchymal cells and influences Fgf-mediated retinal patterning in zebrafish. Dev Biol. 2009;332:287–298. doi: 10.1016/j.ydbio.2009.05.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suriben R, Kivimäe S, Fisher DAC, Moon RT, Cheyette BNR. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009;41:977–985. doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Maminishkis A, Zahn G, Vossmeyer D, Miller SS. Integrin α5β1 mediates attachment, migration, and proliferation in human retinal pigment epithelium: Relevance for proliferative retinal disease. Invest Ophthalmol Vis Sci. 2009;50:5988–5996. doi: 10.1167/iovs.09-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami S, Ohki-Hamazaki H, Watanabe K, Ikenaka K, Ono K. Netrin 1 provides a chemoattractive cue for the ventral migration of GnRH neurons in the chick forebrain. J Comp Neurol. 2010;518:2019–2034. doi: 10.1002/cne.22319. [DOI] [PubMed] [Google Scholar]
- 25.Yebra M, et al. Recognition of the neural chemoattractant Netrin-1 by integrins α6β4 and α3β1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.