Abstract
Tumor cells require a constant supply of macromolecular precursors, and interrupting this supply has been proposed as a therapeutic strategy in cancer. Precursors for lipids, nucleic acids, and proteins are generated in the tricarboxylic acid (TCA) cycle and removed from the mitochondria to participate in biosynthetic reactions. Refilling the pool of precursor molecules (anaplerosis) is therefore crucial to maintain cell growth. Many tumor cells use glutamine to feed anaplerosis. Here we studied how “glutamine-addicted” cells react to interruptions of glutamine metabolism. Silencing of glutaminase (GLS), which catalyzes the first step in glutamine-dependent anaplerosis, suppressed but did not eliminate the growth of glioblastoma cells in culture and in vivo. Profiling metabolic fluxes in GLS-suppressed cells revealed induction of a compensatory anaplerotic mechanism catalyzed by pyruvate carboxylase (PC), allowing the cells to use glucose-derived pyruvate rather than glutamine for anaplerosis. Although PC was dispensable when glutamine was available, forcing cells to adapt to low-glutamine conditions rendered them absolutely dependent on PC for growth. Furthermore, in other cell lines, measuring PC activity in nutrient-replete conditions predicted dependence on specific anaplerotic enzymes. Cells with high PC activity were resistant to GLS silencing and did not require glutamine for survival or growth, but displayed suppressed growth when PC was silenced. Thus, PC-mediated, glucose-dependent anaplerosis allows cells to achieve glutamine independence. Induction of PC during chronic suppression of glutamine metabolism may prove to be a mechanism of resistance to therapies targeting glutaminolysis.
Keywords: cancer metabolism, Warburg effect, metabolic flux analysis, metabolomics
Among the many biological characteristics of malignant transformation, a reprogramming of metabolic activity to support dysregulated cell growth is vital (1, 2). The energy and biomass required for cell growth are provided by enhanced uptake of nutrients like glucose and glutamine, both of which are consumed avidly by tumor cells in vivo. Glycolysis, historically the most widely studied hallmark of cancer metabolism (3), provides energy and some essential biosynthetic precursor molecules. Glutamine provides a major substrate for respiration (4) as well as nitrogen for the production of proteins, hexosamines, and macromolecules. The notion of targeting glucose and glutamine metabolism in cancer, originally rationalized by the number of pathways fed by these two nutrients, has been reinforced by more recent studies demonstrating that metabolism of both nutrients is regulated by oncogenes (5). These findings have begun to unify the metabolic and molecular bases of malignant transformation.
Producing macromolecular precursors is an essential component of cell growth. Many of the precursors used to synthesize lipids, nonessential amino acids, and nucleotides are generated within the mitochondrial tricarboxylic acid (TCA) cycle. If these intermediates are withdrawn to feed biosynthetic pathways, the cycle would cease to function unless additional pathways are engaged to supply oxaloacetate (OAA), the molecule whose condensation with acetyl-CoA forms the cycle's canonical entry point. These OAA-generating pathways are termed anaplerosis (6). An active anaplerotic flux enables levels of TCA cycle intermediates to remain constant during the continuous efflux of biosynthetic precursors for growth.
Because anaplerosis contributes specifically to the production of biomass during tumor cell proliferation, anaplerotic pathways are compelling therapeutic targets in cancer. However, it is unclear in general which carbon sources feed these pathways and how much flexibility exists to allow cancer cells to choose among potential anaplerotic precursors. In principle, glucose and glutamine are excellent anaplerotic nutrients because both are abundant and both can be converted to OAA without first entering the TCA cycle as acetyl-CoA. Glutamine is the preferred anaplerotic precursor in some transformed cell lines, contributing up to 90% of the OAA pool (7, 8). Depriving such cells of glutamine leads to depletion of TCA cycle intermediates and growth failure (9, 10). The oncogene c-myc exacerbates glutamine dependence and stimulates expression of GLS, the enzyme that converts glutamine to glutamate in the first step of glutamine-dependent anaplerosis (9–11). These studies provided the first mechanistic link between an oncogene and regulation of anaplerosis. GLS in particular has been proposed as a therapeutic target in cancer, because antisense and chemical inhibition of GLS reduces cell growth, transformation, and tumorigenesis in various models (12, 13). By contrast, even though the vast majority of tumor cell lines consume glucose more rapidly than any other nutrient, only a few studies have implicated glucose as an anaplerotic precursor for tumors (14).
Here we used stable isotope tracing and metabolic flux analysis to perform a systematic study of the effects of GLS suppression on intermediary metabolism and growth in tumor cells. The work uncovered a compensatory relationship between GLS and PC, the enzyme that allows glucose-derived pyruvate to serve as an anaplerotic precursor. The findings show that glucose-dependent anaplerosis provides an alternative to glutamine dependence and they uncover a mechanism of resistance to inhibition of glutamine metabolism.
Results
Targeting GLS Limits Growth of Glioblastoma Cells in Culture and in Vivo.
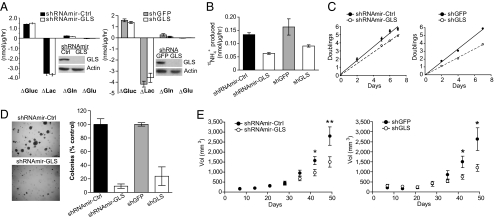
To test whether GLS activity is required for glioblastoma cell growth, two independent shRNAs were expressed in LN229 and SF188 cells (Fig. 1 and SI Appendix, Fig. S2). We chose these cell lines because both use glutamine as the preferred anaplerotic precursor when given access to a physiological mixture of nutrients. Furthermore neither cell line expresses appreciable amounts of the glutaminase isoform GLS2 (SI Appendix, Fig. S1), increasing the likelihood of reducing cell growth by targeting GLS. Suppressing GLS expression modestly reduced net glutamine utilization without affecting glucose consumption, lactate production, or the utilization of other amino acids (Fig. 1A and SI Appendix, Fig. S2 and Table S1). GLS silencing reduced alanine secretion, presumably because of a decrease in intracellular glutamate available for transamination (SI Appendix, Table S1). Release of glutamine's γ nitrogen as NH4+, an indicator of GLS flux, was suppressed during GLS silencing (Fig. 1B). GLS suppression also reduced cell proliferation but not cell-cycle distribution (Fig. 1C and SI Appendix, Fig. S3). Anchorage-independent growth (Fig. 1D) and growth of s.c. xenografts (Fig. 1E and SI Appendix, Fig. S2B) were both reduced in cells with GLS suppression.
Fig. 1.
Glutaminase is required for maximal growth of glioblastoma cells in culture and in vivo. (A) Effect of GLS knockdown on nutrient utilization and metabolite secretion in LN229 cells. Control (Ctrl) or GLS-targeting shRNAmirs (Left) and shRNAs (Right) were tested. Positive values reflect utilization and negative values reflect secretion. Data were averaged over 7 h. (B) GLS flux (production of 15NH4+ from L-[γ-15N]-glutamine) was determined for each cell line over 7 h. Data are the average ± SD of three independent cultures. (C) Cell growth in 2D culture. Each time point is the average ± SD of three parallel cultures. (D) Colony formation in soft agar. Data are the average ± SD of three independent wells. (E) Growth of s.c. xenografts. n = 9 tumors of each cell line for shRNAmirs (Left), and 4 tumors of each cell line for shRNAs (Right). Average volume ± SE is shown for each cell line. *P < 0.05, **P < 0.005. Gluc, glucose; Lac, lactate; Gln, glutamine; Glu, glutamate.
GLS Suppression Is Accompanied by an Increase in Glucose-Dependent Anaplerosis by Pyruvate Carboxylase.
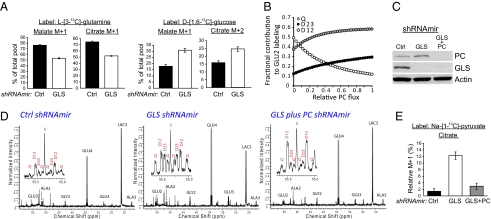
The residual growth of GLS-deficient cells prompted us to identify alternative pathways that might compensate for the reduction in glutamine-dependent anaplerosis. GLS silencing caused a modest reduction in the contribution of glutamine-derived carbon into lipids, whereas glucose-dependent lipid synthesis was marginally increased (SI Appendix, Fig. S4A). GLS suppression also reduced labeling of the TCA cycle intermediates malate and citrate with 13C originating on glutamine (Fig. 2A, Left and SI Appendix, Fig. S4B). To identify alternative nutrients that supply these pools, we cultured cells with D-[1,6-13C]-glucose and used mass spectrometry to analyze malate and citrate. Both intermediates contained more glucose-derived carbon when GLS was silenced (Fig. 2A, Right and SI Appendix, Fig. S4C). The fraction of the citrate pool containing two additional mass units (citrate m+2) increased by over 50% in GLS-suppressed LN229 cells.
Fig. 2.
Glutaminase silencing increases glucose-dependent anaplerosis through pyruvate carboxylase (PC). (A) Gas chromatography/mass spectrometry was used to measure enrichment of citrate and malate in cells cultured with L-[3-13C]-glutamine (Left) and D-[1,6-13C]-glucose (Right). Results are from a representative experiment with duplicate 7-h cultures. (B) Predicted effect of increasing PC flux on the fractional contribution of the quartet (Q), 2,3 doublet (D23), and 1,2 doublet (D12) to overall labeling in glutamate C2 (GLU2), during culture with D-[U-13C]-glucose. The data were generated using the modeling software tcaSIM assuming an active TCA cycle, 95% enrichment of pyruvate, and active anaplerosis. The simulation was performed for 35 turns of the TCA cycle. (C) Western blots of LN229 cells expressing a control shRNAmir, an shRNAmir directed against GLS, or shRNAmirs against both GLS and PC. (D) NMR spectroscopy of metabolites extracted from the cell lines derived in C. Cells were cultured with 10 mM D-[U-13C]-glucose and 4 mM unlabeled glutamine for 8 h. Insets highlight the GLU2 multiplet. Splitting of individual peaks is the result of long-range coupling. (E) Labeling of citrate from [1-13C]-pyruvate in the same three cell lines. The percentage of citrate m+1 relative to an unlabeled standard (where m+1 = 0%) is plotted on the y axis. Each culture contained 5 mM Na-[1-13C]-pyruvate, 4 mM glutamine, and no glucose. Data are the average ± SD of three 7-h cultures. Glu, glutamate; Ala, alanine; Gly, glycine; Lac, lactate; S, singlet.
There are multiple potential explanations for the increase in citrate m+2. The simplest is that more citrate molecules containing a single 13C from glucose-derived acetyl-CoA (citrate m+1) are retained in the cycle, yielding a larger fraction of OAA m+1 and increasing the likelihood of condensing a labeled OAA with a labeled acetyl-CoA (SI Appendix, Fig. S5). This is plausible because glutamine is normally the major source of OAA (7), and reducing GLS flux is predicted to reduce the overall production of unlabeled OAA. An equally plausible alternative is that GLS suppression is accompanied by increased activity of PC, which produces OAA directly from glucose-derived pyruvate and yields citrate m+2 after this OAA m+1 condenses with glucose-derived acetyl-CoA m+1 (SI Appendix, Fig. S5). Because mass spectrometry cannot easily distinguish between these two possibilities, we used the program tcaSIM to simulate the effects of varying glutamine-dependent anaplerosis and PC flux on 13C isotopomer formation (15). Increasing PC flux is predicted to enhance the fractional contribution of the doublet of doublets (or quartet, Q) and 2-3 doublet (D23) at glutamate carbon 2 (GLU2) if cells are cultured in D-[U-13C]-glucose (Fig. 2B; detailed labeling scheme in SI Appendix, Fig. S6). By contrast, a simple reduction in anaplerosis with no change in PC activity suppresses D23 (SI Appendix, Fig. S7). This distinction yields a quantitative marker of relative PC flux that can be monitored by NMR. LN229-derived cell lines were generated, which contained suppression of GLS alone or of both GLS and PC (Fig. 2C), and these cells were cultured in D-[U-13C]-glucose. NMR analysis of glutamate isotopomers (16) revealed that the overall anaplerotic flux fell by ∼45% when GLS was suppressed, but still accounted for a substantial fraction of overall TCA cycle activity (SI Appendix, Table S2). Inspection of GLU2 revealed an enhancement of both Q and D23 in the GLS-suppressed cells, and these resonances returned to normal if PC was also suppressed (Fig. 2D), consistent with enhanced PC flux and glucose-dependent anaplerosis during GLS suppression.
As an independent test of PC activity, cells were cultured with [1-13C]-pyruvate and metabolites were analyzed by mass spectrometry. Pyruvate C1 is released as CO2 by pyruvate dehydrogenase, but is retained if pyruvate enters the TCA cycle via PC (SI Appendix, Fig. S8). GLS suppression was associated with a sixfold increase in 13C transfer from [1-13C]-pyruvate into citrate, and this was reversed if PC was also suppressed (Fig. 2E). Together, the data indicate that chronic GLS suppression causes an increase in PC activity, partially offsetting the reduction of glutamine-dependent anaplerosis.
PC Is Required for Cell Growth During Glutamine Deprivation.
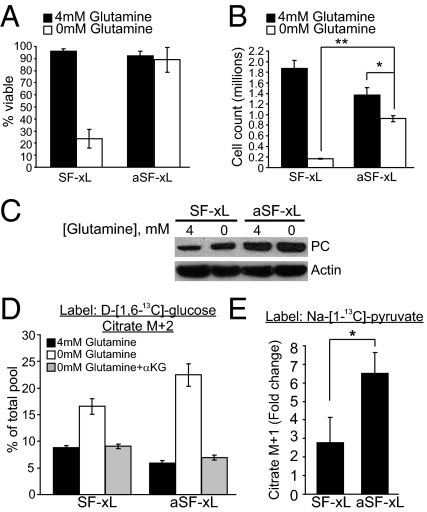
We next tested whether PC was necessary to support growth when glutamine metabolism was impaired. SF188 glioblastoma cells are “addicted” to glutamine for survival and growth (10). We previously used SF188-derived cells overexpressing Bcl-xL (SF-xL) to examine the effects of glucose deprivation and Akt inhibition on intermediary metabolism (17). These cells retain the glutamine-addicted phenotype and lose viability when deprived of glutamine for several days (Fig. 3 A and B). Consistent with observations from other studies (9, 18), glutamine-deprived cells had reduced pools of glutamate and TCA cycle intermediates and modestly increased cellular ATP content (SI Appendix, Fig. S9). Addition of either glutamate or dimethyl-αKG completely rescued cell viability and partially rescued growth in glutamine-deprived cells (SI Appendix, Fig. S10 A and B). We also tested whether other amino acids normally absent in DMEM could be used for anaplerosis. Proline, aspartate, and asparagine were supplemented to DMEM containing L-[U-13C]-glutamine, and metabolites were analyzed by GC/MS. Addition of these amino acids did not substantially alter labeling in TCA cycle intermediates (SI Appendix, Fig. S10C), demonstrating that none of them were metabolized in the TCA cycle to an appreciable extent. Furthermore, none of these amino acids, individually or in combination, could rescue cell growth in the absence of glutamine (SI Appendix, Fig. S10D).
Fig. 3.
PC activity is induced during adaptation to low-glutamine conditions. (A and B) Viability and growth of parental SF-xL glioblastoma cells and cells adapted to low glutamine (aSF-xL), under conditions of glutamine abundance or deprivation. Data are the average ± SD of three independent cultures of 2 × 105 cells grown in the presence or absence of glutamine for 72 h. (C) PC protein expression in parental SF-xL cells and aSF-xL cells. The parental cells were withdrawn from glutamine for 7 h, and the adapted cells were given glutamine-replete medium for 7 h. (D) Abundance of citrate m+2 in parental and adapted cells, in the presence and absence of glutamine and 4 mM dimethyl α-KG for 7 h. (E) Increased transfer of 13C from [1-13C]-pyruvate to citrate in adapted cells. Each culture contained 5 mM Na-[1-13C]-pyruvate and no glucose. The ratio shows the fold change between glutamine-deprived (0 mM) and glutamine-replete (4 mM) conditions during a 7-h experiment. The final citrate m+1 fraction in glutamine-deprived parental and aSF-xL cells were 11% and 16.5%, respectively. *P < 0.05, **P < 0.005.
By gradually adapting SF-xL cells to growth in progressively lower glutamine concentrations, we obtained a subline that maintained viability in 0 mM glutamine (Fig. 3A), providing an opportunity to study glutamine-independent metabolism in the absence of cell death. Unlike the parental cells the adapted cells proliferated in the absence of glutamine, although their growth could still be stimulated modestly by reintroducing glutamine (Fig. 3B). These cells had higher PC protein expression than the parental cells (Fig. 3C), but the mRNA levels did not differ significantly (SI Appendix, Fig. S11A). Although acute glutamine deprivation did not affect glucose consumption in either the parental or the adapted cells (SI Appendix, Fig. S11B), it increased the abundance of citrate m+2 when either cell line was cultured with D-[1,6-13C]-glucose, and the relative increase was higher in the adapted cells (Fig. 3D). Addition of dimethyl-αKG suppressed citrate m+2 to normal levels in both cell lines (Fig. 3D). Incorporation of pyruvate C1 into citrate was also enhanced in both cell lines when glutamine was acutely deprived, but the relative enhancement was higher in the adapted cells (Fig. 3E), paralleling the abundance of PC protein. Together these data demonstrate that glutamine deprivation induces an increase in the abundance of PC protein and the extent to which glucose/pyruvate is used for anaplerosis.
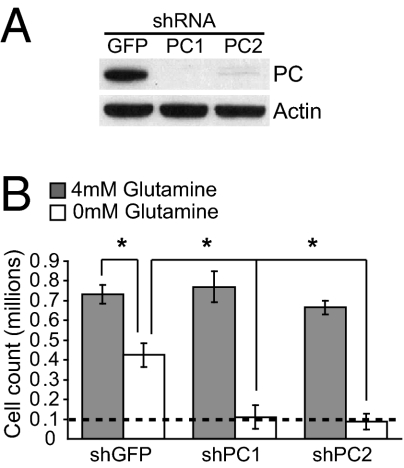
To determine whether PC is required for growth in glutamine-deprived conditions, PC was stably depleted from the adapted cells (Fig. 4A) and these cells were challenged to grow in the presence and absence of glutamine (Fig. 4B). In the presence of glutamine, there was no difference in growth of cells containing a control shRNA or either of two shRNAs directed against PC. However, in the absence of glutamine, silencing of PC expression severely limited cell growth (Fig. 4B). Furthermore, the transfer of carbon from glucose into macromolecules was suppressed when PC was silenced, but only in glutamine-free conditions (SI Appendix, Fig. S12), paralleling the effects on cell growth. Thus, PC is dispensable for growth of glutamine-replete glioblastoma cells, but required when glutamine supply is limited.
Fig. 4.
PC is required for cells to escape glutamine addiction. (A) Suppression of PC protein expression in SF-xL cells adapted to growth in low glutamine, then infected with lentiviruses expressing a control shRNA (GFP) or shRNAs directed against PC (PC1, PC2). (B) Cell growth in the presence and absence of extracellular glutamine. Cells were plated at a density of 1 × 105 /well (dashed line) and cultured for 72 h. Data are the average ± SD of three independent cultures. *P < 0.05.
PC Activity Predicts Resistance to Glutamine Withdrawal.
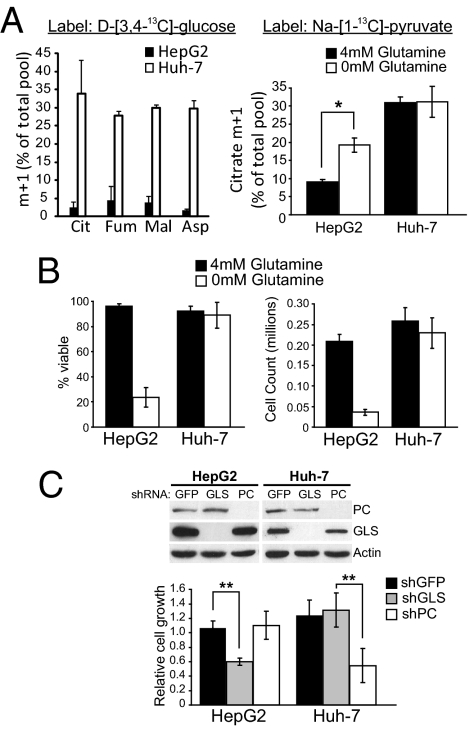
Next, we tested whether PC activity in nutrient-replete conditions could predict glutamine dependence. We cultured two hepatocellular carcinoma cell lines, HepG2 and Huh-7, with various 13C-labeled nutrients to evaluate their use of glutamine and PC for anaplerosis. First, each cell line was cultured in medium containing D-[U-13C]-glucose and unlabeled glutamine, or in unlabeled glucose and L-[U-13C]-glutamine. Metabolite analysis for 13C enrichment revealed that HepG2 cells, but not Huh-7 cells, used glutamine as the predominant anaplerotic precursor (SI Appendix, Fig. S13). Next, each cell line was cultured with unlabeled glutamine and D-[3,4-13C]-glucose, which is metabolized to [1-13C]-pyruvate and provides a convenient readout of PC activity (SI Appendix, Fig. S8). Only Huh-7 cells displayed significant entry of 13C into the TCA cycle (Fig. 5A). The cells were then cultured with [1-13C]-pyruvate in the presence and absence of glutamine. HepG2 cells displayed minimal 13C labeling in citrate when glutamine was present, and a modest increase in labeling during acute glutamine deprivation (Fig. 5A). By contrast, Huh-7 cells had higher levels of citrate labeling regardless of whether glutamine was present (Fig. 5A). These data demonstrate that Huh-7 cells have high PC activity but HepG2 cells do not.
Fig. 5.
PC activity predicts cellular independence from glutamine and glutaminase. (A, Left) Labeling of intracellular metabolites from D-[3,4-13C]-glucose in HepG2 and Huh-7 cells grown in complete medium, including 4 mM glutamine, for 7 h. (Right) Labeling of citrate from [1-13C]-pyruvate in HepG2 and Huh-7 cells grown in the presence and absence of glutamine for 7 h. (B) Viability and growth of HepG2 and Huh-7 cells in the presence and absence of glutamine for 72 h. (C) Protein expression in HepG2- and Huh-7-derived cell lines expressing shRNAs directed against GFP (control), GLS or PC (Top), and growth of each cell line in medium containing both glucose and glutamine. Data are the average ± SD of three independent cultures grown for 72 h. *P < 0.05, **P < 0.005.
HepG2 cells were much more dependent on glutamine than Huh-7 cells, which displayed no reduction in viability or growth in glutamine-free conditions (Fig. 5B). To determine the reliance of each cell line on either anaplerotic enzyme, GLS and PC were suppressed individually in HepG2 and Huh-7 cells. When grown in medium containing both glucose and glutamine, HepG2 cells could tolerate loss of PC expression but displayed reduced growth if GLS was suppressed (Fig. 5C). Huh-7 cells displayed the opposite result, with no loss of growth when GLS was suppressed but poor growth when PC was targeted (Fig. 5C). These data indicate that PC is required for the growth of some tumor cells under nutrient-replete conditions, and that glutamine independence can be predicted by basal PC activity.
Discussion
Here we determined the effects of stably suppressing the anaplerotic enzyme GLS on the growth of glutamine-dependent tumor cells. The results demonstrated that, whereas GLS is required for these cells to achieve maximal growth in culture and s.c. tumors, even glutamine-addicted cells can compensate by rerouting carbon from glucose into metabolite pools normally supplied by glutamine. The enzyme responsible for this form of glucose-dependent anaplerosis, PC, was essential for growth of cells adapted to low-glutamine conditions. PC also provides a preferred anaplerotic flux in some glutamine-independent cancer cell lines, and targeting GLS in these cells had no effect on growth.
The findings emphasize the importance of anaplerosis in tumor cell growth, because inhibiting flux through one anaplerotic pathway induces an alternative pathway that achieves essentially the same goal—production of OAA. Of note, the two pathways studied here enable cells to deliver carbon from the two most abundant nutrients into the OAA pool. Although many nutrients can supply the OAA pool in principle, sustained cell growth likely requires the ability to convert the most readily available nutrients, glutamine and glucose, into the net amounts of OAA required to maintain TCA cycle flux during robust biosynthesis. The findings are consistent with the hypothesis that anaplerosis is one of the key functions of glutamine/glucose consumption in live tumors, and suggest that efforts to manipulate anaplerosis in vivo should focus on these two nutrients.
GLS silencing had several effects on intermediary metabolism. It suppressed but did not eliminate anaplerosis (SI Appendix, Table S2), and the residual anaplerosis was increasingly dependent on PC. It also increased the contribution of glucose to the acetyl-CoA pool (SI Appendix, Table S2), as expected because glutamine is a minor source of carbon for this pool when glutaminase is active (17). It is noteworthy that even near-complete elimination of GLS protein had only modest effects on the delivery of glutamine-derived carbon into the malate and citrate pools (Fig. 2A and SI Appendix, Fig. S4B). This reflects the fact that conversion of glutamine to glutamate, the initial step in glutamine-dependent anaplerosis, is catalyzed not only by glutaminases but also by amidotransferases in pathways for nucleotide and hexosamine biosynthesis (19). Activity of these enzymes, which yield glutamate but not free NH4+, probably helps explain why glutamine consumption exceeds NH4+ secretion in some glioblastoma cells (10, 17). The finding raises the possibility of metabolic cooperativity between anaplerosis and these other biosynthetic pathways. For example, 5-phosphoribosyl pyrophosphate amidotransferase and carbamoyl phosphate synthetase II generate glutamate from glutamine early in the synthesis of purines and pyrimidines, respectively. This glutamate might provide a carbon source for anaplerosis independently of GLS.
PC has a well-known role in producing OAA during gluconeogenesis (20), and global, constitutive PC deficiency in rare inborn errors of metabolism causes fasting intolerance, hypoglycemia, lactic acidosis, and other disturbances of intermediary metabolism (21). However, the function of PC in tumor cells has not been studied extensively. Rat hepatomas typically display suppression of PC relative to surrounding liver, whereas GLS expression correlates with the tumor growth rate (22, 23). In contrast, recent work in human tumors points to a more prominent role for PC. In lung cancer patients, infusion of D-[U-13C]-glucose before surgical resection allowed the intermediary metabolism of the tumor to be analyzed (14). In metabolites extracted from those tumors, NMR spectroscopy revealed a pattern of 13C labeling strongly suggesting that PC provided a significant anaplerotic flux. PC protein was highly expressed in these tumors relative to surrounding lung tissue. Therefore, PC may catalyze a preferred route of anaplerosis in some human tumors.
To our knowledge, this study is a unique description of the relationship between PC and glutamine metabolism in cancer cells. Primary astrocytes use PC-mediated anaplerosis to maintain pools of neurotransmitters like glutamate (20, 24, 25). Astrocytes typically do not consume glutamine, but instead scavenge glutamate from the extracellular space and convert it to glutamine using glutamine synthetase. Thus, the extracellular pool of glutamate remains low, reducing the risk of chaotic excitatory neurotransmission (25). In culture, supplementing astrocytes with glutamate suppresses PC activity, implying that these cells can sense excess glutamate and respond by reducing PC-mediated anaplerosis (26). The current work suggests that similar mechanisms exist in cancer cells, because either glutamine or α-KG suppressed PC flux, even in cells conditioned to grow without glutamine. Thus, given the choice between glutamine- and glucose-dependent anaplerosis, these cells chose glutamine. On an energetic basis, glutamine-dependent anaplerosis is favorable because PC consumes one molecule of ATP per OAA formed, whereas GLS does not use ATP; rather, the metabolism of glutamine to OAA via enzymes in the TCA cycle generates reducing equivalents for oxidative phosphorylation.
The mechanism of PC induction appears to be complex. PC protein abundance increased during glutamine deprivation, but this did not require increased mRNA abundance. The reduced availability of metabolites downstream of glutamine may be involved in inducing PC activity, because some of these metabolites suppress PC in vitro or in cultured cells (26, 27). The modest increase in ATP, a PC activator, may contribute (28). Recent work also demonstrated that PC is subject to lysine acetylation in the liver (29), but the effect of this modification on PC activity is unknown. Further work is needed to understand the importance of each of these factors in regulating anaplerosis.
The current work implies that PC can allow cancer cells to resist glutaminase inhibition or, in some cases, glutamine deprivation. This could provide a mechanism of resistance during chronic exposure to agents that interfere with glutamine uptake or metabolism. Alternatively, some cells like Huh-7 use PC as the default mechanism of anaplerosis, rendering them insensitive to treatments that impair the use of glutamine as a mitochondrial substrate. True glutamine independence likely requires an additional mechanism to produce small quantities of glutamine for protein synthesis and to supply nitrogen for hexosamines and nucleotides (30). It will be interesting to determine whether PC and glutamine synthetase are coregulated in cancer cells.
Metabolic flux analysis using stable isotopes should continue to provide insight into the metabolic pathways at work in proliferating tumor cells in culture, in animal models, and in human cancer. The potential for human studies is of particular interest, because 13C infusion could be orchestrated with conventional metabolic imaging to produce a broad view of tumor metabolism and to clarify findings on 1H magnetic resonance spectroscopy, positron emission tomography, and other clinical approaches. By carefully choosing the labeled nutrient, it may be possible to correlate metabolic activity with tumor stage, tumor genetics, and response to therapy. This approach could be used to define key metabolic pathways for growth in vivo and to predict the effects of metabolic blockade on tumor growth.
Materials and Methods
Cell Culture, Growth, and Viability Assays.
SF-xL, LN229, HepG2, and Huh-7 cells were cultured in Dulbecco's modified eagle medium (DMEM) supplemented with 10% FBS (HyClone), penicillin/streptomycin, and 6 mM L-glutamine. Nutrient deprivation and metabolic labeling experiments used DMEM with dialyzed FBS (17). Cell growth was monitored by trypsinization and counting with a hemacytometer or automated cell counter (BioRad; TC10). Viability was determined by trypan blue exclusion. In all stable knockdown experiments, very few detached cells were noted in the culture, and these were not included in growth and viability counts. Phase contrast images were obtained with a Nikon Eclipse TE300 microscope and processed with MetaMorph (Universal Imaging). To adapt SF-xL cells to low glutamine, the cells were grown in DMEM as above, but with progressively less glutamine (3 mM for 1 wk, then 1 mM for 1 wk, then 0.5 mM for 1 wk). After >1 mo of growth in 0.5 mM glutamine, the cells were transferred to medium containing 0 mM glutamine. The cells that survived this challenge and continued to proliferate were considered to be glutamine independent (“aSF-xL cells”).
Metabolic Assays.
Concentrations of glucose, lactate, glutamine, and glutamate were determined from 0.6-mL aliquots of medium using an automated electrochemical analyzer (BioProfile Basic-4 analyzer; NOVA). NH4+ secretion was measured with a spectrophotometric assay (Megazyme). Consumption and secretion of amino acids were measured by HPLC using an amino acid analyzer (Hitachi; L8900). Metabolic rates were determined by normalizing absolute changes in metabolite abundances to protein content.
RNA Interference.
All RNA interference (RNAi) experiments used pools of cells. Vectors for RNAi were obtained from commercial libraries based on the pGIPZ (shRNAmir) or pLKO.1 (shRNA) backbones (Open Biosystems). The sequences used for RNAi are in SI Appendix. Lentiviral particles were produced by cotransfecting 293T cells with the lentiviral construct, pCMVΔR8.91, and pMD2.G using Fugene HD (Roche). Virus-containing supernatant was collected 2 d posttransfection and used to infect cells. Puromycin (1 μg/mL) was added 2 d after infection, and selection was continued for 10 d before any experiments. Stably infected pools with adequate silencing were maintained in 1 μg/mL puromycin.
Protein Expression.
Whole-cell lysates were prepared in RIPA buffer and quantified using the BCA protein assay (Thermo Scientific). Protein was separated by SDS/PAGE, transferred to a PVDF membrane, and probed with antibodies against PC (Santa Cruz Biotechnology), β-actin (Sigma), GLS, or GLS2 (prepared in the Matés laboratory).
Soft Agar Colony Formation Assays.
Anchorage-independent colony formation was determined by plating cells at low density (cell count 2 × 103) and high density (1 × 104) in 12-well plates, using 0.33% SeaKem GTG agar (Cambrex BioScience). After 2 wk, the cultures were stained with 0.05% crystal violet in 20% methanol. Low-density wells were used to count colonies >200 μm in diameter, and high-density wells were used for photography.
Xenograft Growth Assays.
SF-xL or LN229 cells were resuspended in DMEM at a concentration of 200 × 106/mL, mixed 1:1 with Matrigel (Becton-Dickinson), and implanted s.c. into the flanks of male NCRNU mice aged 6–8 wk (20 × 106 cells per injection). Cells expressing nontargeting and targeting shRNAs were injected into contralateral flanks. Tumor volume was calculated according to the formula [(length) × (width)2]/2. When a tumor exceeded 2,000 mm3 in volume, the mouse was killed.
Metabolic Experiments Using Stable Isotopes.
Unless indicated, all cultures contained 10 mM glucose and 2–4 mM glutamine. Dishes of 80–90% confluent cells were rinsed twice in PBS, then overlaid with medium containing the isotopically enriched nutrient, and cultured for 7–8 h. GLS flux was measured by monitoring the transfer of 15N from L-[γ-15N]-glutamine (Cambridge Isotope Laboratories) to 15NH4+ (details in SI Appendix). For analysis of intracellular metabolites by GC/MS, cells labeled in 6-cm dishes were rinsed in ice-cold normal saline and lysed with three freeze-thaw cycles in cold 50% methanol. The lysates were centrifuged to remove precipitated protein, a standard (50 nmols of sodium 2-oxobutyrate) was added, and the samples were evaporated and derivatized by trimethylsilylation (Tri-Sil HTP reagent, Thermo Scientific). Three microliters of the derivatized material was injected onto an Agilent 6970 gas chromatograph equipped with a fused silica capillary GC column (30-m length, 0.25-mm diameter) and networked to an Agilent 5973 mass selective detector. Retention times of all metabolites of interest were validated using pure standards. The abundance of the following ions was monitored: m/z 245–249 for fumarate, m/z 335–339 for malate, m/z 334–338 for aspartate, and m/z 465–471 for citrate. An explanation of these ions is provided in SI Appendix. The measured distribution of mass isotopomers was corrected for natural abundance of 13C (31).
NMR spectroscopy experiments used larger cultures (eight 15-cm dishes per sample). Metabolites were extracted and lyophilized as described (17). NMR spectroscopy was performed on a Varian ANOVA 14.1 T spectrometer (Varian Instruments) equipped with a 3-mm broadband probe with the “observe” coil tuned to 13C (150 MHz). Proton decoupling was performed using a standard WALTZ-16 pulse sequence. Carbon spectra were acquired under the following conditions: pulse flip angle 45°, repetition time 1.5 s, spectral width 35 kHz, number of data points 104,986, and number of scans 7,000–30,000, requiring 6–25 h. Free induction decays were zero filled to 131,072 points and apodized with exponential multiplication. Relevant peak areas were determined using ACDLabs SpecManager (Advanced Chemistry Development).
Supplementary Material
Acknowledgments
We thank Craig Malloy, Mark Jeffrey, and Aron Jaffe for feedback on the data and manuscript. Kartik Rajagopalan measured amino acid levels. Carolina Cardona purified the glutaminase antibodies. R.J.D. is supported by grants from the National Institutes of Health (NIH) (DK072565), the Cancer Prevention and Research Institute of Texas (CPRIT) (HIRP100437), the Welch Foundation (I-1733), and the Children's Clinical Research Advisory Committee. E.S.J. is supported by an NIH grant (DK078933). J.M.M. is supported by a grant from Ministerio de Ciencia y Tecnología of Spain (SAF2010-17573). T.C. is supported by a CPRIT training grant and A.R.M. is supported by an NIH training grant (5T32GM083831-02).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.V.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016627108/-/DCSupplemental.
References
- 1.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 4.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 5.Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 6.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portais JC, Voisin P, Merle M, Canioni P. Glucose and glutamine metabolism in C6 glioma cells studied by carbon 13 NMR. Biochimie. 1996;78:155–164. doi: 10.1016/0300-9084(96)89500-9. [DOI] [PubMed] [Google Scholar]
- 9.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao P, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JB, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo C, et al. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem J. 2000;348:257–261. [PMC free article] [PubMed] [Google Scholar]
- 14.Fan TW, et al. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM) Mol Cancer. 2009;8:41. doi: 10.1186/1476-4598-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffrey FM, Rajagopal A, Malloy CR, Sherry AD. 13C-NMR: A simple yet comprehensive method for analysis of intermediary metabolism. Trends Biochem Sci. 1991;16:5–10. doi: 10.1016/0968-0004(91)90004-f. [DOI] [PubMed] [Google Scholar]
- 16.Malloy CR, Sherry AD, Jeffrey FM. Carbon flux through citric acid cycle pathways in perfused heart by 13C NMR spectroscopy. FEBS Lett. 1987;212:58–62. doi: 10.1016/0014-5793(87)81556-9. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, et al. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choo AY, et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBerardinis RJ, Cheng T. Q's next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jitrapakdee S, Wallace JC. Structure, function and regulation of pyruvate carboxylase. Biochem J. 1999;340:1–16. doi: 10.1042/bj3400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marin-Valencia I, Roe CR, Pascual JM. Pyruvate carboxylase deficiency: Mechanisms, mimics and anaplerosis. Mol Genet Metab. 2010;101:9–17. doi: 10.1016/j.ymgme.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Chang LO, Morris HP. Enzymatic and immunological studies on pyruvate carboxylase in livers and liver tumors. Cancer Res. 1973;33:2034–2041. [PubMed] [Google Scholar]
- 23.Linder-Horowitz M, Knox WE, Morris HP. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969;29:1195–1199. [PubMed] [Google Scholar]
- 24.Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- 25.McKenna MC. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- 26.Qu H, Eloqayli H, Unsgård G, Sonnewald U. Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. J Neurosci Res. 2001;66:1127–1132. doi: 10.1002/jnr.10032. [DOI] [PubMed] [Google Scholar]
- 27.Scrutton MC, White MD. Pyruvate carboxylase. Inhibition of the mammalian and avian liver enzymes by alpha-ketoglutarate and L-glutamate. J Biol Chem. 1974;249:5405–5415. [PubMed] [Google Scholar]
- 28.Keech B, Barritt GJ. Allosteric activation of sheep kidney pyruvate carboxylase by the magnesium ion (Mg2+) and the magnesium adenosine triphosphate ion (MgATP2-) J Biol Chem. 1967;242:1983–1987. [PubMed] [Google Scholar]
- 29.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng M, Chen S, Lao T, Liang D, Sang N. Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells. Cell Cycle. 2010;9(19):3921–3932. doi: 10.4161/cc.9.19.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 1996;31:255–262. doi: 10.1002/(SICI)1096-9888(199603)31:3<255::AID-JMS290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.