Abstract
Ionizing radiation and interstrand DNA crosslinking compounds provide important treatments against cancer due to their extreme genotoxicity for proliferating cells. Both the efficacies of such treatments and the mutagenic potential of these agents are modulated by the ability of cells to repair the inflicted DNA damage. Here we demonstrate that homologous recombination-deficient mRAD54–/– mice are hypersensitive to ionizing radiation at the embryonic but, unexpectedly, not at the adult stage. However, at the adult stage mRAD54 deficiency dramatically aggravates the ionizing radiation sensitivity of severe combined immune deficiency (scid) mice that are impaired in DNA double-strand break repair through DNA end-joining. In contrast, regardless of developmental stage, mRAD54–/– mice are hypersensitive to the interstrand DNA crosslinking compound mitomycin C. These results demonstrate that the two major DNA double-strand break repair pathways in mammals have overlapping as well as specialized roles, and that the relative contribution of these pathways towards repair of ionizing radiation-induced DNA damage changes during development of the animal.
Keywords: DNA-dependent protein kinase/DNA double-strand breaks/DNA end-joining/DNA interstrand crosslinks/ionizing radiation
Introduction
DNA double-strand breaks (DSBs) are extremely genotoxic DNA lesions, because they cause problems for DNA transcription, replication and segregation. Improper processing of DSBs gives rise to chromosomal instability that can result in carcinogenesis through activation of proto-oncogenes or inactivation of tumor suppressor genes. DSBs are caused by exogenous sources such as ionizing radiation, and by endogenous sources such as radicals generated during metabolic processes. In addition, it is likely that a predominant source of DSBs in dividing cells is the process of DNA replication itself (Kogoma, 1997; Cox, 1998; Haber, 1999). The adverse effects of DSBs have triggered the evolution of multiple pathways for their repair. The two major pathways are homologous recombination and DNA end-joining (Kanaar et al., 1998; Tsukamoto and Ikeda, 1998). The fundamental difference between these pathways is their dependence on DNA homology and accuracy of repair. In general, homologous recombination ensures accurate repair by using the un– damaged sister chromatid or homologous chromosome as a template. DNA end-joining, on the other hand, uses no or extremely limited sequence homology to rejoin ends in a manner that need not be error free. Both major pathways can be divided into subpathways that can result in different outcomes of DSB repair and might require common as well as subpathway-specific genes. Homologous recombination includes single-strand annealing, gene conversion and break-induced replication (Paques and Haber, 1999). DNA end-joining includes precise end-joining and micro– homology-directed end-joining (Critchlow and Jackson, 1998).
Mutants in the Saccharomyces cerevisiae RAD52 epistasis group display hypersensitivity to ionizing radiation and are defective in DSB repair through homologous recombination (Petes et al., 1991; Game, 1993; Smith and Nicolas, 1998). Key proteins in the RAD52 epistasis group are Rad51, Rad52 and Rad54. Biochemical analyses have shown that the central events of recombination, homologous DNA pairing and strand exchange are mediated by Rad51 (Sung, 1994; Bianco et al., 1998), and that both the Rad52 and Rad54 proteins can stimulate the recombination activities of the Rad51 protein (Sung, 1997; New et al., 1998; Petukhova et al., 1998; Shinohara and Ogawa, 1998). The functional significance of DSB repair through homologous recombination is underscored by the conservation of the RAD52 pathway from fungi to humans. Mammalian homologs of RAD51, RAD52 and RAD54 have been identified (Petrini et al., 1997; Baumann and West, 1998; Kanaar et al., 1998) and the human Rad51, Rad52 and Rad54 proteins have been shown to possess similar activities to their yeast counterparts (Baumann et al., 1996; Mortensen et al., 1996; Reddy et al., 1997; Benson et al., 1998; Petukhova et al., 1998, 1999; Shinohara and Ogawa, 1998; Sugiyama et al., 1998; Swagemakers et al., 1998; Tan et al., 1999). While homologous recombination is the predominant DSB repair pathway in bacteria and yeast, DNA end-joining is believed to be the principal DSB repair pathway in vertebrate cells (Kanaar et al., 1998). However, experiments using cells containing a combination of mutations effecting both DNA repair pathways revealed that homologous recombination and DNA end-joining can both contribute to repair of ionizing radiation-induced DNA damage in S.cerevisiae, Drosophila melanogaster and chicken cells (Siede et al., 1996; Takata et al., 1998; Kooistra et al., 1999). Genes involved in DNA end-joining include XRCC4, Ku70, Ku80 and DNA-PKcs (Critchlow and Jackson, 1998; Jeggo, 1998; Lieber, 1999). The DNA-PKcs gene, encoding the catalytic subunit of DNA-dependent protein kinase (DNA-PK), is defective in severe combined immune deficiency (scid) mice (Blunt et al., 1995; Kirchgessner et al., 1995; Peterson et al., 1995). The scid mutation confers immuno– deficient and ionizing radiation hypersensitive phenotypes due to deficiencies in processing DSB intermediates during development of the immune system and repair of ionizing radiation-induced DNA damage (Schuler and Bosma, 1989; Fulop and Phillips, 1990; Biedermann et al., 1991; Hendrickson et al., 1991; Smith and Jackson, 1999).
We have shown previously that mRAD54–/– embryonic stem (ES) cells are hypersensitive to ionizing radiation and the DNA interstrand crosslinking agent mitomycin C. The mRAD54–/– ES cells display a reduced level of homologous recombination compared with wild-type ES cells (Essers et al., 1997). These results have revealed that in addition to DNA-PK-mediated DNA end-joining, homologous recombination can contribute to the repair of ionizing radiation-induced DNA damage in mammalian cells. In contrast to homozygous disruption of other genes implicated in DSB repair through homologous recombination, including mRAD51, BRCA1 and BRCA2 (Lim and Hasty, 1996; Tsuzuki et al., 1996; Sharan et al., 1997; Zhang et al., 1998; Moynahan et al., 1999), disruption of mRAD54 results in viable mice (Essers et al., 1997). Thus, mRAD54–/– mice provide the opportunity to study the biological relevance of homologous recombin– ation in mammalian DNA damage repair.
Results and discussion
mRAD54–/– mice are hypersensitive to ionizing radiation at the embryonic but not the adult stage
To determine whether the ionizing radiation hypersensitive phenotype of mRAD54–/– ES cells was also displayed by mRAD54–/– mice, we treated 2- to 4–month-old mRAD54-proficient and –deficient mice with different doses of ionizing radiation. Four groups of five mRAD54–/– and five mRAD54+/– mice were irradiated with doses of 6, 7, 7.5 and 8 Gy, respectively. Unexpectedly, only one mRAD54–/– mouse irradiated with 7 Gy died within 3 weeks, whereas none of the mice irradiated with 6 Gy and 7.5 Gy died. Irradiation with 8 Gy was lethal for all mice, irrespective of mRAD54 status (data not shown).
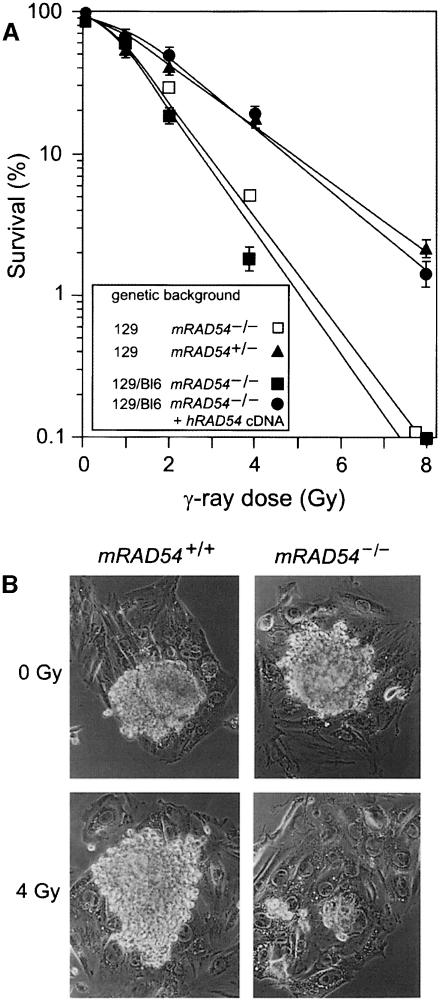
We subsequently investigated whether this dramatic difference in ionizing radiation hypersensitivity between mRAD54–/– ES cells and mice might be due to a difference in genetic background between the ES cells and the mice. The mRAD54–/– ES cells are derived from a 129 mouse strain (Essers et al., 1997), while the mRAD54–/– mice were C57Bl6/129 hybrids. Possibly, the difference in genetic background could mask the effect of mRAD54 disruption in the mice. To test this hypothesis, we isolated de novo ES cells from the mRAD54–/– C57Bl6/129 mice. The C57Bl6/129-derived mRAD54–/– ES cells showed a sensitivity to ionizing radiation similar to 129 mRAD54–/– ES cells (Figure 1A). Expression of the human RAD54 (hRAD54) cDNA in the C57Bl6/129 mRAD54–/– ES cells corrected the ionizing radiation sensitivity of the cells to the level of 129 mRAD54-proficient ES cells. We conclude that mRAD54–/– mice are not hypersensitive to ionizing radiation and that this lack of ionizing radiation hyper– sensitivity compared with that of mRAD54–/– ES cells is not due to a difference in genetic background. Interestingly, opposite results have been obtained for DNA-PKcs. DNA-PKcs–/– ES cells are not ionizing radiation hypersensitive, whereas scid mice are (Biedermann et al., 1991; Gao et al., 1998).
Fig. 1. Effect of ionizing radiation on ES cells and day 3.5 embryos. (A) Effect of ionizing radiation on 129 and C57Bl6/129 mRAD54-proficient and -deficient ES cells. The percentage of surviving cells as measured by their colony-forming ability is plotted as a function of ionizing radiation dose. The 129 mRAD54-deficient ES cells were generated by gene targeting (Essers et al., 1997). The C57Bl6/129 mRAD54-deficient ES cells were isolated de novo. A RAD54-proficient derivative of this ES cell line was generated by selecting a cell line that stably expressed an hRAD54 cDNA construct. (B) After isolation, day 3.5 embryos were irradiated with an ionizing radiation dose of 0 and 4 Gy and cultured for 10 days. Outgrowth of the inner cell mass and trophoblast cells from the embryos was determined.
Next, we determined whether, in contrast to the adult stage, mRAD54 deficiency resulted in ionizing radiation hypersensitivity at the embryonic stage. We isolated mRAD54–/– and mRAD54+/+ embryos at day 3.5 of gestation. Embryos were treated with ionizing radiation doses of 0 or 4 Gy and subsequently cultured for 10 days (Figure 1B). Without irradiation the percentage of embryos showing outgrowth of the inner cell mass and trophoblast cells was similar for both wild-type and mutant embryos (Table I). After exposure to 4 Gy the percentage of wild-type embryos showing outgrowth of the inner cell mass was slightly reduced. However, after 10 days the inner cell mass of all 13 irradiated mRAD54–/– embryos was completely ablated. The presence of trophoblast cells provided a control for initial attachment of the embryos to the culture dish. Thus, in contrast to adult mRAD54–/– mice, mRAD54–/– embryos are hypersensitive to ionizing radiation.
Table I. The absence of mRad54 results in ionizing radiation hypersensitive embryos.
| Outgrowth of inner cell mass from day 3.5 embryos | ||
|---|---|---|
| Dose (Gy) | Genotype |
|
| mRAD54+/+ | mRAD54–/– | |
| 0 | 100% (3/3) | 93% (14/15) |
| 4 | 77% (10/13) | <7% (0/13) |
Quantitation of the results shown in Figure 1B. mRAD54-proficient and -deficient embryos were treated with an ionizing radiation dose of 0 or 4 Gy and cultured for 10 days. The percentage of embryos showing outgrowth of the inner cell mass was obtained by dividing the number of embryos showing outgrowth of the inner cell mass and the trophoblast cells by the number of embryos showing outgrowth of the trophoblast cells only. Absolute numbers are shown in parentheses.
Disruption of mRAD54 augments the ionizing radiation hypersensitivity of scid mice
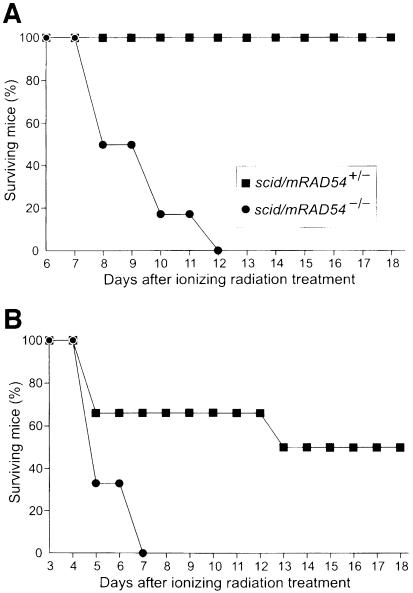
The lack of a severe ionizing radiation hypersensitivity of mRAD54–/– mice compared with mRAD54–/– ES cells could be explained if mRAD54 ceases to function after a certain developmental stage; either because its function is restricted to embryonic cells or because homologs of mRAD54 might be able to substitute for its function later during development (Dresser et al., 1997; Klein, 1997; Shinohara et al., 1997; Hiramoto et al., 1999). Alternatively, in contrast to the embryonic stage, the role of mRAD54-dependent DSB repair could be masked by the DNA end-joining pathway at later developmental stages. To discriminate between these possibilities, the ionizing radiation sensitivity of scid mice and scid/mRAD54–/– mice was compared. Consistent with previous observations (Biedermann et al., 1991), half of the scid/mRAD54+/– mice died after irradiation with 3 Gy, while all mice survived a dose of 2 Gy. In contrast, even at the low irradiation dose of 2 Gy all scid/mRAD54–/– mice died within 2 weeks (Figure 2). Therefore, scid/mRAD54–/– mice are extremely radiosensitive, even more so than Ku80–/– and ATM–/– mice (Barlow et al., 1996; Nussenzweig et al., 1997). We conclude that mRAD54 is functional in adult mice and that its role in radioprotection is masked by the DNA-PK-dependent DNA end-joining pathway.
Fig. 2. Ionizing radiation sensitivity of scid/mRAD54+/– and scid/mRAD54–/– mice. Shown are survival curves of two groups of six scid/mRAD54+/– and six scid/mRAD54–/– mice after irradiation with 2 Gy (A) and 3 Gy (B), respectively. Each curve represents three male and three female mice.
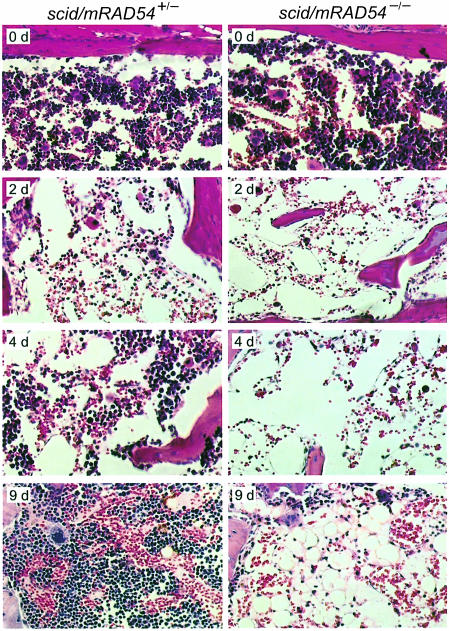
To determine whether the extreme radiosensitivity of the scid/mRAD54–/– mice was due to global radiation toxicity or to selective toxicity of specific organs, scid/mRAD54+/– and scid/mRAD54–/– mice were irradiated with 2 Gy and tissues were examined histologically at 0.12, 1, 2, 3, 4, 9 and 11 days post-irradiation. No histological abnormalities were found in the majority of tissues, including brain, heart, kidney, liver and lung. A complete list of tissues investigated can be found in Materials and methods. Moderate to severe radiation-induced damage in the form of apoptotic cells was detected in stomach, colon, jejunum, ileum and rectum. These tissues completely recovered from the radiation effects after 9 days, although the kinetics of recovery, as measured by bromodeoxy uridine (α–BrdU) incorporation, was delayed in the absence of mRAD54 (data not shown). In contrast, a dramatic difference in the effect of the irradiation on the bone marrow of scid/mRAD54+/– and scid/mRAD54–/– mice was observed (Figure 3). Two days after ionizing radiation exposure an equally severe depletion of cells in the bone marrow of mRAD54-proficient and -deficient mice was apparent. The cellularity of the bone marrow of scid/mRAD54+/– mice completely recovered, as was evident from the hyper-proliferation 9 days after the irradiation (Figure 3). In contrast, the bone marrow of scid/mRAD54–/– mice remained devoid of cells even at 11 days postirradiation. Similar effects on cellular depletion and lack of recovery to those observed in the bone marrow were seen in the spleen (data not shown). We conclude that scid/mRAD54–/– mice do not display a global radiation toxicity, rather their extreme radiosensitivity results from effects of bone marrow failure.
Fig. 3. Histological appearance of bone marrow after ionizing radiation exposure. Two-month-old scid/mRAD54+/– and scid/mRAD54–/– mice were irradiated with 2 Gy and euthanized at 0.12, 1, 2, 3, 4, 9 and 11 days postirradiation. Haematoxylin/eosin-stained sections of bone marrow before (day 0) and postirradiation (day 2, 4 and 9) are shown. Two days after irradiation (2 d), the depletion of cells seen in the bone marrow of mRAD54-proficient and –deficient scid mice was comparable. Four days after irradiation, the cellularity of the bone marrow of scid/mRAD54+/– mice was recovering (4 d). In contrast, no recovery was observed in the bone marrow of scid/mRAD54–/– mice (4 d and 9 d).
Adult mRAD54–/– mice are hypersensitive to mitomycin C
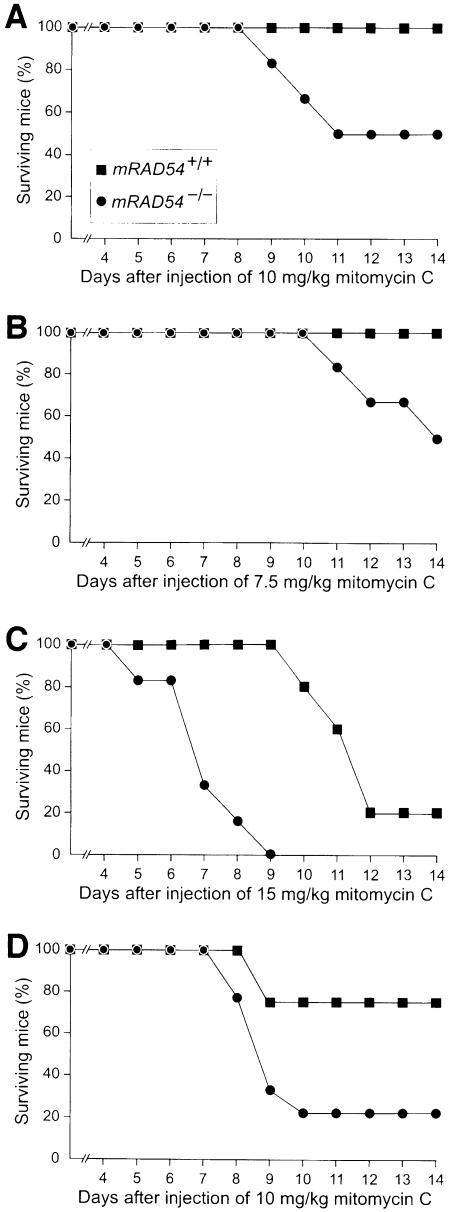
The demonstration that the contribution of mRAD54 towards repair of ionizing radiation-induced DNA damage is revealed in a scid background eliminates the possibility that mRAD54 expression is shut off at a certain developmental stage (Figure 2). Our observation generates the question of whether disruption of mRAD54 in an otherwise repair-proficient background causes hypersensitivity of adult mice towards DNA-damaging agents other than ionizing radiation. A critical difference between the homologous recombination-deficient mRAD54–/– ES cells and DNA end-joining-deficient scid cells is their sensitivity to mitomycin C. While mRAD54–/– ES cells are hypersensitive to mitomycin C, cells derived from scid mice are not (Biedermann et al., 1991; Hendrickson et al., 1991; Essers et al., 1997). Therefore, we investigated the mitomycin C sensitivity of mRAD54-proficient and –deficient mice. After peritoneal injection of 10 and 7.5 mg of mitomycin C per kg body weight, half of the mRAD54–/– female and male mice died within 2 weeks (Figure 4A and C). All mRAD54+/+ mice survived the treatment. Enhanced sensitivity and shorter latency periods in mRAD54–/– mice were seen at higher mitomycin C doses (Figure 4B and D). We conclude that mRAD54–/– mice are hypersensitive to mitomycin C.
Fig. 4. Mitomycin C sensitivity of mRAD54–/– mice. Shown are survival curves of mRAD54+/– and mRAD54–/– mice after a single intraperitoneal injection of the amount of mitomycin C indicated. (A) Survival curve of six mRAD54+/– female and six mRAD54–/– female mice after injection at day 0 with a dose of 10 mg/kg mitomycin C. (B) Survival curve of five mRAD54+/– female and six mRAD54–/– female mice after injection with 15 mg/kg mitomycin C. (C) Survival curve of eight mRAD54+/– male and nine mRAD54–/– male mice after injection with 7.5 mg/kg mitomycin C. (D) Survival curve of six mRAD54+/– male and eight mRAD54–/– male mice after injection with 10 mg/kg mitomycin C.
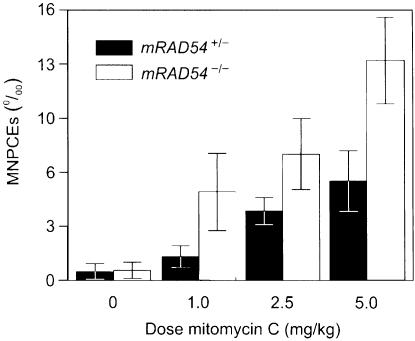
The bone marrow is a major target for mitomycin C-inflicted damage in vivo. Therefore, we tested whether mitomycin C treatment differentially affected cells in the blood of mRAD54+/– and mRAD54–/– mice using the peripheral blood micronucleus assay. The presence of micronuclei in polychromatic erythrocytes provides a measure of chromosomal aberrations. Two-month-old mRAD54–/– and mRAD54+/– mice were injected with 1.0, 2.5 or 5.0 mg/kg mitomycin C. This treatment resulted in dose-related increases in the frequency of micronuclei-containing polychromatic erythrocytes (Figure 5). Before the mitomycin C treatment micronuclei-containing polychromatic erythrocyte levels were similar in mRAD54–/– and mRAD54+/– mice. Consistent with the mitomycin C hypersensitivity of mRAD54–/– mice, the crosslinking agent induced significantly higher levels of micronucleicontaining polychromatic erythrocytes in mRAD54–/– mice compared with mRAD54+/– mice (Figure 5).
Fig. 5. Induction of micronuclei by mitomycin C in polychromatic erythrocytes. mRAD54+/– and mRAD54–/– mice were intraperitoneally injected with a single dose of 1.0, 2.5 and 5.0 mg/kg bodyweight mitomycin C. At 24 h before and 48 h after mitomycin C treatment, 25 μl of peripheral blood were collected by orbita puncture. For each animal, 1000 polychromatic erythrocytes were observed and the number of cells with micronuclei was recorded. Plotted are the number of micronuclei-containing polychromatic erythrocytes (MNPCEs) per 1000 polychromatic erythrocytes. Data points represent an average from four independently treated animals. The standard error of the mean is indicated.
Overlapping and specialized roles of homologous recombination and DNA end-joining in mice
Our results show that mRAD54-mediated homologous recombination and DNA-PK-mediated DNA end-joining have overlapping roles in providing ionizing radiation resistance in mice. At the cellular level it has been shown that both DSB repair pathways contribute to repair of ionizing radiation-induced DNA damage in a specialized immunological cell-type derived from the chicken (Takata et al., 1998). For metazoan embryos similar results have been reported using D.melanogaster (Kooistra et al., 1999). The pivotal insight gained from the study presented here is that in mammals the relative contribution of the two major DSB repair pathways, homologous recombination and DNA end-joining, changes during development of the animal.
While homologous recombination provides protection against ionizing radiation-induced DNA damage in embryos its contribution in adults is not detected, unless DNA end-joining is disabled (Figures 1 and 2). In this regard the mouse embryo resembles S.cerevisiae cells (Siede et al., 1996). We consider three possible reasons for these findings. First, the major contribution of homologous recombination to ionizing radiation resistance early in mouse development, as opposed to in the adult animal, might be due to the greater efficiency of homologous recombination in rapidly dividing cells because of the availability of the sister chromatid as a repair template in the S and/or G2 phases of the cell cycle (Sonoda et al., 1999) or because the presence of active components of the DNA replication machinery is necessary to complete homologous recombination efficiently. A second reason for a preference of homologous recombination over DNA end-joining for repair of a DSB might be found in the difference in repair fidelity between the pathways. Accurate repair, ensured by homologous recombination but not by DNA end-joining, might be more important for cells early in development compared with terminally differentiated somatic cells. Inaccurate repair could be more easily tolerated by differentiated somatic cells because a large fraction of their genome is no longer functional. For a similar reason homologous recombination could be the preferred mechanism of DSB repair in a unicellular organism such as S.cerevisiae because most of its genome contains coding information.
A third reason for the existence of at least two separate DSB repair pathways is their specialized function. Although their functions can overlap for the processing of certain types of DNA damages such as those produced by ionizing radiation, they also have pathway-specific functions. DNA end-joining is involved in processing the DSB intermediates required for proper immunoglobulin and T–cell receptor gene expression (Critchlow and Jackson, 1998; Jeggo, 1998; Lieber, 1999), whereas mRAD54-mediated homologous recombination is not (Essers et al., 1997). In contrast, our experiments show that mRAD54 protects adult mice from the deleterious effects of the interstrand DNA crosslinking agent mitomycin C (Figures 4 and 5), while experiments with cells derived from scid mice suggest that DNA-PKcs does not appear to contribute to the repair of mitomycin C-induced DNA damage (Biedermann et al., 1991; Hendrickson et al., 1991). The underlying reason for these observations might be the greater versatility of homologous recombination compared with DNA end-joining. DNA end-joining has evolved to deal specifically with DSBs. Homologous recombination on the other hand can also repair DNA lesions that do not necessarily involve a DSB intermediate, as is thought to be the case during interstrand DNA crosslink repair in Escherichia coli (Friedberg et al., 1995). Although some interstrand DNA crosslinks are processed into DSBs (Vock et al., 1998), it is not certain whether this is the case for a mitomycin C-induced interstrand DNA crosslink. Even if it was processed into a DSB, the DNA end-joining pathway will not be optimally suited for its repair. While homologous recombination can bypass a crosslink starting from a single DSB to one side of the crosslink, DNA end-joining cannot. The latter pathway will require more or less simultaneously produced DSBs on either side of the crosslink before it can be repaired in an error-prone manner.
In summary, we have demonstrated that mRAD54–/– mice are not hypersensitive to ionizing radiation, while mRAD54–/– embryos are. However, at the adult stage mRAD54 deficiency exacerbates the ionizing radiation sensitivity of scid mice. In contrast, regardless of developmental stage, mRAD54–/– mice are hypersensitive to mitomycin C. These results suggest that the relative contribution of two major DSB repair pathways, homolo– gous recombination and DNA end-joining, can differ depending on mammalian developmental stage (i.e. cell type) and on the specific type of DNA damage.
Materials and methods
Cell culture
ES cells, isolated from a 129 mouse strain, were cultured and electro– porated as described (Weeda et al., 1997). To isolate de novo C57Bl6/129 ES cells (Hogan et al., 1994) mRAD54–/– blastocysts were isolated from a C57Bl6/129 mRAD54–/– cross. These mRAD54–/– mice were littermates from an mRAD54+/– cross, obtained by backcross three of an mRAD54 chimera to C57Bl6 mice. The PGK-hRAD54 construct (Swagemakers et al., 1998) was electroporated into C57Bl6/129 mRAD54–/– ES line mRAD54307neo/neo (Essers et al., 1997). Both disrupted mRAD54 alleles in this line contain the neomycin selectable marker gene. The PGK-hRAD54 construct was co-electroporated with a plasmid carrying a puromycin selectable marker. Puromycin resistant clones were isolated and screened for hRad54 expression by immunoblot analysis as described (Essers et al., 1997; Swagemakers et al., 1998).
Generation of scid/mRAD54+/– and scid/mRAD54–/– mice
mRAD54+/– mice (backcross three of a 129 mRAD54 chimera to C57Bl6 mice; Essers et al., 1997) were bred with homozygous mutant C.B.–17 scid mice (Jackson Laboratories) to obtain F1 mRAD54+/–/scid+/– offspring. The scid mutation was fixed by crossbreeding F1 animals. Homozygosity for the scid mutation was confirmed by determining the level of the IgM subclass in the serum as described (Essers et al., 1997). mRAD54 status was determined by PCR analysis of tail DNA.
DNA damage sensitivity assays
The sensitivity of ES cells to increasing doses of ionizing radiation was determined by measuring their colony-forming ability after irradiation with a 137Cs source (Essers et al., 1997). To determine the ionizing radiation sensitivity of mice, 2- to 4–month-old animals were exposed to ionizing radiation from a 137Cs source. Single doses between 2 and 8 Gy were used. Ionizing radiation sensitivity of day 3.5 embryos was determined as described (Sharan et al., 1997; Luo et al., 1999). To determine the mitomycin C sensitivity of mice, 2–month-old mRAD54+/+ and mRAD54–/– animals were injected intraperitoneally with 7.5, 10 or 15 mg/kg bodyweight mitomycin C. After exposure to ionizing radiation or mitomycin C, mice were kept in sterile isolators and observed for 28 and 14 days, respectively. After these periods surviving animals were euthanized. In none of the assays employed to date was a difference observed between mRAD54+/+ and mRAD54+/– cells and mice (data not shown).
Micronucleus assay
Two-month-old mRAD54+/– and mRAD54–/– animals were injected intraperitoneally with 1.0, 2.5 or 5.0 mg/kg bodyweight mitomycin C. At 24 h before and 48 h after mitomycin C treatment, 25 μl of peripheral blood were collected by orbita puncture. The blood was placed on an acridine orange-coated glass slide, covered with a coverslip, and allowed to stain (Hayashi et al., 1990). Erythrocytes with a red fluorescing reticulum in the cytoplasm were observed. One thousand erythrocytes were observed per animal by fluorescence microscopy within a few days of slide preparation. The number of cells with micronuclei displaying greenish yellow fluorescence was recorded.
Pathological analysis
Collected tissues were fixed in 10% buffered formalin, embedded in paraffin blocks, sectioned, and stained using standard methods, including haematoxylin/eosin staining and α–BrdU immunohistochemistry. Sections were examined and photographed using a light microscope. After irradiation with 2 Gy no histological abnormalities were found in adrenal gland, bone, bladder, brain, cartilage, heart, kidney, liver, lung, pancreas, pituitary gland, prostate, thyroid, tongue, salivary gland, seminal vesicles, skeletal muscle, skin and smooth muscle.
Acknowledgments
Acknowledgements
We thank C.van Oostrom, M.Boeve and B.Hoebee for assistance with the experiments shown in Figures 4 and 5. This research was supported by grants from the Dutch Cancer Society (KWF), the Netherlands Organization for Scientific Research (NWO) and the Human Frontier Science Program Organization. R.K. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
References
- Barlow C., et al. (1996)Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell, 86, 159–171. [DOI] [PubMed] [Google Scholar]
- Baumann P. and West, S.C. (1998) Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci., 23, 247–251. [DOI] [PubMed] [Google Scholar]
- Baumann P., Benson, F.E. and West, S.C. (1996) Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell, 87, 757–766. [DOI] [PubMed] [Google Scholar]
- Benson F.E., Baumann, P. and West, S.C. (1998) Synergistic actions of Rad51 and Rad52 in recombination and DNA repair. Nature, 391, 401–404. [DOI] [PubMed] [Google Scholar]
- Bianco P.R., Tracy, R.B. and Kowalczykowski, S.C. (1998) DNA strand exchange proteins: a biochemical and physical comparison. Front. Biosci., 3, 570–603. [DOI] [PubMed] [Google Scholar]
- Biedermann K.A., Sun, J.R., Giaccia, A.J., Tosto, L.M. and Brown, J.M. (1991) scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc. Natl Acad. Sci. USA, 88, 1394–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt T., et al. (1995)Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell, 80, 813–823. [DOI] [PubMed] [Google Scholar]
- Cox M.M. (1998) A broadening view of recombinational DNA repair in bacteria. Genes Cells, 3, 65–78. [DOI] [PubMed] [Google Scholar]
- Critchlow S.E. and Jackson, S.P. (1998) DNA end-joining: from yeast to man. Trends Biochem. Sci., 23, 394–398. [DOI] [PubMed] [Google Scholar]
- Dresser M.E., Ewing, D.J., Conrad, M.N., Dominguez, A.M., Barstead, R., Jiang, H. and Kodadek, T. (1997) DMC1 functions in a Saccharomyces cerevisiae meiotic pathway that is largely independent of the RAD51 pathway. Genetics, 147, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J., Hendriks, R.W., Swagemakers, S.M.A., Troelstra, C., de Wit, J., Bootsma, D., Hoeijmakers, J.H.J. and Kanaar, R. (1997) Disruption of mouse RAD54 reduces ionizing radiation resistance and homologous recombination. Cell, 89, 195–204. [DOI] [PubMed] [Google Scholar]
- Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. American Society for Microbiology, Washington, DC. [Google Scholar]
- Fulop G.M. and Phillips, R.A. (1990) The scid mutation in mice causes a general defect in DNA repair. Nature, 347, 479–482. [DOI] [PubMed] [Google Scholar]
- Game J.C. (1993) DNA double-strand breaks and the RAD50–RAD57 genes in Saccharomyces. Semin. Cancer Biol., 4, 73–83. [PubMed] [Google Scholar]
- Gao Y., Chaudhuri, J., Zhu, C., Davidson, L., Weaver, D.T. and Alt, F.W. (1998) A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity, 9, 367–376. [DOI] [PubMed] [Google Scholar]
- Haber J.E. (1999) DNA recombination: the replication connection. Trends Biochem. Sci., 24, 271–275. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Morita, T., Kodama, Y., Sofuni, T. and Ishidate, M. (1990) The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res., 245, 245–249. [DOI] [PubMed] [Google Scholar]
- Hendrickson E.A., Qin, X.-Q., Bump, E.A., Schatz, D.G., Oettinger, M. and Weaver, D.T. (1991) A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc. Natl Acad. Sci. USA, 88, 4061–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto T., et al. (1999)Mutations of a novel human RAD54 homologue, RAD54B, in primary cancer. Oncogene, 18, 3422–3426. [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington,R., Constantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Jeggo P.A. (1998) DNA breakage and repair. Adv. Genet., 38, 185–218. [DOI] [PubMed] [Google Scholar]
- Kanaar R., Hoeijmakers, J.H.J., van Gent, D.C. (1998) Molecular mechanisms of DNA double strand break repair. Trends Cell Biol., 8, 483–489. [DOI] [PubMed] [Google Scholar]
- Kirchgessner C.U., Patil, C.K., Evans, J.W., Cuomo, C.A., Fried, L.M., Carter, T., Oettinger, M.A. and Brown, J.M. (1995) DNA-dependent protein kinase (p350) as a candidate gene for the murine SCID defect. Science, 267, 1178–1183. [DOI] [PubMed] [Google Scholar]
- Klein H.L. (1997) RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics, 147, 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T. (1997) Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol. Mol. Biol. Rev., 61, 212–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra R., Pastink, A., Zonneveld, J.B., Lohman, P.H. and Eeken, J.C. (1999) The Drosophila melanogaster DmRAD54 gene plays a crucial role in double-strand break repair after P-element excision and acts synergistically with Ku70 in the repair of X-ray damage. Mol. Cell. Biol., 19, 6269–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M.R. (1999) The biochemistry and biological significance of nonhomologous DNA end joining: an essential repair process in multicellular eukaryotes. Genes Cells, 4, 77–85. [DOI] [PubMed] [Google Scholar]
- Lim D.-S. and Hasty, P. (1996) A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol., 16, 7133–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Yao, M.S., Bender, C.F., Mills, M., Bladl, A.R., Bradley, A. and Petrini, J.H. (1999) Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc. Natl Acad. Sci. USA, 96, 7376–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen U.H., Bendixen, C., Sunjevaric, I. and Rothstein, R. (1996) DNA strand annealing is promoted by the yeast Rad52 protein. Proc. Natl Acad. Sci. USA, 93, 10729–10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan M.E., Chiu, J.W., Koller, B.H. and Jasin, M. (1999) Brca1 controls homology-directed DNA repair. Mol. Cell, 4, 511–518. [DOI] [PubMed] [Google Scholar]
- New J.H., Sugiyama, T., Zaitseva, E. and Kowalczykowski, S.C. (1998) Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature, 391, 407–410. [DOI] [PubMed] [Google Scholar]
- Nussenzweig A., Sokol, K., Burgman, P., Li, L. and Li, G.C. (1997) Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: the effects of ionizing radiation on growth, survival, and development. Proc. Natl Acad. Sci. USA, 94, 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques F. and Haber, J.E. (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev., 63, 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S.R., Kurimasa, A., Oshimura, M., Dynan, W.S., Bradbury, E.M. and Chen, D.J. (1995) Loss of the catalytic subunit of the DNA-dependent protein kinase in DNA double-strand-break-repair mutant mammalian cells. Proc. Natl Acad. Sci. USA, 92, 3171–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T.D., Malone,R.E. and Symington,L.S. (1991) Recombination in yeast. In Broach,J.R., Pringle,J.R. and Jones,E.W. (eds), The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 407–521. [Google Scholar]
- Petrini J.H.J., Bressan, D.A. and Yao, M.S. (1997) The RAD52 epistasis group in mammalian double strand break repair. Semin. Immunol., 9, 181–188. [DOI] [PubMed] [Google Scholar]
- Petukhova G., Stratton, S. and Sung, P. (1998) Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature, 393, 91–94. [DOI] [PubMed] [Google Scholar]
- Petukhova G., Van Komen, S., Vergano, S., Klein, H. and Sung, P. (1999) Yeast Rad54 promotes Rad51-dependent homologous DNA pairing via ATP hydrolysis-driven change in DNA double helix conformation. J. Biol. Chem., 274, 29453–29462. [DOI] [PubMed] [Google Scholar]
- Reddy G., Golub, E.I. and Radding, C.M. (1997) Human Rad52 protein promotes single-strand DNA annealing followed by branch migration. Mutat. Res., 377, 53–59. [DOI] [PubMed] [Google Scholar]
- Schuler W. and Bosma, M.J. (1989) Nature of the scid defect: a defective VDJ recombinase system. Curr. Top. Microbiol. Immunol., 152, 55–62. [DOI] [PubMed] [Google Scholar]
- Sharan S.K., Morimatsu, M., Albrecht, U., Lim, D.-S., Regel, E., Dinh, C., Sands, A., Eichele, G., Hasty, P. and Bradley, A. (1997) Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature, 386, 804–810. [DOI] [PubMed] [Google Scholar]
- Shinohara A. and Ogawa, T. (1998) Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature, 391, 404–407. [DOI] [PubMed] [Google Scholar]
- Shinohara M., Shita-Yamaguchi, E., Buerstedde, J.-M., Shinagawa, H., Ogawa, H. and Shinohara, A. (1997) Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics, 147, 1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siede W., Friedl, A.A., Dianova, I., Eckardt-Schupp, F. and Friedberg, E.C. (1996) The Saccharomyces cerevisiae Ku autoantigen homologue affects radiosensitivity only in the absence of homologous recombination. Genetics, 142, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.C. and Jackson, S.P. (1999) The DNA-dependent protein kinase. Genes Dev., 13, 916–934. [DOI] [PubMed] [Google Scholar]
- Smith K.N. and Nicolas, A. (1998) Recombination at work for meiosis. Curr. Opin. Genet. Dev., 8, 200–211. [DOI] [PubMed] [Google Scholar]
- Sonoda E., Sasaki, M.S., Morrison, C., Yamaguchi-Iwai, Y., Takata, M. and Takeda, S. (1999) Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol., 19, 5166–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., New, J.H. and Kowalczykowski, S.C. (1998) DNA annealing by Rad52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc. Natl Acad. Sci. USA, 95, 6049–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P. (1994) Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science, 265, 1241–1243. [DOI] [PubMed] [Google Scholar]
- Sung P. (1997) Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem., 272, 28194–28197. [DOI] [PubMed] [Google Scholar]
- Swagemakers S.M.A., Essers, J., de Wit, J., Hoeijmakers, J.H.J. and Kanaar, R. (1998) The human Rad54 recombinational DNA repair protein is a double-stranded DNA-dependent ATPase. J. Biol. Chem., 273, 28292–28297. [DOI] [PubMed] [Google Scholar]
- Takata M., et al. (1998)Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J., 17, 5497–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.L.R., Essers, J., Citterio, E., Swagemakers, S.M.A., de Wit, J., Benson, F.E., Hoeijmakers, J.H.J. and Kanaar, R. (1999) Mouse Rad54 affects DNA conformation and DNA-damage-induced Rad51 foci formation. Curr. Biol., 9, 325–328. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y. and Ikeda, H. (1998) Double-strand break repair mediated by DNA end-joining. Genes Cells, 3, 135–144. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T., Fujii, Y., Sakumi, K., Tominaga, Y., Nakao, K., Sekiguchi, M., Matsushiro, A., Yoshimura, Y. and Morita, T. (1996) Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl Acad. Sci. USA, 93, 6236–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vock E.H., Lutz,W.K., Hormes,P., Hoffmann,H.D. and Vamvakas,S. (1998) Discrimination between genotoxicity and cytotoxicity in the induction of DNA double-strand breaks in cells treated with etopo– side, melphalan, cisplatin, potassium cyanide, Triton X-100, and γ-irradiation. Mutat. Res., 413, 83–94. [DOI] [PubMed] [Google Scholar]
- Weeda G., Donker, I., de Wit, J., Morreau, H., Janssens, R., Vissers, C.J., Nigg, A., van Steeg, H., Bootsma, D. and Hoeijmakers, J.H.J. (1997) Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr. Biol., 7, 427–439. [DOI] [PubMed] [Google Scholar]
- Zhang H., Tombline, G. and Weber, B.L. (1998) BRCA1, BRCA2, and DNA damage response: collision or collusion? Cell, 92, 433–436. [DOI] [PubMed] [Google Scholar]