Abstract
Axin proteins are key negative regulators of the canonical Wnt signal transduction pathway. Although Axin2 null mice are viable, we identified an unusual ENU-induced recessive allele of Axin2, canp, that causes midgestation lethality in homozygotes. We show that the Axin2canp mutation is a V26D substitution in an invariant N-terminal sequence motif and that the Axin2canp protein is more stable than wild type. As predicted for an increased level of a negative regulator, the Axin2canp mutation leads to decreased Wnt signaling in most tissues, and this can account for most of the morphological phenotypes of Axin2canp mutants. In contrast, there is a paradoxical increase in canonical Wnt activity in the late primitive streak of all Axin2canp mutant embryos that is associated with the formation of an ectopic tail in some mutants. Treatment of wild-type embryos with an inhibitor of Tankyrase that stabilizes Axin proteins also causes inhibition of Wnt signaling in anterior regions of the embryo and a gain of Wnt signaling in the primitive streak. The results indicate that although increased stability of Axin2 leads to a loss of canonical Wnt signaling in most tissues, stabilized Axin2 enhances Wnt pathway activity in a specific progenitor population in the late primitive streak.
Keywords: axis formation, Axin1, LRP6, IWR-1, cardia bifida
Canonical Wnt signaling is required for the development of nearly every stage and organ system in mammals, whereas inappropriate activation of the pathway contributes to colorectal cancer and a variety of other tumors (1). In the absence of Wnt ligand, cytoplasmic β-catenin is degraded; degradation depends on a destruction complex that includes Axin, APC, CK1, and GSK3β. When Wnt proteins bind the Frizzled and LRP5/6 coreceptors, Axin is removed from the destruction complex, and stable β-catenin moves to the nucleus where, in combination with LEF/TCF DNA binding proteins, it activates target gene expression.
Axin is a crucial component of the cytoplasmic complex that targets β-catenin for degradation in the absence of ligand. Loss of function of mouse Axin1 causes early embryonic lethality associated with a variety of malformations, including duplication of the anterior–posterior body axis, as the result of excess activity of the canonical Wnt pathway (2, 3). In both Drosophila and mammalian cells, Axin is degraded in response to ligand, and overexpression of Axin blocks signaling (4–6), supporting the view that the concentration of Axin can define the level of Wnt signaling. Two groups recently identified small molecule inhibitors of Wnt signaling that act by stabilizing Axin proteins (7, 8). These molecules inhibit the activity of tankyrase, a poly-ADP ribosylating enzyme that binds to an N-terminal domain of Axin and promotes its turnover (8). These inhibitors reduce Wnt signaling in cancer cell lines, and it has been suggested that they provide a new option for therapy of Wnt-based tumors (9).
Although most attention has focused on Axin proteins in the β-catenin destruction complex, Axin also binds to the Lrp5/6 Wnt receptors, where Axin appears to have a positive role in activation of the receptor complex (10–13). However, the significance of this positive role for Axin in the Wnt signaling pathway has not been defined in vivo.
In vertebrates, a second Axin gene also regulates Wnt signaling (14). In contrast to the ubiquitous expression of Axin1, Axin2 is induced by canonical Wnt signaling and its expression pattern marks the cells exposed to Wnt signals (15, 16). Because Axin2 is a direct transcriptional target of Wnt signaling, it could act in a negative feedback loop to limit Wnt signaling. Axin2 null mutants are viable and have no defects in embryonic patterning; the defects in Axin2 null mice in skull formation (17) and tooth development (18) appear to be due to tissue-specific increases in canonical Wnt signaling. Despite their differences in expression, Axin1 and Axin2 both inhibit the stabilization and nuclear translocation of β-catenin when overexpressed in cells (14), and Axin2 can fully replace the function of Axin1 during mouse embryogenesis when knocked into the Axin1 locus (19).
We identified an unusual recessive allele of mouse Axin2, canopus (canp), which leads to midgestation lethality associated with defects in morphogenesis of the heart, tail, and primitive streak. The canp mutation is a missense substitution in the evolutionarily conserved N-terminal motif that was implicated in the binding of tankyrase and the control of Axin stability (8). We find that embryonic Axin2canp protein is more stable than the wild-type protein, demonstrating the in vivo significance of this domain for Axin2 stability. As predicted for an increase in the level of a negative regulator of the pathway, Axin2canp mutant embryos show decreased canonical Wnt signaling in most tissues. However, we show that the canp allele leads to enhanced Wnt signaling in the late primitive streak. Stabilization of Axin proteins by treatment with a small molecule inhibitor of Tankyrase also enhances canonical Wnt signaling in the primitive streak. The findings demonstrate that, in addition to its role as a negative regulator of the pathway, Axin2 also plays a positive role in canonical Wnt signaling pathway in vivo in a progenitor population in the primitive streak of the mouse embryo.
Results
Allele of Axin2 Disrupts Embryonic Morphogenesis.
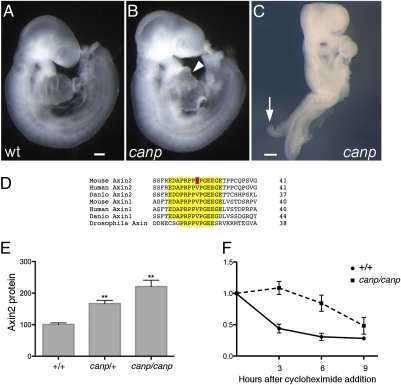
The canopus (canp) mutation was identified in a genomewide N-ethyl N-nitrosourea (ENU)-mutagenesis screen for mouse developmental mutants (20), on the basis of the abnormal morphology of mutant homozygotes. Most homozygous canp embryos (75–80%) arrested at midgestation with abnormal hearts and slightly shortened tails (Fig. 1 A and B). The remaining 20–25% of homozygotes showed a stronger phenotype that we call the double tail phenotype, in which the entire spinal neural tube failed to close and a short tail-like structure protruded from the dorsal side of the neural plate (Fig. 1C, arrow).
Fig. 1.
A mutation in Axin2 disrupts embryonic morphogenesis and slows protein turnover. (A–C) The e9.5 Axin2canp mutant phenotype. Unlike the wild-type embryo (A), Axin2canp mutants have abnormal hearts (arrowhead), slightly shorter tails, and about 30% are exencephalic, like the one shown here (B). (C) A homozygous Axin2canp embryo of the double tail class, with a short tail-like protrusion on the dorsal side of the embryo (arrow). (D) The V26D missense mutation (red) in Axin2canp lies in a highly conserved motif in Axin2 (yellow). (E) Western blot data (Fig. S1) from three e9.0 litters showed that the level of Axin2 was increased 1.6-fold in heterozygous and 2.2-fold in homozygous Axin2canp embryos relative to wild type. The amount of Axin2 protein in both homozygous and heterozygous embryos was significantly higher than in wild type (P < 0.001). (F) Turnover of Axin2 protein in MEFs. Wild-type and Axin2canp homozygous MEFs were cultured with 20 μg/mL cycloheximide (CHX) for the indicated number of hours and analyzed by Western blot (Fig. S1). The Axin2canp mutation increased the half-life of Axin2 from 1.4 h to 4.6 h.
We mapped the canp mutation by meiotic recombination to a 3-Mb region on chromosome 11 that contained 24 annotated transcripts (Materials and Methods). We sequenced the coding region of all genes in the interval except those in which null mutants were viable, but did not identify any sequence changes. We therefore considered the possibility that canp might be an unusual allele of a gene in the interval not required for viability. One of the genes in the interval was Axin2, which encodes a negative regulator of canonical Wnt signaling but is not essential for embryonic development (17). Because of the important roles of Wnt signaling in development of the heart, tail, and body axis, we sequenced the coding region of Axin2 from canp embryos and identified a T-to-C change that would cause a V26D substitution in an evolutionarily conserved sequence near the N terminus of Axin2 (Fig. 1D). The mutation created a BamH1 restriction fragment-length polymorphism that we used to confirm the linkage of the mutation to the canp phenotypes (Materials and Methods). Because mice lacking Axin2 survive to adulthood (17) and the canp homozygotes died as embryos, the results suggested that canp was an unusual allele of Axin2.
canp Is a Recessive Hypermorphic Mutation That Stabilizes Axin2 Protein.
To test how the V26D mutation affected Axin2 function, we prepared embryonic extracts from wild type, heterozygous, and homozygous canp embryos at embryonic day (e) e9.0, and analyzed Axin2 protein levels. Western blot analysis showed that homozygous canp embryos had 2.2 times as much Axin2 protein as wild-type embryos (Fig. 1E and Fig. S1A); canp/+ heterozygous embryos also showed an increase in the amount of Axin2 to 1.6 times the level in wild type. Both in situ hybridization and quantitative real-time PCR (qPCR) showed that the level of Axin2 mRNA was not elevated in the mutants (Fig. S2), suggesting that the increase in Axin2 protein in the embryo could be caused by increased protein stability.
To test whether the canp mutation altered protein stability, we examined the rate of turnover of the mutant and wild-type forms of Axin2 in mouse embryonic fibroblasts (MEFs) derived from wild-type and mutant e10.0 embryos. We found that there was twofold more Axin2 protein present in homozygous canp mutant MEFs than in wild-type MEFs (Fig. S1B). The MEFs were treated with cycloheximide to block protein synthesis and the level of Axin2 was determined by Western blotting at subsequent time points. The half-life of wild-type Axin2 was ∼1.4 h in the wild-type MEFs, whereas the half-life of Axin2canp increased to 4.6 h in homozygous canp mutant MEFs (Fig. 1F and Fig. S1C), demonstrating that canp encodes a more stable form of Axin2. Axin2canp/Axinnull mice (n = 15) were viable and fertile and did not exhibit the abnormal skull development seen in null homozygotes, which indicates that canp is a hypermorphic allele of Axin2.
Increased Stability of Axin2 Lowers the Level of Canonical Wnt Signaling in Axin2canp Embryos.
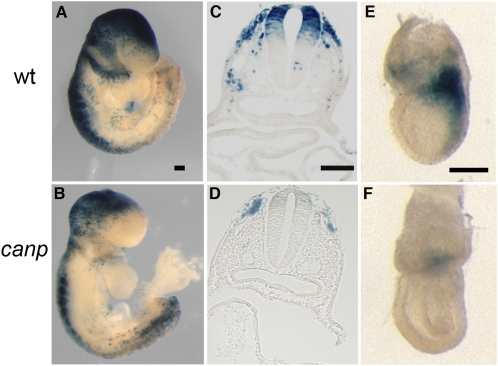
To test whether the increased stability of Axin2 in Axin2canp mutants affected Wnt signaling in the embryo, we generated Axin2canp homozygotes that carried one copy of TOPGAL, a transgenic reporter of canonical Wnt activity, to assess pathway activity (21). TOPGAL activity was reduced in most tissues of e9.0 Axin2canp embryos, including the brain, branchial arches, somites, migrating neural crest cells, and dorsal neural tube (Fig. 2 A–D), consistent with the increased levels of the negative regulator Axin2.
Fig. 2.
Reduced canonical Wnt activity in Axin2canp mutants. Wild type (A) and homozygous Axin2canp (B) mutants (16–20 somite stage) carrying one copy of the TOPGAL reporter transgene, stained for β-galactosidase activity. Axin2canp mutants displayed reduced TOPGAL staining in the brain, branchial arches, dorsal neural tube, and somites. Cross-sections through X-Gal–stained wild-type (C) and homozygous Axin2canp embryos (D) at the level of branchial arches. (Scale bars, 300 mm in A–D; 50 mm in C and D.) (E and F) TOPGAL expression in gastrulating e7.25 embryos was greatly reduced in the primitive streak in Axin2canp embryos (F) compared with wild type (E).
Most of the Axin2canp phenotypes were consistent with a partial loss of Wnt signaling. All e9.5 Axin2canp embryos had a somewhat shorter tail than wild type (Figs. 1B and 2B), a phenotype that is also seen in embryos homozygous for vestigial tail, a partial loss-of-function allele of Wnt3a (22), which is expressed in the tail bud and is required for tail development. All Axin2canp embryos showed some degree of cardia bifida (Fig. S3), which was likely to be the cause of their midgestation lethality. Genetic removal of β-catenin in migrating precardiac mesoderm using the MesP1-Cre caused defects in heart looping (23) that appear to include the failure of the two lateral heart fields to fuse correctly, which suggests that the cardia bifida phenotype of Axin2canp embryos may be due to a requirement for high levels of canonical Wnt signaling in cardiac progenitors.
Canonical Wnt signaling is required at gastrulation to initiate and maintain the primitive streak (24, 25). All e7.25 Axin2canp embryos showed decreased Wnt signaling in the primitive streak, as assayed by the activity of the TOPGAL reporter (Fig. 2 E and F). Expression of Tbx6, a marker of nascent mesoderm, was reduced in all Axin2canp embryos and was not detectable at e7.5 in the most strongly affected mutants (Fig. S4D). Most Axin2canp mutants showed no obvious defects in gastrulation. However, about one-quarter of the mutants had an abnormal primitive streak at e7.5 (23/101; 22.7%) (Fig. S4 E–H) and showed reduced expression of Brachyury (T), a marker of the primitive streak and a direct target of Wnt3a signaling (Fig.S4C) (26). Both the morphology and marker gene expression in the strong e7.5 Axin2canp embryos resembled that seen in Lrp5+/−; Lrp6−/− embryos and Lrp5−/−; Lrp6−/− mutants, in which canonical Wnt signaling is reduced or blocked due to the loss of the Lrp 5/6 Wnt coreceptors (27).
Gain of Wnt Activity in Late Primitive Streak of Axin2canp Embryos.
Other mouse mutants with an early loss of Wnt signaling or defects in early mesoderm specification arrest at ∼e8.0 and lack mesoderm-derived structures (27). In contrast, we did not observe either early lethality or a gross deficit in mesoderm-derived structures, such as somites, in e9.5 Axin2canp mutants (Fig. 1B). The striking recovery of the early deficit in mesoderm in Axin2canp embryos between e7.5 and e9.5 and the positive role of Wnt signaling in mesoderm specification prompted us to examine canonical Wnt signaling in Axin2canp mutants during this time window.
In contrast to the loss of activity of the canonical Wnt pathway seen in e7.25 Axin2canp embryos (Fig. 2 E and F), a low level of TOPGAL activity was detected in the primitive streak of e7.5 Axin2canp embryos (Fig. 3D). By e8.5, there were striking region-specific differences in canonical Wnt activity in Axin2canp embryos: TOPGAL activity was reduced in the head and all other tissues rostral to the presomitic mesoderm (PSM), whereas TOPGAL expression was elevated in the caudal region of all Axin2canp embryos (Fig. 3 and Fig. S5). TOPGAL expression was also significantly increased in the caudal region of Axin2canp/+ embryos (Fig. S5). Cross-sections through the e9.0 TOPGAL-stained Axin2canp mutants showed ectopic TOPGAL+ cells in the primitive streak and the presomitic mesoderm (Fig. 3F), although TOPGAL activity was reduced in the posterior notochord (Fig. 3 C and F), showing that Wnt activity was increased in a cell type-specific, rather than a regional, manner.
Fig. 3.
Elevated Wnt signaling in the late primitive streak of Axin2canp embryos. (A–H) Canonical Wnt activity assayed by TOPGAL staining is elevated in the late streak of Axin2canp embryos. (A and B) TOPGAL is strong in the wild-type primitive streak of e7.5 embryos and decreases by e8.5. (C) TOPGAL expression in a section through an e9.0 wild-type embryo (14–16 somites) at the anterior limit of the presomitic mesoderm. (D) TOPGAL activity is detected in the primitive streak of e7.5 Axin2canp embryos. (E) Elevated TOPGAL activity in the primitive streak of e8.5 Axin2canp embryos. (F) Section through an e9.0 Axin2canp embryo at the same level as in C shows that TOPGAL is elevated in the epiblast and presomitic mesoderm (arrows), but relatively low in the mutant notochord (arrowheads). (G and H) β-Galactosidase activity assays show that canonical Wnt activity is low in the anterior (G), but not posterior region (H) of mutant embryos. (I–L) Elevated Wnt activity leads to the formation of a duplicated primitive streak in the canp-doubletail embryos. (I and J) Expression of T. (I) T is expressed in the primitive streak of wild-type embryos. (J) In canp-double tail embryos, both a normal-appearing primitive streak and a smaller, dorsal protrusion shaped like a primitive streak express T (arrow) (J). Meox1, expressed in the wild-type somites and presomitic mesoderm (K), is expressed in the mutant double tail (L, arrow). (Scale bars, 100 mm.)
To quantitate the changes of TOPGAL activity in the mutant canp embryos, e9.0 standard Axin2canp (not double tail) embryos were cut into anterior and posterior halves at the level of the rostral limit of the PSM. Analysis of β-galactosidase enzyme activity in the embryonic extracts showed that the anterior half of Axin2canp embryos had 49% the level of β-galactosidase activity found in wild-type littermates (P < 0.001) (Fig. 3G). The activity of TOPGAL was not significantly different in the posterior wild-type and mutant extracts (Fig. 3H), suggesting that the gain of TOPGAL activity in the PSM and primitive streak of Axin2canp mutants was balanced by a loss of TOPGAL activity in the node and caudal notochord. Thus, the Axin2canp mutation had opposite effects on TOPGAL activity in the primitive streak and PSM at different stages of development: a loss of TOPGAL activity at e7.25 and a gain of TOPGAL activity at e8.5.
Tankyrase Inhibitors Elevate Wnt Signaling in the Primitive Streak.
The Axin2canp mutation is likely to stabilize Axin2 by disrupting the domain that binds Tankyrase. We therefore tested how Tankyrase inhibitors affected canonical Wnt activity in wild-type embryos carrying the TOPGAL reporter. In initial experiments, both IWR-1 and XAV939 inhibited TOPGAL activity, but IWR-1 gave stronger and more consistent results, as seen previously in explanted mouse kidneys (28); we therefore used IWR-1 in our experiments.
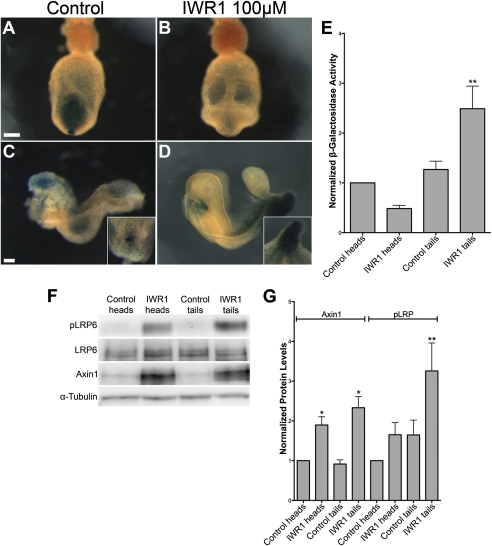
Wild-type e7.5 embryos cultured for 18 h in the presence of 100 μM IWR-1 showed reduced TOPGAL activity in the primitive streak compared with control embryos (Fig. 4 A and B), similar to the reduction in TOPGAL at this stage in Axin2canp mutants (Fig. 2 E and F and Fig. 3 A and D). When e8.0 wild-type embryos (late headfold-early somite stage) were cultured either in the presence or absence of IWR-1, they developed 6–10 somites and had beating hearts after 18 h in culture. As expected, the level of Axin1 protein was elevated in both the anterior and posterior of embryos cultured in the presence of 100 μM IWR-1 (Fig. 4 F and G). TOPGAL expression was diminished in the head and somite region in embryos cultured in the presence of IWR-1 compared with controls (Fig. 4 C and D), as expected due to the increased level of Axin. In contrast to the inhibition of Wnt signaling in the anterior of the embryo, TOPGAL expression appeared to be elevated in the primitive streak of early somite stage embryos cultured in the presence of IWR-1 (Fig. 4 C and D), similar to the phenotype of the Axin2canp embryos at this stage (Fig. 3E).
Fig. 4.
IWR-1 decreases or increases TOPGAL activity in wild-type embryos, depending on stage and tissue. (A–D) TOPGAL activity in cultured control (A and C) and IWR-1–treated (B and D) wild-type embryos that carry one copy of the TOPGAL transgene. (A and B) Posterior views of late headfold embryos after 18 h of culture, showing decreased expression of TOPGAL in the primitive streak of IWR-1–treated embryos. (C and D) Lateral views of 6-somite embryos after 18-h culture. At this stage, IWR-1–treated embryos showed reduced TOPGAL expression in the head, but increased expression in the primitive streak and PSM. The Insets in C and D are dorsal and ventral views of the primitive streak, respectively. The unusual shape of the tail is characteristic of embryos treated with the tankyrase inhibitors. (Scale bars, 100 μm.) (E) β-Galactosidase activity in anterior (head) and posterior (tail) halves of control and IWR-1–treated embryos, normalized to the activity in control heads. β-Galactosidase activity was measured in three independent experiments, with six to eight embryos per condition in each experiment. (F) Representative Western blots showing the increased level of Axin1 in embryos treated with 100 μM IWR-1 compared with DMSO controls. Embryos treated with IWR-1 also have a higher level of pLRP6 in the tails. (G) Analysis of Western blot data from six independent experiments in which four to eight embryos were cultured per condition in each experiment. Protein levels were normalized to α-tubulin, and values were normalized to protein levels in DMSO-treated heads. Axin1 levels were increased 1.90-fold in IWR-1–treated heads (P < 0.05) and 2.33-fold in treated tails (P < 0.05). The change in pLRP6 levels in treated heads was variable and not significant, whereas the 3.26-fold increase in IWR-1–treated tails was significant (P < 0.01).
To quantitate the TOPGAL activity, embryos were cut into anterior and posterior halves at the anterior border of the PSM after culture and β-galactosidase activity in each lysate was assayed. β-galactosidase activity in the anterior half of IWR-1–treated embryos was about 50% of that in controls, as in Axin2canp embryos (Fig. 3G). In contrast, the TOPGAL activity in the posterior half of IWR-1–treated embryos was 2.5-fold greater than in either the anterior or posterior of control embryos (P < 0.01) and 4.0-fold greater than in the anterior half of drug-treated embryos (P < 0.01) (Fig. 4E). Thus, inhibition of Tankyrase, like the Axin2canp mutation, decreased canonical Wnt activity in the head, but increased Wnt activity in the primitive streak of early somite-stage embryos.
It has been proposed that, in addition to its negative role in Wnt signaling, Axin may play a positive role in canonical Wnt signaling by recruitment of an Axin–GSK3 complex to the activated LRP5/6 receptor (10–13); in this model, phosphorylation of LRP5/6 is important for downstream signaling. We therefore assayed the level of phospho-LRP6 and found that phospho-LRP6 was elevated in the posterior, but not the anterior of IWR-1–treated embryos (Fig. 4 F and G).
Elevated Wnt Signaling Produces an Ectopic Primitive Streak in a Fraction of Axin2canp Embryos.
Although the morphology of the streak and mesoderm was normal in most Axin2canp embryos, ∼15% of the mutants developed an ectopic tail-like structure (Fig. 1C and Fig. S6). Because Wnt signaling is required for formation of the primitive streak and tail (24, 25) and excess Wnt activity can cause the formation of ectopic body axes (3, 29), we investigated the nature of the tail-like structure. Cross-sections through double tail embryos showed that the protrusion was composed of mesenchymal cells that protruded dorsally from the midline of the neural plate (Fig. S6J). The protrusions expressed T, which marks the primitive streak and notochord, Meox1, a marker of presomitic mesoderm, and a high level of TOPGAL (Fig. 3 I–L and Fig. S5). We therefore conclude that these ectopic protrusions are second tails induced by elevated Wnt activity in an expanded population of tail progenitors in the primitive streak.
Discussion
An N-Terminal Domain Controls the Stability of Axin2 in Vivo.
The Axin2canp mutation is recessive, but it increases the activity of Axin2 protein by increasing its stability. Recessive gain-of-function mutations are unusual and must represent cases where the elevation of activity associated with a single copy of the mutant allele is compatible with normal development, but double the dosage of the mutant allele is not. The Axin2canp mutation is an excellent example of this type of mutation, as one copy of the mutant allele increases the steady-state level of Axin2 1.6-fold, which is compatible with survival of all heterozygotes, whereas two copies of the Axin2canp allele doubles the steady-state level of the Axin2, which decreases Wnt signaling to an extent that causes completely penetrant midgestation lethality.
The Axin2canp mutation causes a nonconservative valine-to-glutamate substitution that disrupts an N-terminal PRPPVPGEE motif, which is perfectly conserved in all Axin family members, from Drosophila to humans. Our results support the importance of the N-terminal region identified by Huang et al. (8) as a critical regulator of Axin2 stability. Huang et al. named this motif the tankyrase-binding domain (TBD), as they showed that this domain can bind Tankyrase, an enzyme with poly-ADP ribosylation activity, and they presented data that modification of Axin by tankyrase can direct ubiquitin-mediated, proteosome-dependent degradation of Axin1 and Axin2. The analysis of the effects of the Axin2canp mutation confirms that this protein domain regulates Axin2 stability in vivo and that disruption of this domain decreases Wnt signaling in most embryonic tissues.
Our data suggest that the PRPPVPGEE motif has functions in addition to binding tankyrase. There are two tankyrase genes in mammalian genomes, and it was noted previously that the phenotype of mouse tankyrase1,2 double mutants (30) does not suggest an obvious effect on Wnt signaling (8). In contrast, Axin2canp embryos arrest at least one day earlier than tankyrase1,2 double mutant embryos and show clear Wnt-related phenotypes not described in the tankyrase double mutant embryos. In addition, the canp V26D mutation stabilizes the endogenous Axin2 protein to a greater extent than blocking tankyrase activity with the small molecule XAV939 (compare Fig. S1C to Huang et al. ref. 8). Thus, it is likely that the PRPPVPGEE motif controls the stability of Axin2 through interactions with proteins in addition to tankyrase.
Stabilized Axin2 Activates Wnt Signaling in Primitive Streak Progenitors.
All Axin2canp mutants show decreased Wnt signaling in the early primitive streak, similar to the effect on Wnt signaling seen in other tissues. However, in contrast to the early loss of Wnt reporter activity, all Axin2canp homozygotes show elevated activity of the TOPGAL canonical Wnt reporter in the e8.5 primitive streak. Whereas mutants in other genes associated with decreased canonical Wnt signaling in the primitive streak arrest by e9.0 with reduced or absent mesodermal cell types, all Axin2canp embryos differentiate a nearly normal number of somites at e9.5, which provides independent evidence that Wnt signaling is restored in the late Axin2canp streak.
Wnt signaling in the late primitive streak does not simply return to the normal level in the Axin2canp embryos; it surpasses the level of signaling in the wild-type streak. Inhibition of Axin2 turnover by the tankyrase inhibitor IWR-1 has a similar tissue-specific effect: canonical Wnt signaling is decreased in anterior regions of the embryo and elevated in the primitive streak. The increased TOPGAL activity in the posterior of IWR-1–treated embryos is greater than that in Axin2canp embryos, which suggests that both Axin1 and Axin2 can activate Wnt signaling in the streak. Thus, two independent approaches demonstrate that stabilized Axin proteins promote, rather than inhibit, canonical Wnt signaling in the primitive streak.
Our results provide in vivo support for studies that suggested that Axin can play a positive role in canonical Wnt signaling through its interaction with activated LRP5/6 (10–13). In these models, binding of Wnt ligand leads to recruitment of an Axin–GSK3 complex to LRP5/6 and phosphorylation of LRP5/6 by GSK3, and phosphorylated LRP5/6 can recruit additional Axin in a positive feedback loop that amplifies receptor phosphorylation (13). Our findings show that stabilization of Axin is accompanied by an elevated level of phosphorylated LRP6 specifically in the posterior of the e8.5 embryo and suggest that the increased phospho-LRP6 activates Wnt signaling in the late primitive streak.
The time of transition between decreased (e7.5) and increased activity (e8.5) of the canonical Wnt pathway in Axin2canp mutants represents a developmental transition at the primitive streak. Before e7.5, Wnt3 is expressed in the streak and is required for the formation of the streak, which gives rise to a variety of cell types, including the definitive endoderm and all types of mesoderm (extraembryonic, axial, paraxial, and lateral plate) (9). At e7.5, Wnt3 expression shuts off and Wnt3a expression turns on in the streak, where it is required to maintain progenitors that give rise primarily to paraxial mesoderm (25, 31). Wnt5a and its receptor Ror2 are also expressed in the streak at this stage (32, 33) and may modulate the canonical pathway (34, 35). We propose that the increased Wnt signaling activity in the late streak of Axin2canp embryos reflects a positive action of an Axin2–Lrp5/6 complex in the response of progenitor cells in the late streak to Wnt signals. We suggest that the balance of regulators in the primitive streak is such that increased amount of Axin protein has a greater impact on the positive roles of Axin than on the β-catenin destruction complex.
Cells in primitive streak are thought to act as a stem/progenitor pool (36, 31), and our data suggest that stabilization of Axin2 activates signaling in this progenitor population. It has been proposed that tankyrase inhibitors, which stabilize the Axin proteins, may be useful for therapy of Wnt-dependent tumors, but our findings suggest it will be important to test whether stabilization of Axin proteins can activate Wnt signaling in other stem and progenitor populations, as well as in in vivo tumor models.
Materials and Methods
Mouse Strains.
The canp mutation mapped between D11Mit333 and D11Mit301. PCR amplification from genomic DNA (F: 5′ GTTTGGTGGACTGGACCTTG 3′; R: 5′ AGACGCTCTCCCTCACCAT 3′) generated a fragment containing an RFLP cleaved by BamH1 digestion in the mutant but not wild-type sequence. The phenotype was analyzed in the C3H congenic background. Axin2tm1Wbm (lacZ knock-in), was obtained from Dr. Frank Costantini (Columbia University, New York) (17). TOPGAL transgenic mice were from Dr. Elaine Fuchs (Rockefeller University, New York) (21).
Phenotypic Analysis and Embryo Culture.
In situ hybridization was carried out as described (37). Embryos were cultured in 50% rat serum/50% DMEM/F12 at 37 °C with 5% CO2 in static culture. A stock of IWR-1 in DMSO was diluted in culture medium to 100 μM (0.5% DMSO); control embryos were cultured in 0.5% DMSO. A stock of XAV939 in DMSO was diluted in culture medium to 200 μM (0.1% DMSO); control embryos were cultured in 0.1% DMSO. IWR-1 and XAV939 were from Sigma.
Wnt Reporter Assays.
TOPGAL males carrying two copies of the TOPGAL transgene and one copy of canp mutant allele were crossed to canp heterozygous females TOPGAL activity in embryos was detected as described (38). In each experiment, 4–21 embryos of each genotype were stained for TOPGAL; results were consistent between embryos of the same genotype at the same stage. β-Galactosidase activity in embryo extracts was measured using the β−galactosidase Enzyme Assay system (Promega); 8–10 embryos of each genotype were analyzed. Activity was normalized to total protein concentration, determined by the Pierce BCA Protein Assay kit. The Newman–Keuls multiple comparison test was used to compare the significance of differences in β-galactosidase activity.
Western Blot Analysis.
Protein lysates were prepared using RIPA buffer. The anti-Axin2 antibody was a gift from Dr. Frank Costantini (15); the pLRP6, LRP6, and Axin1 antibodies were from Cell Signaling; the γ-tubulin and α-tubulin antibodies were from Sigma. To calculate the Axin2 half-life, the level of Axin2 was quantified by Western blotting and normalized to that at time 0. The Axin2 levels were fit to the first-order decay and the half-life was calculated with PRISM (39). For Western blot analysis of IWR-1–treated embryos, 10 litters of e8.0 embryos in eight independent cultures were divided equally between DMSO and IWR-1 conditions. Gels were run with extracts from one to three litters. Protein levels were quantified with ImageJ and were calculated as percentage of the amount in embryos in the same culture (averages ± SD) and analyzed by one-way ANOVA followed by Newman–Keuls posttest.
Supplementary Material
Acknowledgments
We thank Frank Costantini for the Axin2 antibody and the Axin2-lacZ mice, Ed Espinoza for assistance with photography, Min Jung Kim and Nick Tolwinski for discussions, and Sarah Goetz, Isabelle Migeotte, and Tim Bestor for comments on the manuscript. The work was supported by National Institutes of Health Grant HD035455 (to K.V.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100328108/-/DCSupplemental.
References
- 1.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool. 1949;110:47–76. doi: 10.1002/jez.1401100105. [DOI] [PubMed] [Google Scholar]
- 3.Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 4.Tolwinski NS, et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 5.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto H, et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 7.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, He X. Destruction of a destructor: A new avenue for cancer therapeutics targeting the Wnt pathway. J Mol Cell Biol. 2010;2:70–73. doi: 10.1093/jmcb/mjp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao J, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 11.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 13.Zeng X, et al. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 15.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu HM, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammi L, et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chia IV, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-García MJ, et al. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc Natl Acad Sci USA. 2005;102:5913–5919. doi: 10.1073/pnas.0501071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 22.Greco TL, et al. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- 23.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci USA. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 28.Karner CM, et al. Tankyrase is necessary for canonical Wnt signaling during kidney development. Dev Dyn. 2010;239:2014–2023. doi: 10.1002/dvdy.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill BJ, et al. Tcf3: A transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- 30.Chiang YJ, et al. Tankyrase 1 and tankyrase 2 are essential but redundant for mouse embryonic development. PLoS ONE. 2008;3:e2639. doi: 10.1371/journal.pone.0002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson V, Beddington RS. Cell fate and morphogenetic movement in the late mouse primitive streak. Mech Dev. 1996;55:79–89. doi: 10.1016/0925-4773(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda T, et al. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev. 2001;105:153–156. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 34.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: Orphans no more. Trends Cell Biol. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 36.Snow M. Gastrulation in the mouse: Growth and regionalization of the epiblast. J Embryol Exp Morphol. 1977;42:291–303. [Google Scholar]
- 37.Belo JA, et al. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 38.Hogan BL, Blessing M, Winnier GE, Suzuki N, Jones CM. Growth factors in development: The role of TGF-beta related polypeptide signalling molecules in embryogenesis. Dev Suppl. 1994;1994:53–60. [PubMed] [Google Scholar]
- 39.Belle A, Tanay A, Bitincka L, Shamir R, O'Shea EK. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci USA. 2006;103:13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.