Abstract
In insects, odor cues are discriminated through a divergent family of odorant receptors (ORs). A functional OR complex consists of both a conventional odorant-binding OR and a nonconventional coreceptor (Orco) that is highly conserved across insect taxa. Recent reports have characterized insect ORs as ion channels, but the precise mechanism of signaling remains unclear. We report the identification and characterization of an Orco family agonist, VUAA1, using the Anopheles gambiae coreceptor (AgOrco) and other orthologues. These studies reveal that the Orco family can form functional ion channels in the absence of an odor-binding OR, and in addition, demonstrate a first-in-class agonist to further research in insect OR signaling. In light of the extraordinary conservation and widespread expression of the Orco family, VUAA1 represents a powerful new family of compounds that can be used to disrupt the destructive behaviors of nuisance insects, agricultural pests, and disease vectors alike.
Keywords: olfaction, mosquito, malaria, electrophysiology
In insects, olfactory cues are in part sensed through the activation of a family of cell-surface odorant receptors (ORs). In vivo, insect ORs form heteromeric complexes of unknown stoichiometry consisting of a conventional OR, and a universal coreceptor, now collectively referred to as OR coreceptor (Orco) (1, 2). In this model, highly divergent conventional ORs provide coding specificity to the complex and have broad ligand specificities (3–5). In contrast, the functionally requisite Orco has not been shown to bind odorants and is extremely well conserved across taxa (6). Orco is required for neuronal cell-surface trafficking and proper signal transduction, and it has been demonstrated that orthologues are functionally equivalent in vivo and in vitro (7). Although it is known that Orco is essential for OR-mediated chemoreception, the precise mechanism of signaling has remained unclear. Recent evidence from two studies supports two alternative—and not necessarily exclusive—models for insect olfactory signal transduction. In both models, the OR complex signals ionotropically through odorant-gated ion channels; however, one study demonstrates complex gating through cyclic nucleotides, whereas the other does not (8, 9).
ORs from the principal Afro-tropical malaria vector, Anopheles gambiae, which partially dictate their host-seeking behavior, were used to examine the function of insect ORs. In An. gambiae, 78 conventional Agam\Ors (hereafter referred to as AgOrs) have been described and their coding specificities have been extensively characterized (5, 10–13). A single conventional AgOr is expressed in every olfactory receptor neuron (ORN) in conjunction with the An. gambiae Orco orthologue Agam\Orco, hereafter referred to as AgOrco (2, 14).
Results
In the context of a larger effort to identify broadly effective insect repellents, we carried out high-throughput, calcium-imaging screens for novel modulators of the AgOrco+AgOr10 complex expressed in human embryonic kidney (HEK293) cell lines. AgOr10 was chosen in particular on the basis of its molecular and functional conservation across multiple mosquito species (15).
Unlike the many novel agonists identified in our small-molecule screens against AgOrco+AgOr10-expressing cells, only one of the 118,720 compounds tested (denoted here as VUAA1; Fig. 1A) elicited activity consistent with allosteric agonism. Classification as an allosteric agonist was based on VUAA1’s intrinsic efficacy and capacity to potentiate the complex's response to a natural ligand. The chemical identity of VUAA1 was verified using high-resolution MS as well as 1H and 13C NMR (Methods). VUAA1 was revalidated against AgOrco+AgOr10 cells and elicited concentration-dependent responses that were not seen in control cells (Fig. 1B). In addition, VUAA1 activated several other AgOrco+AgOrx cell lines in the context of other, ongoing high-throughput screens. We pursued VUAA1 on the basis of its novelty, as a probe for AgOR pharmacology, and in light of its potential role as a modulator of olfactory driven behaviors in An. gambiae.
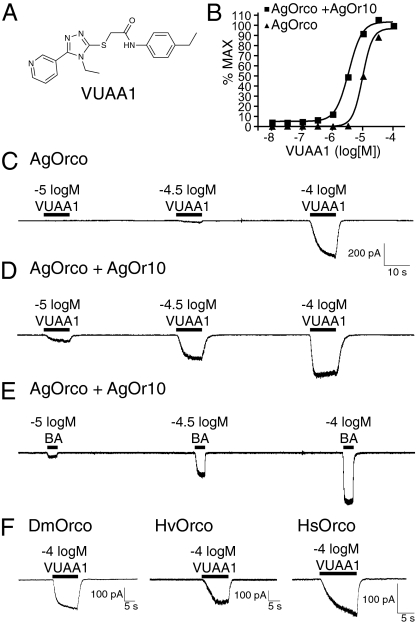
Fig. 1.
VUAA1 evokes macroscopic currents in HEK293 cells expressing AgOrco and its orthologues. (A) Structure of VUAA1. (B) Concentration–response curves generated from Fluo-4 acetoxymethyl ester-based Ca2+ imaging with AgOrco and AgOrco+AgOr10 cell lines in response to VUAA1. (C and D), Whole-cell patch-clamp recordings of concentration-dependent responses to VUAA1 in cells stably expressing AgOrco alone (C) and AgOrco+AgOr10 (D). (E) Benzaldehyde (BA), an AgOr10 agonist, elicits concentration-dependent responses in AgOrco+AgOr10 cells. (F) Whole-cell current responses to VUAA1 in HEK293 cells expressing DmOrco, HvOrco, and HsOrco. Holding potentials of −60 mV were used in C–F.
As AgOrco was the common element among the functional responses of numerous AgOrco+AgOrx cell lines, we postulated that VUAA1 was a potential AgOrco agonist. To test this hypothesis, whole-cell patch-clamp responses were examined in AgOrco+AgOr10-expressing cells and HEK293 cells stably expressing AgOrco alone. In these experiments, VUAA1 elicited concentration-dependent inward currents in both AgOrco- and AgOrco+AgOr10-expressing cells (Fig. 1 C and D) demonstrating that VUAA1 is an AgOrco agonist and that currents were AgOrco-dependent. The VUAA1-induced currents in AgOrco+AgOr10 cells resembled those resulting from application of benzaldehyde, an AgOr10 agonist (Fig. 1E). AgOrco+AgOr10 cells were more sensitive to VUAA1 than AgOrco cells, producing inward currents at −5.0 logM, a concentration at which AgOrco had no response. All currents induced by VUAA1 were AgOrco-dependent; no responses were observed in control cells (Fig. S1).
To further investigate the specificity of VUAA1 agonism, and to determine if it was capable of activating related orthologs, we tested Orco orthologues across Dipteran, Lepidopteran, and Hymenopteran taxa. When we transiently transfected HEK cells with the Orco orthologues of Drosophila melanogaster (Dmel\Orco hereafter referred to DmOrco), Heliothis virescens (Hvir\Orco hereafter referred to HvOrco), and Harpegnathos saltator (Hsal\Orco hereafter referred to HsOrco), VUAA1 elicited robust inward currents similar to AgOrco-expressing cells (Fig. 1F). These results demonstrate that VUAA1 is a broad-spectrum Orco family agonist capable of activating Orcos within and across multiple insect orders. This is consistent with the high sequence identity that is characteristic of Orco family members (76% to DmOrco, 67% to HvOrco, and 62% to HsOrco), as well as their previously demonstrated functional overlap (6).
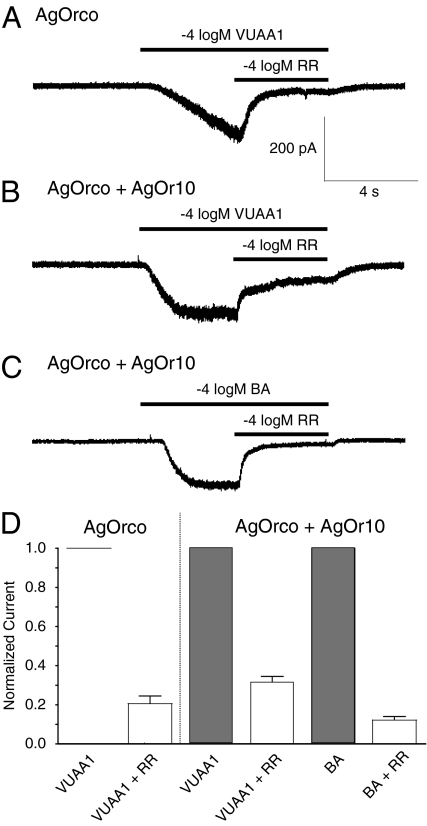
It has been previously demonstrated that insect OR complexes function ionotropically, so we set out to characterize the conductive properties of the anopheline complex in response to VUAA1 (8, 9). By using whole-cell patch-clamp experiments, we determined the current–voltage relationships of AgOrco on its own and in complex with AgOr10. Currents induced by VUAA1 in AgOrco-expressing cells as well as those induced by VUAA1 or benzaldehyde in AgOrco+AgOr10 cells were all nearly symmetrical (Fig. S2 A–C). The reversal potential of AgOrco alone in the presence of VUAA1 was −4.74 ± 3.17 mV, whereas AgOrco+AgOr10 reversal potentials were +0.18 ± 0.02 mV with VUAA1 and −0.81 ± 2.12 mV with benzaldehyde (mean ± SEM; Fig. S2D). These current–voltage relationships do not indicate any voltage-dependent gating, and the near-zero reversal potentials are consistent with previous reports that described nonselective cation conductance (8, 9). We next examined whether VUAA1-induced responses could be attenuated by ruthenium red (RR), a general cation channel blocker previously found to inhibit insect OR currents (9). Application of RR reduced the VUAA1-elicited currents of AgOrco cells by 79.4 ± 4.0%, whereas RR reduced VUAA1 and benzaldehyde responses of AgOrco+AgOr10 cells by 68.3 ± 2.8% and 87.8 ± 1.8%, respectively (Fig. 2). Taken together, these data indicate that AgOrco exhibits channel-like properties consistent with a nonselective cation channel.
Fig. 2.
RR blocks inward currents of AgOrco alone and in complex. (A–C) Representative traces of RR-blocked inward currents in AgOrco (A) and AgOrco+AgOr10 (B and C) cells. Holding potential was −60 mV for A–C. (D) Analysis of RR blockage of VUAA1 and BA-induced currents from A (n = 5), B (n = 5), and C (n = 4).
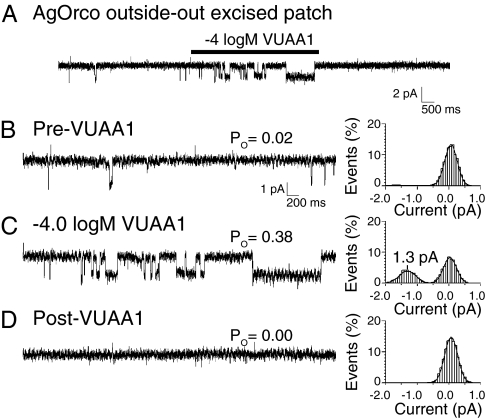
In the next set of studies, outside-out membrane patches were excised from AgOrco-expressing cells to examine single-channel currents evoked by VUAA1 (Fig. 3A). Here, spontaneous channel opening was observed before VUAA1 stimulation, but with very low probability (Po = 0.02; Fig. 3B). During a 5-s application of VUAA1, channel opening probability increased to Po = 0.38 (Fig. 3C). Subsequent to agonist washout, channel opening probability decreased to 0.00 (Fig. 3D). The average unitary current of AgOrco was 1.3 ± 0.3 pA (mean ± SD; Fig. 3C, Inset), which is comparable to previous single-channel studies of insect ORs (9). Taken together, these data support the hypothesis that VUAA1 agonizes AgOrco in the absence of other intracellular components and provide additional support for the ionotropic nature of this channel as well as the role of VUAA1 as a direct agonist of AgOrco and other Orco family members.
Fig. 3.
AgOrco is a functional channel and responds to VUAA1 in outside-out membrane patches. (A) Single-channel recording from an outside-out excised patch pulled from a cell-expressing AgOrco. (B–D) Expansions of trace A before (B), during (C), and after (D) a 5-s application of −4.0 logM VUAA1. All-point current histograms of trace expansions are on the right sides of B–D. Excised membrane patch was held at −60 mV.
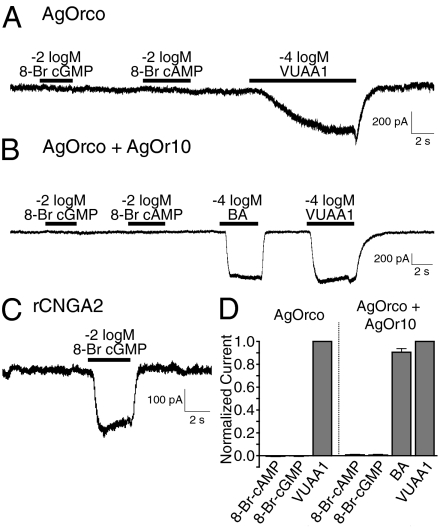
We then investigated whether activation of AgOrco involves second-messenger–based signaling, which has been reported to contribute to insect olfactory signaling (8). In these studies, which are consistent with a previously published report, two cyclic nucleotide analogues (8-Br-cAMP and 8-Br-cGMP) were unable to evoke whole-cell currents in AgOrco or AgOrco+AgOr10 cells (Fig. 4 A and B) (9). Importantly, Rattus norvegicus cyclic-nucleotide gated channel A2 (rCNGA2) demonstrated robust responses to 8-Br cGMP (Fig. 4C) (16). These data support a hypothesis in which an ionotropic mechanism is the principal, if not sole, signaling mechanism of functional AgOR complexes.
Fig. 4.
8-Br-cAMP and 8-Br-cGMP did not elicit currents in AgOrco or AgOrco+AgOr10 cells. (A) Representative trace from whole-cell recordings from cells expressing AgOrco-expressing cells with application of 8-Br-cAMP, 8-Br-cGMP, and VUAA1. (B) Representative trace from cells expressing AgOrco+AgOr10 with application of 8-Br-cAMP, 8-Br-cGMP, BA, and VUAA1. (C) Representative trace from cells expressing rCNGA2 with application of 8-Br-cGMP. Holding potentials for all recordings were −60 mV. (D) Histogram of normalized currents from cyclic nucleotide and control responses (n = 4). All currents normalized to VUAA1 responses.
In addition to demonstrating that AgOrco alone and AgOrco+AgOr10 complexes act as functional, ligand-gated ion channels, these studies have shown that VUAA1 elicits AgOR currents similar to those evoked by odorants. As an additional indicator of the specificity of VUAA1 for Orcos, we tested VUAA1 on another nonselective cation channel, the rat transient receptor potential vanilloid receptor 1 (TRPV1) (17, 18). In these controls, VUAA1 failed to evoke any response, whereas capsaicin elicited robust responses in TRPV1-expressing cells, thereby demonstrating that VUAA1 does not act as a general cation channel agonist (Fig. S2F).
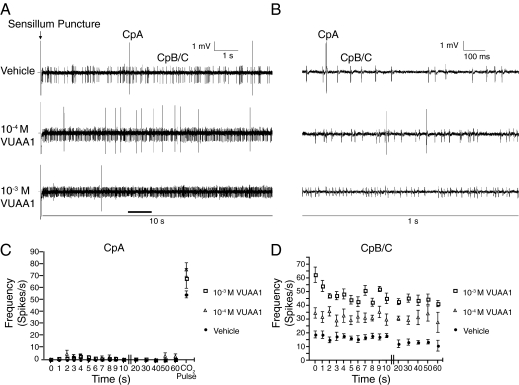
We next performed single-unit, extracellular electrophysiological recordings on the maxillary palp of adult female An. gambiae to determine whether VUAA1 could activate AgOrco-expressing ORNs in vivo. We have previously demonstrated that the maxillary palp, an elongate olfactory appendage emanating from the head, contains only a single sensilla type, the capitate peg (Cp), and that all Cps contain three chemosensory neurons (10). The highly stereotypic Cp sensilla contain two AgOrco/conventional OR-expressing ORNs (CpB and CpC), as well as a CO2-sensitive neuron (CpA), which does not express AgOrco. Single sensillum recordings (SSRs) involve puncturing the sensillum wall with a glass electrode, which enables the passive sampling of all sensillum neurons simultaneously. The activities of individual neurons are discriminated from each other based on compound response profiles and action potential amplitudes. In these preparations, CpA spike activity is clearly distinguished from those of CpB/C by its large action potential amplitude. CpB/C spikes were much smaller, and, in some preparations, indistinguishable from each other. Consequently, the spike activities of CpB/C neurons were binned for data analysis. Accordingly, we reasoned that if VUAA1 acts as a specific AgOrco agonist, we would expect it to selectively increase the spike frequency of the CpB/C neurons, but have no effect on CpA responses.
Because of its relatively high molecular weight, and despite multiple attempts, volatile delivery of VUAA1 was not feasible. As a result, VUAA1 was directly introduced to each sensillum via the glass-recording electrode, rather than through the more conventional method of volatile delivery. When VUAA1 was added to the electrode solution, it increased the spike frequency of CpB/C neurons in a dose-dependent manner, whereas vehicle alone had no effect (Fig. 5 A, B, and D). Differential CpB/C spike activity was observed immediately after puncturing each sensillum, suggesting millisecond compound diffusion rates into the sensillum (Fig. 5 A, B, and D). At the completion of each assay, a CO2 pulse was delivered to the sensillum to test whether VUAA1 affected the CpA neuron; in contrast to the responsiveness of the AgOrco-expressing CpB/C neurons, CpA activity was unchanged in the presence of vehicle and/or VUAA1 (Fig. 5 A–C). The ability of VUAA1 to activate AgOrco-expressing ORNs in vivo further demonstrates that AgOrco is an accessible biological target in An. gambiae, which is not obscured by other proteins or cofactors involved in olfactory signal transduction.
Fig. 5.
VUAA1 activates AgOrco-expressing neurons in An. gambiae females. (A) Representative traces of SSR recordings from Cp sensilla upon electrode puncture. VUAA1 or vehicle alone (DMSO) was delivered through the glass recording electrode. CpA is discernible from the smaller CpB/C action potentials. (B) Expansions of traces in A. (C) Activity of CpA neuron in response to VUAA1. Spike frequency was calculated every second for the first 10 s after sensillum puncture and every 10 s thereafter. After 60 s, the preparation was pulsed for 2 s with atmospheric air to confirm a functional CpA neuron (n = 6). (D) Activity of CpB/CpC neurons in response to VUAA1, as in C.
Discussion
Here we report the identification and characterization of VUAA1, an Orco family agonist that is capable of gating orthologues across multiple insect taxa. In contrast to previous models, these data support a hypothesis whereby AgOrco and related Orco orthologues act as ion channels, which can function independently of their heteromeric partners and are indifferent to cyclic nucleotide modulation (8, 9). Other than the unique activity of VUAA1, there are currently no known natural ligands for Orco family members. Therefore, we suggest that AgOrco and other Orco family members should be recognized as independently gated ion channels or channel subunits rather than ORs.
As Orco functionality is required for OR-mediated chemoreception across all insects, an Orco agonist would theoretically be capable of activating all OR-expressing ORNs. Accordingly, Orco agonism would be expected to severely impact the discrimination of odors across all insect taxa, affecting a broad range of chemosensory driven behaviors. In An. gambiae females, universal ORN activation would likely disrupt a variety of olfactory-driven behaviors, most notably human host-seeking, which serves as the foundation for their ability to transmit malaria (19). The discovery of a universal Orco agonist is also an important step toward the development of a new generation of broad-spectrum agents for integrated management of nuisance insects and agricultural pests.
The in vivo VUAA1-mediated activation of AgOrco-expressing cells serves as a proof of principle that targeting AgOrco and other Orco orthologues is a viable approach for the development of behaviorally disruptive olfactory compounds to control a wide range of insect pests and vectors. Although it is premature to speculate as to the ultimate utility of VUAA1 as an antimalarial behaviorally disruptive olfactory compound or as a general modulator of insect chemosensory-driven behaviors, VUAA1 nevertheless represents an important tool for the direct study of AgOrco and other Orco orthologues in insect chemosensory signal transduction.
Materials and Methods
Cell Culture and Ca2+ Imaging.
Transient transfections of pCI (Promega)-containing OR constructs were performed with FuGENE 6 (Roche) into FLP-IN T-REX 293 (Invitrogen) cell lines. TRPV1 cells were a gift from D. Julius (University of California, San Francisco, CA) (17), and the pCIS-rCNGA2 construct was a gift from R. Reed (16) (Johns Hopkins, Baltimore, MD). Construction of the AgOrco+AgOr10 cell line has been previously described (15). Fluo-4AM dye–loaded cells were assayed for ligand response in an FDSS6000 (Hamamatsu) as previously described (15).
Chemicals.
Benzaldehyde (CAS 100–52-7) and capsaicin (CAS 404–86-4) were purchased from Sigma-Aldrich. 8-Br-cAMP and 8-Br-cGMP were obtained from Enzo Life Sciences. VUAA1 (N-(4-ethylphenyl)-2-((4-ethyl-5-(3-pyridinyl)-4H-1,2,4-triazol-3-yl)thio)acetamide) was purchased from the Sigma-Aldrich Rare Chemical Library (CAS no. 525582–84-7; product discontinued as of the time of manuscript submission). To ensure that observed activity was elicited from VUAA1, and not from a contaminant present in the mixture, we performed preparative HPLC. Briefly, 20 mg VUAA1 was dissolved in a 50/50 mixture of methanol and DMSO, and HPLC was performed on a Phenomenex Luna 30 × 50-mm C18 prep column with 0.1% TFA in H2O coupled to an acetonitrile gradient. Appropriate fractions were pooled and passed over a TFA scavenger column (StratoSpheres SPE PL-HCO3 MP-resin; Polymer Laboratories). The solvent was removed by rotary evaporation with a Biotage V10 rotary evaporator, yielding white powder. VUAA1 was subsequently redissolved in DMSO and assayed as described.
Characterization of Chemical Materials.
Hydrogen-1 NMR spectra (400 MHz, DMSO-d6): δ 8.73 (d, J = 1.8 Hz, 1H), 8.65 (dd, J = 1.5, 4.8 Hz, 1H), 7.97 (dt, J= 1.9, 8.0 Hz, 1H), 7.49 (dd, J = 2.5, 8.3 Hz,1H), 7.37 (d, J = 8.4 Hz, 2H), 7.04 (d, J = 8.4 HZ, 2H), 4.10 (s, 1H), 3.95 (q, J = 7.2 Hz, 2H), 2.43 (q, J = 7.6 Hz, 2H), 1.13 (t, J = 8.0 Hz, 3H), 1.04 (t, J = 8.0 Hz, 3H).Carbon-13 NMR spectra (400 MHz, DMSO-d6): δ 165.71, 152.92, 151.32, 150.95, 149.07, 139.35, 136.87, 136.33, 128.38, 124.34, 123.90, 119.58, 37.91, 27.97, 16.05, 15.42. The high-resolution MS (m/z) [M]+ calculated for C19H22N5OS was 368.1544 and 368.1545 was found.
Patch-Clamp Recording in HEK Cells.
Currents from OR-expressing HEK293 cells were amplified using an Axopatch 200b amplifier (Axon Instruments) and digitized through a Digidata 1322A digitizer (Axon Instruments). Electrophysiological data were recorded and analyzed using pCLAMP 10 (Axon Instruments). Electrodes were fabricated from quartz tubing (Sutter Instruments) and pulled to 4 to 6 MΩ for whole-cell recording. Electrodes were filled with internal solution [120 mM KCl, 30 mM D-glucose, 10 mM Hepes, 2 mM MgCl2, 1.1 mM EGTA, and 0.1 CaCl2 (pH 7.35, 280 mOsm)]. External (bath) solution contained 130 mM NaCl, 34 mM D-glucose, 10 mM Hepes, 1.5 mM CaCl2, 1.3 mM KH2PO4, and 0.5 MgSO4 (pH 7.35, 300 mOsm). Compounds were diluted in external solution and locally perfused to the recording cell using Perfusion Pencil (Automate Scientific) and controlled by a ValveLink 8.2 controller (Automate Scientific). Whole-cell recordings were sampled at 10 kHz and filtered at 5 kHz. Outside-out patches were obtained using 10- to 15-MΩ electrodes pulled from standard glass capillaries (World Precision Instruments) and fire-polished with an MF-830 microforge (Narishige). Single-channel recordings were sampled at 20 kHz. Recordings were reduced to 1 kHz and low-pass filtered at 500 Hz for display and analysis using QuB (State University of New York at Buffalo).
Cloning of HsOrco.
The full-length coding sequence of HsOrco was PCR amplified from H. saltator antennal cDNA using the primers 5′-ATGATGAAGATGAAGCAGCAGGGCCT-3′ and 5′-TTTCATGGTGCTGGTACAACTGAAGTGA-3′. The subsequent PCR fragment was then cloned into pENTR/d-TOPO (Invitrogen) and then subcloned into the pCI mammalian expression vector.
SSRs.
SSRs were performed on 4- to 7-d-old, non–blood-fed An. gambiae females maintained on 10% sucrose and a 12 h/12 h light/dark cycle. Legs, wings and antennae were removed from cold-anesthetized females that were then restrained on double-stick tape with thread. A glass reference electrode filled with sensillar lymph Ringer solution was placed in the eye, and the recording electrode filled with DMSO or VUAA1 diluted in sensillar lymph Ringer solution was used to puncture sensilla at their base (20). Preparations were kept under a steady stream of humidified, synthetic air (21% O2/79% N2) to limit the basal activity of CpA. Sensilla that did not respond to CO2 or 1-octen-3-ol were excluded from analysis. Responses were recorded and digitized using a Syntech IDAC-4 and analyzed with AutoSpike software (Syntech). A new glass recording pipette was used for every recording. Data were sampled at 12 kHz.
Supplementary Material
Acknowledgments
We thank P. Reid, P. Portonovo, G. A. Sulikowski, C. J. Denicola, and D. L. Hachey of the Vanderbilt Institute for Chemical Biology for assistance in preparative HPLC purification, MS, and NMR; K. Erreger, C. C. Hernandez, A. Galli, and R. L. Macdonald for assistance and advice in patch-clamp electrophysiology; D. C. Dorset, E. L. Days, C. Farmer, and C. D. Weaver for high-throughput screening and technical and data analysis assistance; D. Julius for providing TRPV1-expressing HEK293 cells; J. Liebig for providing H. saltator antennal cDNA; and R. R. Reed for providing the rCNGA2 construct. We also thank members of the Zwiebel laboratory for critical discussions, Z. Li for assistance in mosquito rearing, and L. Sun, J. G. Camp, and M. Rützler for cell culture and screening assistance. This work was supported by a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (to L.J.Z.).
Footnotes
Conflict of interest statement: Preliminary patents have been filed on the use of this compound for insect applications.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102425108/-/DCSupplemental.
References
- 1.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vosshall LB, Hansson BS. A unified nomenclature system for the insect olfactory coreceptor. Chem Senses. 2011 doi: 10.1093/chemse/bjr022. 10.1093/chemse/bjr022. [DOI] [PubMed] [Google Scholar]
- 3.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones WD, Nguyen T-AT, Kloss B, Lee KJ, Vosshall LB. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 2005;15:R119–R121. doi: 10.1016/j.cub.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 10.Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox AN, Pitts RJ, Zwiebel LJ. A cluster of candidate odorant receptors from the malaria vector mosquito, Anopheles gambiae. Chem Senses. 2002;27:453–459. doi: 10.1093/chemse/27.5.453. [DOI] [PubMed] [Google Scholar]
- 12.Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill CA, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 14.Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohbot JD, et al. Conservation of indole responsive odorant receptors in mosquitoes reveals an ancient olfactory trait. Chem Senses. 2011;36:149–160. doi: 10.1093/chemse/bjq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 17.Bohlen CJ, et al. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141:834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 19.Zwiebel LJ, Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu P, Atkinson R, Jones DN, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.