CELL BIOLOGY Correction for “Itch E3 ubiquitin ligase regulates large tumor suppressor 1 tumor-suppressor stability,” by King Ching Ho, Zhonghua Zhou, Yi-Min She, Alex Chun, Terry D. Cyr, and Xiaolong Yang, which appeared in issue 12, March 22, 2011 of Proc Natl Acad Sci USA (108:4870–4875; first published March 7, 2011; 10.1073/pnas.1101273108).
The authors note that the title appeared incorrectly. The title should instead appear as “Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability.” The title in the online version has been corrected.
There were also some textual errors. On page 4870, right column, first full paragraph of the Results section, line 20 “(Fig. 1A)” should instead appear as “(Fig. 1, A and B).” On page 4871, left column, first partial paragraph, line 1 “(Fig. 1A)” should instead appear as “(Fig. 1C).” On page 4871, left column, first partial paragraph, line 10 “(Fig. 1B)” should instead appear as “(Fig. 1D).”
Additionally, the legends for Figures 2 and 5 appeared incorrectly. The figures and their corrected legends appear below.
Fig. 2.
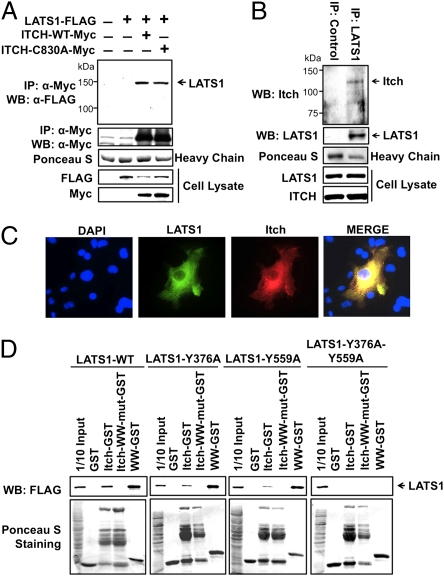
Interaction of LATS1 and Itch in vivo and in vitro. (A) Interaction of ectopically expressed LATS1 and Itch. COS7 lysates expressing either LATS1-FLAG alone or together with Itch-Myc or Itch-C830A-Myc were immunoprecipitated with anti-Myc antibody, followed by Western blotting with anti-FLAG antibody. Ponceau S staining of antibody heavy chain indicates equal amounts of anti-Myc antibody were used. (B) Interaction of endogenous LATS1 and Itch. Protein lysates from MDA-MB-231 cells were immunoprecipitated with either control anti-FLAG antibody or anti-LATS1 antibody, followed by Western blotting with anti-Itch antibody. (C) Immunostaining analysis of LATS1 and Itch. LATS1-FLAG and Itch-Myc were cotransfected into COS7 cells, followed by immunostaining with anti-FLAG and anti-Myc primary antibodies and AF488 anti-mouse IgG and AF555 anti-rabbit IgG secondary antibodies. (D) GST-pulldown analysis of interaction of LATS1 and Itch in vitro. COS7 lysates expressing wild-type (LATS1-WT-FLAG), single-PPxY mutants (LATS1-Y376F-FLAG or LATS1-Y559F-FLAG), or double PPxY mutant (LATS1-Y376F-Y559F-FLAG) of LATS1 were pulled down with GST, GST-Itch, GST-Itch-WW-mutant, or GST-WW, followed by Western blotting for LATS1-FLAG using anti-FLAG antibody. 1/10 input (10 μg) represents 1/10 of protein lysate (100 μg) used for GST pulldown.
Fig. 5.
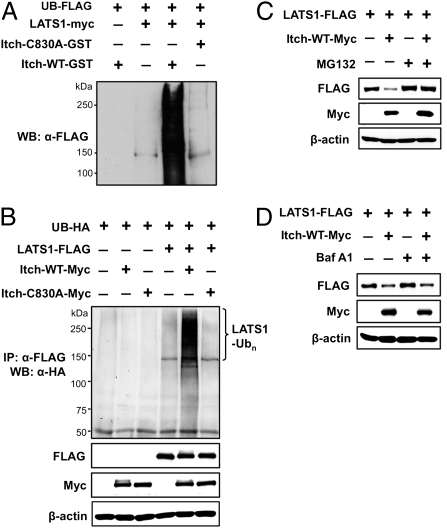
Itch promotes polyubiquitination and subsequent degradation of LATS1 by 26S proteasome. (A) In vitro ubiquitination of LATS1 by Itch. Immunoprecipitated LATS1-myc on beads was used as a substrate in an ubiquitination assay with a ligase buffer containing E1, E2, Ubiquitin-FLAG, ATP, and Itch-GST or Itch-C830A-GST. After reaction, the beads containing LATS1-myc were washed extensively with modified RIPA buffer, followed by Western blot analysis using anti-FLAG antibody. (B) In vivo ubiquitination of LATS1 by Itch. Ubiquitin-HA and different combinations of Itch-Myc, Itch-ligase-dead mutant (Itch-C830A-Myc), and LATS1-FLAG were transfected into COS7 cells. Ubiquitinated LATS1 was detected by immunoprecipitation of LATS1 with anti-FLAG antibody, followed by detection of ubiquitin using anti-HA antibody. (C) Proteasome inhibitor blocks Itch-induced LATS1 degradation. COS7 cells transfected with either LATS1-FLAG alone or LATS1-FLAG together with Itch-Myc were treated with either DMSO (control) or proteasome inhbitor (MG132). (D) Lysosome inhibitor fails to block Itch-induced LATS1 degradation. COS7 cells transfected with either LATS1-FLAG alone or LATS1-FLAG together with Itch-Myc were treated with either DMSO (control) or lysosome inhibitor (Baf A1).