Abstract
It is well known that early disruption of sensory input from one modality can induce crossmodal reorganization of a deprived cortical area, resulting in compensatory abilities in the remaining senses. Compensatory effects, however, occur in selected cortical regions and it is not known whether such compensatory phenomena have any relation to the original function of the reorganized area. In the cortex of hearing cats, the auditory field of the anterior ectosylvian sulcus (FAES) is largely responsive to acoustic stimulation and its unilateral deactivation results in profound contralateral acoustic orienting deficits. Given these functional and behavioral roles, the FAES was studied in early-deafened cats to examine its crossmodal sensory properties as well as to assess the behavioral role of that reorganization. Recordings in the FAES of early-deafened adults revealed robust responses to visual stimulation as well as receptive fields that collectively represented the contralateral visual field. A second group of early-deafened cats was trained to localize visual targets in a perimetry array. In these animals, cooling loops were surgically placed on the FAES to reversibly deactivate the region, which resulted in substantial contralateral visual orienting deficits. These results demonstrate that crossmodal plasticity can substitute one sensory modality for another while maintaining the functional repertoire of the reorganized region.
Keywords: vision, single-unit electrophysiology, orienting behavior, cooling deactivation
Aremarkable property of the brain is its capacity to respond to change. This neuroplastic process endows the nervous system with the ability to adjust itself to the loss of an entire set of sensory inputs or even two (1). Under these conditions, it is clearly adaptive for inputs from an intact modality to substitute for those that have been lost, such as auditory navigation in the blind. Crossmodal plasticity can also enhance perceptual performance within the remaining sensory modalities. Numerous reports document improvement over sighted subjects in auditory and somatosensory tasks in blind individuals (2–7), as well as enhanced performance in visual and tactile behaviors in the deaf (8–11). However, with the accumulation of studies examining such compensatory effects following early sensory loss, it is becoming evident that not all features of the replacement sensory modalities are equally represented. For example, early-deaf subjects exhibit supranormal abilities for visual localization (10) and visual motion detection (11, 12), but not visual brightness discrimination (13), contrast sensitivity (14), visual shape detection (15), grating acuity, vernier acuity, orientation discrimination, motion direction, or velocity discrimination (11). Thus, rather than a generalized overall improvement, it seems that only specific features of the replacement modality are affected by crossmodal plasticity.
Crossmodal plasticity itself does not appear to be a uniformly distributed effect. Although it seems plausible that the entire territory vacated by a damaged sensory modality might be available for crossmodal innervation, this assumption is not supported by evidence from studies of early deafness. Following early deafness, crossmodal inputs appear to avoid (5, 16) or only partially innervate (17) the primary auditory cortex. In contrast, early deafness induces visual reorganization of the posterior auditory field and the dorsal auditory zone (11). The factors that select a deprived region for reorganization, and the specific sensory modalities to be involved, are unknown. One clue might be the observation that the posterior auditory field of hearing animals is involved in auditory localization (18) whereas the same region in congenitally deaf animals underlies their improvement in visual localization of peripheral targets (11). Similarly, following early blindness, the lexigraphic components of Braille reading provide activation of visual cortex (19). Therefore, it seems possible that the behavioral role of a crossmodally reorganized area is related to its role in hearing/sighted individuals. This hypothesis was examined in the present study.
The auditory field of the anterior ectosylvian sulcus (FAES) of the cat is a higher-level component of auditory cortex (20) that has extensive connections with the orienting centers of the brainstem (20, 21). Unilateral cooling deactivation of the FAES results in severe acoustic localization deficits in the contralateral field (18), and its neurons show sensitivity to sound location (22). The region also contains a subset of auditory neurons whose activity can be modulated by the presence of visual (23) or somatosensory (24) stimulation, as well as bimodal visual–auditory neurons (25, 26). These multisensory properties suggest that a substrate is present for crossmodal reorganization should auditory inputs be damaged or lost. The present experiment sought to determine whether neurons in FAES of early-deafened cats become crossmodally reorganized and whether deactivation of the reorganized area results in behavioral localization deficits mediated by the replacement modality.
Results
Sensory Activity of Deafened FAES Cortex.
Single-unit recordings made from the FAES of early-deafened cats (n = 3), summarized in Fig. 1, revealed physiologically active neurons (n = 415), the majority of which (67.7% ± 7.6 SD) were responsive to visual stimulation. By contrast, similar recordings from the FAES in adult hearing cats (n = 3) in this and in previous studies (2, 22, 23, 25–28) showed a strong preference auditory responsivity, although a small proportion of nonauditory responses also occurred (Fig. 1). In early-deafened FAES, responses were detected using either manually presented visual cues or, as in Fig. 1C, by repeatable, electronically gated visual stimuli. When stimulating with the latter, it was clear that responses to visual stimuli were robust and reliable. Most visually responsive neurons were sensitive to movement direction and preferred high-velocity movement (e.g., >100°/s), thus demonstrating response properties characteristic of visual cortical neurons. As illustrated in Fig. 2A, these visually responsive neurons exhibited receptive fields that were collectively distributed across the contralateral visual hemifield, sparing only the most superior and inferior extremes, although no clear retinotopy was observed. In addition, the majority (89%) of visual receptive fields extended into the ipsilateral visual field (average 12.7° ± 10.4 SD). Thus, most receptive fields included a representation of central visual space and were quite large, averaging 63.9° (±18 SD) in diameter. Visual receptive fields of hearing animals exhibited similar size and position distributions to those of deafened FAES animals (Fig. 2B), but were far fewer in occurrence.
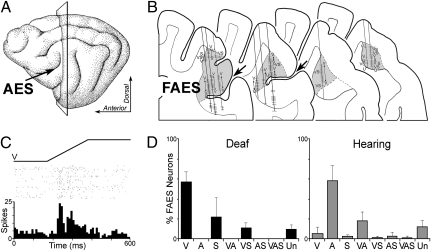
Fig. 1.
(A) On the lateral view of the cat brain, the arrow indicates the anterior ectosylvian sulcus (AES) and the plane of section corresponds with the coronal sections displayed in B. (B) Portions of serially arranged coronal sections containing the AES (large arrows) and the field of the anterior ectosylvian sulcus (FAES; shaded). Representative recording penetrations are shown traversing the FAES. Unlike hearing animals, where the FAES is largely auditory, recordings from adults that were postnatally deafened revealed responses driven primarily by visual stimulation (labeled V; also somatosensory, S; unresponsive, U). (C) Typical single-unit neuronal responses (raster dot = 1 spike; row = 1 presentation; histogram is 10-ms time bin) to visual stimulation showed vigorous and reliable activation by a moving bar of light (ramp labeled V). For neurons histologically verified in the FAES, D summarizes the sensory modality patterns found in postnatally deafened (solid bars; three cats, n = 415 neurons) and in hearing (shaded bars; three cats, n = 205 neurons) cats. These data indicate that the FAES is crossmodally reorganized from the auditory to visual modality in early-deafened animals.
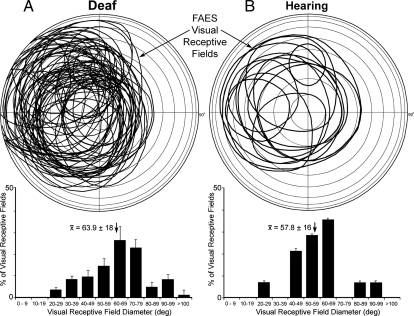
Fig. 2.
(A) On a representation of the visual field, visual receptive fields are plotted (solid ovals) for each of the FAES neurons mapped in early-deafened cats. Essentially, all points in contralateral visual space (except extreme superior/inferior aspects) were represented. (A, Lower) The histogram shows that the size (diameter, calculated as average of vertical and horizontal axes) of visual receptive fields in deafened FAES was generally quite large, averaging ~64° (at arrow, error bars = SD). (B) Visual receptive field locations recorded from hearing FAES, with their size ranges (Lower) plotted. Although far fewer visual neurons were identified in hearing FAES, receptive field size and general distribution were similar to those of early-deafened FAES.
Early-deafened animals also revealed a small proportion of FAES neurons responsive to somatosensory stimulation (33.5% ± 12 SD; values sum >100% due to bimodal neurons). These neurons in early-deafened animals were activated by tactile cues, mostly (67%) by low-threshold hair-type receptors whose receptive fields could be readily mapped. The somatosensory receptive fields generally were quite large and were never restricted to a single digit or vibrissa, but frequently encompassed an entire limb or body region. Furthermore, somatosensory receptive fields in early-deafened FAES most often (75.8%) were bilateral, such that portions of both right and left sides of the body surface were represented.
Similar proportions of unresponsive FAES neurons were observed in the deafened and in the hearing animals (Fig. 1D), indicating no significant loss of sensory activation in the latter. Thus, for the FAES region, early deafness induced crossmodal plasticity characterized by vigorous visually evoked activity and distinct receptive fields that collectively represented the contralateral visual field.
Behavioral Role of Reorganized FAES.
Behaviorally, the hearing cats were effective at detecting and orienting to visual or to auditory stimuli (Fig. 3A, Left) in a perimetry arena (Fig. S1), and the early-deafened cats were similarly adept at orienting to visual stimuli in the same apparatus (Fig. 3A, Right). When the cooling coils were implanted, they deactivated the FAES region as the plots of the thermoclines indicate in Fig. 4. As expected (18), after implantation of the cooling loops (Fig. S2) when the FAES in the hearing animals was unilaterally deactivated, acoustic, but not visual, localization behaviors were blocked in the contralateral field, as depicted in Fig. 3B (data summarized in Table S1). However, in the early-deafened animals, unilateral cooling of the FAES resulted in a profound decrement in orienting to visual stimuli (Fig. 3B, Right), consistent with the visual crossmodal reorganization observed at the neuronal level. Furthermore, when the FAES was bilaterally deactivated, profound orienting deficits were observed across the entire visual field for individual deaf cats (Fig. S3B) as well as the group (Fig. S4C). This crossmodal reorganization of localization behaviors did not appear to be applicable to all auditory areas in the deafened animals. Deactivation of primary auditory cortex (A1) in early-deafened cats did not produce visual orienting deficits (Fig. 3C, Right). On the other hand, visual detection behaviors normally mediated by visual areas in hearing animals [posteromedial lateral suprasylvian visual area (PMLS)] (Fig. 3D, Left) were affected by cooling deactivation in early-deafened cats (Fig. 3D, Right). In summary, these data indicate that FAES-mediated orienting behaviors initiated by acoustic stimuli in hearing animals are triggered by visual stimuli in early-deaf animals.
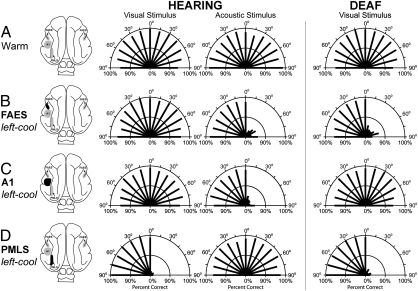
Fig. 3.
Orienting responses to visual or acoustic stimuli during reversible cooling deactivation of selected cortical areas in hearing and deaf animals. Dorsal views of the cat brain (Left) indicate the presence and position of a cryoloop (shading), and its operational status (solid area indicates loop was cooled and that cortex deactivated). The A1 cooling loop is illustrated here as placed on the left hemisphere (actually implanted on the right as depicted in Fig. S2) so that unilateral deactivations can be more easily compared. In the polar plot graphs, the two concentric semicircles represent 50% and 100% correct response levels and the length of each thick line indicates the percentage of correct responses at each location. In hearing animals (Center), responses were measured to either visual or acoustic stimuli. For deafened animals (Right), only responses to visual stimuli were tested. Each row represents the averaged results comparing orienting under the different deactivation conditions. (A) Hearing and deaf cats performed the orienting tasks with similar levels of proficiency. (B) Unilateral cooling of FAES results in contralateral hearing, but not visual, deficits in hearing cats, but induces contralateral visual orienting deficits in early-deafened subjects. (C) Unilateral deactivation of A1 results in contralateral acoustic orienting deficits in hearing cats, but has no effect on visual orienting in early-deaf cats. (D) Unilateral deactivation of visual area PMLS results in contralateral visual orienting deficits in both hearing and deaf cats, but does not affect acoustic orienting behaviors. Numerical values for these results are detailed in Table S1.
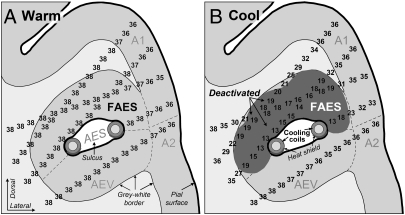
Fig. 4.
Temperature measurements were recorded from identical sites in, and around, the FAES in the cryoloop-implanted behavioral animals while the cryoloop was (A) warm at 38 °C or (B) cooled to 3 °C. As depicted in the coronal section (see Fig. 1A for plane of reference), the FAES occupies a position deep in the cortical surface and the sulcus (AES) separates the FAES from the AEV area. This sulcus extends inward from the cortical surface and is followed by the course of the cooling loop (circles with shading shown in cross-section within the submerged sulcus). In the case depicted, a heat-shielding compound was applied to the ventral surface (crescent with dark shading) of the cryoloop to minimize cooling in that direction. Temperature measurements were made systematically in and around the AES sulcus (illustrated here for deaf behavioral animal no. 3) using microthermocouples. The positions of the temperature measurements were reconstructed using microlesions and depth measurements to determine the temperature profiles. In A, the tissue of the FAES was uniformly warm at 38 °C. In B, with the cryoloop chilled to 3 °C, the region with dark shading indicates the area of cortex that was at, or below, 20 °C, showing that deactivation of the FAES was nearly complete; little or no deactivation of the adjoining AEV was evident. Abbreviations: A1, primary auditory cortex; A2, secondary auditory cortex; AES, anterior ectosylvian sulcus; AEV, ectosylvian visual area.
Discussion
These results support the hypothesis that the behavioral role of a crossmodally reorganized area resulting from early deafness/blindness is related to its role in hearing/sighted individuals. In hearing animals, the auditory FAES is behaviorally involved in auditory localization, but in early-deafened subjects, the FAES was visually reorganized and its deactivation resulted in the loss of visually evoked localization behaviors. Consistent with this finding is the observation that the posterior auditory field, also known for its involvement in auditory localization behaviors (18), is necessary for enhanced visual localization abilities in congenitally deaf cats (11). Furthermore, in early-blind individuals, lexigraphical regions of visual cortex are activated by Braille reading (19) and the nonvisual spatial properties of medial occipital cortex are retained (29). Thus, for a given cortical region, functional specificity is preserved following deafness/blindness despite substitutions among the input modalities.
For the early-deafened FAES, the input modality was changed. The response and receptive field properties of the visually restructured FAES resembled those reported for its neighboring region, the ectosylvian visual area (AEV) (30). Thus, it is tempting to suggest that the AEV simply expanded to fill the territory of the auditory-deprived FAES. Indeed, it may be possible that inputs that activate the AEV spread into the deafened FAES to innervate it as well. However, the outputs from these different areas generate different behavioral effects. Specifically, deactivation of AEV in hearing animals does not have an effect on orienting behaviors (31) whereas cooling of FAES does in both hearing and deaf animals (ref. 18 and this study). Because of these different behavioral effects, it would be inaccurate to regard deafened FAES as an expanded AEV. Therefore, as illustrated in Fig. S5, because the behavioral role of the FAES remains the same, its outputs appear to connect with the same effector regions in hearing as well as deaf subjects. In this way, crossmodal plasticity can substitute for the loss of a sensory input to a region without the necessity of reorganizing the area's outputs in the process.
The present experiments also revealed that the visually reorganized FAES was as significantly involved in visual localization behaviors as visual regions normally tasked to perform this role. The size and distribution of visual receptive fields observed in early-deafened FAES are consistent with visuomotor structures, like the superior colliculus, where neurons with large and spatially overlapping receptive fields discharge in relation to highly precise eye movements (for review, see ref. 32). In the present experiment, deactivation of reorganized FAES blocked visual localization as effectively as cooling visual PMLS in hearing (31) as well as early-deafened animals (present results). These observations, at first, might appear puzzling, where an adaptive modification (crossmodal plasticity) is given the same priority as normal visuomotor circuitry that has evolved through millions of years of evolutionary pressure. However, from the perspective of localization circuitry itself, the FAES has a strong influence whether in deaf (this study) or in hearing animals (18). Therefore, the effect of FAES on the downstream circuitry appears to be preserved even though the modality of the initiating stimulus is different between deaf and hearing subjects.
The neuronal mechanisms underlying this and other examples of crossmodal plasticity remain enigmatic, but are widely accepted to be grouped into two basic categories: physiological (unmasking of existing synapses) and structural (ingrowth/rewiring of new connections) (33). What is known of the FAES suggests that unmasking may have at least a partial role in its reorganization following deafness. In hearing animals, ~30% of FAES neurons show modulation of auditory responses by activation of an adjacent somatosensory area (24), and ~30% of neurons can be influenced by visual stimulation (23, 27). Therefore, with a reduction of auditory inputs after deafness, it would be expected that existing nonauditory inputs to the FAES would be unmasked. In fact, in early-deafened animals, 33% of FAES neurons were activated to suprathreshold levels by somatosensory stimulation, whereas an even larger proportion (67%) were activated by visual stimuli. Thus, the connectional strength of these sensory inputs changed from mostly subthreshold (in hearing animals) to suprathreshold activation (in deafened animals). However, it does not appear that the crossmodal inputs to FAES are present at the developmental stages in which deafness was induced (26, 34). In addition, given that early deafness induced crossmodal plasticity in 89% of FAES neurons (this study) whereas late deafness generated similar levels in adult A1/anterior auditory field (AAF) (87%) (35), the role of age in deafness-induced crossmodal plasticity remains to be determined. Whether the alternative mechanism involving rewiring also participates in these crossmodal phenomena will require directed connectional studies of deaf animals.
Few studies have examined the sensory features of crossmodal plasticity at the neuronal level. Of particular importance has been the observation of neurons in the AEV of visually-deprived cats that exhibit enhanced auditory spatial tuning properties (36). Also, the findings from surgically rerouted visual connections to ferret auditory cortex (37) revealed crossmodal neuronal properties that resembled those of visual cortical neurons. Similarly, deafness induces the somatosensory reorganization of auditory cortices of adult ferrets where the neurons exhibited large receptive fields most often associated with higher-level cortices (30, 31). The present data are consistent with these sensory features of crossmodally innervated neurons.
We are unaware of any studies that have directly examined the behavioral role of an area that has also been electrophysiologically demonstrated to exhibit crossmodal reorganization. The studies of Rauschecker and colleagues identified neurons in visually deprived AEV (28) that showed enhanced auditory spatial sensitivities (36), as well as visually deprived animals that showed parallel enhancements of auditory spatial localization (38). However, at that time it is was not possible to demonstrate that the reorganized AEV was responsible for the observed behavioral effect. The present study was able to make this significant leap. Here, neurons in the FAES of early-deafened cats were electrophysiologically determined to respond to visual stimulation, and their deactivation, through cortical cooling, resulted in the loss of visual detection and localization behaviors. Similar procedures have revealed that, although deactivation of A1 in deaf animals had no effect on visual orientation (this study; see also ref. 11), cooling of the posterior auditory field led to a reduction in the supranormal localization of peripheral visual stimuli, whereas deactivation of the dorsal auditory zone blocked enhancement of visual motion detection (11). Thus, the behavioral effects observed in the present experiment appear not to be a generalized feature of auditory cortex, but to be specific for the reorganized FAES following early deafness. Ultimately, the crossmodal substitution of inputs following deafness appears to drive the established output circuitry to preserve the region's behavioral role.
Materials and Methods
Twelve domestic cats (6 deafened and 6 hearing) were examined. All procedures were performed in compliance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health publication 86-23) and the National Research Council's Guidelines for Care and Use of Mammals in Neuroscience and Behavioral Research (2003) and approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University (electrophysiological and anatomical procedures) and at the University of Western Ontario (behavioral procedures).
Auditory Lesions.
Six normally pigmented kittens (four females and two males) from three litters were given bilateral cochlear lesions at 8 d postnatal (DPN) (onset for auditory cortical activity in cats) (39), using ethacrynate sodium (25 mg/kg, i.v.) and kanamycin (200 mg/kg, s.c.) (see ref. 40 for protocol). After weaning (~40 d), hearing levels were assessed using auditory brainstem responses (ABR) (Nicolet Spirit 2000; Nicolet Biomedical; stimulus, 15–95 dB, 0.1-ms square-wave click; rarefaction, 1/s with 2,000–5,000 repetitions through a calibrated minispeaker; leads, vertex-to-ipsilateral mastoid). At that time, four of the treated animals showed an ~45-dB hearing deficit requiring a second ototoxic treatment that increased their ABR thresholds >90 dB. Thus, all treated animals exhibited hearing thresholds >90 dB by 50 DPN.
Electrophysiological Recording.
The young cats were raised beyond the auditory critical period (~120 DPN) (39) and all were at least 290 d of age before testing. In preparation for electrophysiological recording, three early-deafened animals (and three hearing adults) were anesthetized (sodium pentobarbital, 40 mg/kg, i.p.) and a craniotomy was stereotaxically performed to expose the FAES cortex. A resealable recording well was secured over the opening using screws and dental acrylic. The scalp was sutured closed around the implant and routine postoperative care was provided. Approximately 5–7 d elapsed before the initial recording experiment.
Recording experiments were initiated by anesthetizing the animal (35 mg/kg ketamine and 0.4 mg/kg acepromazine, i.m.) and securing the implant to a supporting bar. The animal was intubated and ventilated; expired CO2 was maintained at ~4.5%. Fluids, supplemental anesthetics (0.5 mg ketamine·kg·h−1 and 0.05 mg acepromazine·kg·h−1, i.v.) and a muscle relaxant (Pancuronium bromide, <1 mg/h, i.v.) were continuously administered through a veinous cannula. This regimen suppressed spontaneous movements during recording sessions and for at least 30 min following cessation of drug infusion at the end of each experiment. The electrode (glass-insulated tungsten, <1 MΩ) was lowered through the recording well to the cortical surface and then advanced with a hydraulic microdrive. Neuronal waveforms of at least 3:1 signal:noise ratio were sought for study. Neurons were identified by their spontaneous activity and by their responses to somatosensory (air puffs, brush strokes and taps, manual pressure, and joint movement) and/or visual search stimuli (flashed or moving spots or bars of light from a hand-held ophthalmoscope projected onto a translucent hemisphere positioned 45 cm in front of the animal). Visual receptive fields were mapped by projecting a spot of light onto the hemisphere, upon which the stimuli were moved in all directions until a responsive area was outlined. Auditory stimuli (hisses, claps, whistles, pops, etc.) were also manually presented to each neuron. Quantitative, electronically generated sensory tests were delivered to selected neurons where visual stimuli were generated by a projector that cast a bright bar through a rotating prism onto a galvanometer-driven mirror. Thus, controlled, repeatable visual stimuli were moved through the visual receptive field while the neuron's response was recorded, digitized (at 25 kHz), and stored on a computer for subsequent analysis using Spike2 software (CED).
For each neuron, its response type (auditory, somatosensory, visual, multisensory, or unresponsive) was determined and an ≥125-μm interval was required between recording sites. Several recording penetrations were performed in a single experiment and successful penetrations were marked with a small electrolytic lesion to assist histological reconstruction.
After a series of experiments, the animal was overdosed (sodium pentobarbital, 80 mg/kg. i.p.) and perfused with saline followed by fixative. The brain was stereotaxically blocked and sections (50 μm) were cut in the coronal plane, processed using standard histological procedures, and counterstained. A projecting microscope was used to trace sections and to reconstruct recording penetrations. Gyral/sulcal patterns, cytoarchitectonic characteristics, and observed physiological properties were used to demarcate functional cortical subdivisions. Using cytoarchitectonic features (41), FAES neurons were distinguished from the AAF at the lateral lip of the anterior ectosylvian gyrus and from the ectosylvian visual area in the ventral bank of the sulcus. Only neurons verified within the FAES were included in this study.
Orienting Behaviors and Reversible Cortical Deactivation by Cooling.
Three mature, postnatally deafened cats and three age-matched hearing cats were trained to detect and localize flashed visual stimuli in a dimly lit visual orienting arena (18). The three hearing cats were also trained in the same arena to detect and localize a brief white noise burst (100 ms duration, 78 dB intensity). Training procedures for both visual and acoustic tasks were identical. The apparatus was a semicircular arena (diameter 90 cm) that consisted of 13 pairs of red, 2-V (DC) light-emitting diodes (LEDs) and miniature speakers (Fig. S1). The LED/speaker combinations were mounted 15° apart along 180° of the azimuthal plane. The pairs were located 45 cm from the animal's start position and positioned at cat's eye level. A food reward tray was located under each LED/speaker pair. For the visual localization task, each cat was trained to stand in the center of the arena and approach the 0° position when the red LED at this position was illuminated. A piece of low-incentive, dry cat chow was then presented from the reward tray below the stimulus. During training, the animal's attention was first attracted to the central LED. Then, the LED was extinguished. After 100 ms, the center LED, or one of the 12 peripheral LEDs, was flashed for 100 ms. The rapid and accurate turning of the head, or head and body, and accurate approach toward the locus of the stimulus (within 2,000 ms of stimulus onset) constituted a correct orienting response. Any response other than a prompt direct approach to the appropriate stimulus was scored as incorrect. The cats were conditioned to approach the 0° position when a stimulus could not be localized and receive the low-incentive food. Premature responses, or a lack of response, were not scored and went unrewarded. Thirty-five trials constituted a block: 2 trials to each of the 12 peripheral positions, 4 trials to the central position, and 7 catch trials. Six blocks of data were collected per session. Catch trials, where no target stimulus was presented, were randomly conducted, and cats were required to approach the 0° position to receive a low-incentive food. Training took ~3 mo and was complete when a criterion performance level of ≥80% correct (average across all trials) was reached on 3 consecutive d.
After training was complete, cooling loops (30) were implanted to reversibly deactivate selected regions of cortex. Cooling loops were placed bilaterally on the FAES and unilaterally on A1 and the posteromedial lateral suprasylvian visual area (PMLS), as shown in Fig. S2. Cryoloops were fabricated by shaping 23-gauge (0.635 mm, outside diameter) stainless steel hypodermic tubing to conform to a designated cortical area. In two of the animals in the deaf group and two of the animals in the hearing group, we bilaterally placed cooling loops in the AES as we have previously done and extensively described (42). Therefore, both the dorsal and ventral banks of the AES sulcus were deactivated. To eliminate the concern that the AEV could be playing a role in the results obtained from the first two animals, in the third animal in each group we painted a ceramic heat-shielding compound (Small Parts; SO-FH6) on the ventral side of the AES loop and, once dried, coated the loop with epoxylite. As illustrated in Fig. 4, this procedure prevented the spread of cooling into the AEV. This approach has been successful in selectively deactivating the posterior but not the anterior bank of the posterior ectosylvian sulcus (11, 42, 43). For implantation, cryoloops were secured to the skull by using stainless steel skull screws and dental acrylic. Dermal incisions were sutured closed and standard postoperative procedures were administered. Following cooling loop implantation and before any experimental testing, baseline performance levels were reestablished.
To assess visual orienting in early-deaf and in hearing animals, a three-step behavioral testing paradigm was used as depicted in Fig. S3: (i) collection of baseline data with all sites warm and active, (ii) testing with the temperature of a cryoloop set to 3 °C [an FAES loop cooled to 3 °C places the 20 °C thermocline (the deactivation extent) at the layer VI/white matter interface as depicted in Fig. 4] (43), and (iii) when cooling ended the tissue was rewarmed and baseline levels were reestablished. Two blocks of 35 trials were conducted for each of the three conditions. Each testing session consisted of 210 trials. A typical daily testing session was ~1 h in duration; 25 testing sessions were conducted for each cooled locus. Therefore, for each deactivation condition, and for each animal, data presented are based on 100 trials at each of the 12 peripheral target positions. For the auditory task, testing procedures were identical to the visual task.
For both orientation tasks (visual and auditory), we calculated the percentage of correct responses. Performance was assessed with a mixed ANOVA with one within-hemisphere variable (warm vs. cold, locus of cooling loop). Orienting responses were assessed with multifactor mixed ANOVA variables (warm vs. cold, azimuth, locus of cooling loop). The order of sessions was counterbalanced between areas (loops), functional states (active vs. deactivated), and hemispheres. Confirmation of cryoloop efficacy and position has been detailed previously (42, 43).
After completion of behavioral testing, the animals were anesthetized (sodium pentobarbital, 25 mg/kg, i.v.) and the cortex and cryoloops were exposed to determine the extent that cooling spread from each loop. Cortical temperatures surrounding the cooling loops were measured using microthermocouples (150 μm in diameter; Omega Engineering). At each temperature recording site, measurements were taken with cortex warm (and uncooled, Fig. 4A) and then with the FAES loop cooled to 3 ± 1 °C (Fig. 4B). Following perfusion and histological processing, the location of each temperature reading was reconstructed on the tissue from which it was taken, resulting in a representation of induced thermoclines like those illustrated in Fig. 4.
Supplementary Material
Acknowledgments
We thank Dr. R. K. Shepherd for advice on ototoxic procedures and Drs. S. Shapiro and A. Rice for use of their auditory brainstem response equipment. This work was supported by National Institutes of Health Grant NS-039640, the Jeffress Foundation (M.A.M.), and the Canadian Institutes of Health Research (S.G.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018519108/-/DCSupplemental.
References
- 1.Obretenova S, Halko MA, Plow EB, Pascual-Leone A, Merabet LB. Neuroplasticity associated with tactile language communication in a deaf-blind subject. Front Hum Neurosci. 2010 doi: 10.3389/neuro.09.060.2009. 10.3389/neuro.09.060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 3.Lessard N, Paré M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395:278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- 4.Grant AC, Thiagarajah MC, Sathian K. Tactile perception in blind Braille readers: A psychophysical study of acuity and hyperacuity using gratings and dot patterns. Percept Psychophys. 2000;62:301–312. doi: 10.3758/bf03205550. [DOI] [PubMed] [Google Scholar]
- 5.Weeks R, et al. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sathian K. Practice makes perfect: Sharper tactile perception in the blind. Neurology. 2000;54:2203–2204. doi: 10.1212/wnl.54.12.2203. [DOI] [PubMed] [Google Scholar]
- 7.Voss P, et al. Early- and late-onset blind individuals show supra-normal auditory abilities in far-space. Curr Biol. 2004;14:1734–1738. doi: 10.1016/j.cub.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 8.Levänen S, Hamdorf D. Feeling vibrations: Enhanced tactile sensitivity in congenitally deaf humans. Neurosci Lett. 2001;301:75–77. doi: 10.1016/s0304-3940(01)01597-x. [DOI] [PubMed] [Google Scholar]
- 9.Bavelier D, et al. Visual attention to the periphery is enhanced in congenitally deaf individuals. J Neurosci. 2000;20:RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavelier D, Neville H. Cross-modal plasticity: Where and how? Nat Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 11.Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 2010;13:1421–1427. doi: 10.1038/nn.2653. [DOI] [PubMed] [Google Scholar]
- 12.Bosworth RG, Dobkins KR. Visual field asymmetries for motion processing in deaf and hearing signers. Brain Cogn. 2002;49:170–181. doi: 10.1006/brcg.2001.1498. [DOI] [PubMed] [Google Scholar]
- 13.Bross M. Residual sensory capacities of the deaf: A signal detection analysis of a visual discrimination task. Percept Mot Skills. 1979;48:187–194. doi: 10.2466/pms.1979.48.1.187. [DOI] [PubMed] [Google Scholar]
- 14.Finney EM, Dobkins KR. Visual contrast sensitivity in deaf versus hearing populations: Exploring the perceptual consequences of auditory deprivation and experience with a visual language. Brain Res Cogn Brain Res. 2001;11:171–183. doi: 10.1016/s0926-6410(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds HN. Effects of foveal stimulation on peripheral visual processing and laterality in deaf and hearing subjects. Am J Psychol. 1993;106:523–540. [PubMed] [Google Scholar]
- 16.Kral A, Schröder JH, Klinke R, Engel AK. Absence of cross-modal reorganization in the primary auditory cortex of congenitally deaf cats. Exp Brain Res. 2003;153:605–613. doi: 10.1007/s00221-003-1609-z. [DOI] [PubMed] [Google Scholar]
- 17.Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: Unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- 19.Burton H, McLaren DG. Visual cortex activation in late-onset, Braille naive blind individuals: An fMRI study during semantic and phonological tasks with heard words. Neurosci Lett. 2006;392:38–42. [Google Scholar]
- 20.Meredith MA, Clemo HR. Auditory cortical projection from the anterior ectosylvian sulcus (field AES) to the superior colliculus in the cat: An anatomical and electrophysiological study. J Comp Neurol. 1989;289:687–707. doi: 10.1002/cne.902890412. [DOI] [PubMed] [Google Scholar]
- 21.Stein BE, Wallace MW, Stanford TR, Jiang W. Cortex governs multisensory integration in the midbrain. Neuroscientist. 2002;8:306–314. doi: 10.1177/107385840200800406. [DOI] [PubMed] [Google Scholar]
- 22.Las L, Shapira AH, Nelken I. Functional gradients of auditory sensitivity along the anterior ectosylvian sulcus of the cat. J Neurosci. 2008;28:3657–3667. doi: 10.1523/JNEUROSCI.4539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith MA, Allman BL. Subthreshold multisensory processing in cat auditory cortex. Neuroreport. 2009;20:126–131. doi: 10.1097/WNR.0b013e32831d7bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meredith MA, Keniston LR, Dehner LR, Clemo HR. Cross-modal projections from somatosensory area SIV to the auditory field of the anterior ecosylvian sulcus (FAES) in cat: Further evidence for subthreshold forms of multisensory processing. Exp Brain Res. 2006;72:472–484. doi: 10.1007/s00221-006-0356-3. [DOI] [PubMed] [Google Scholar]
- 25.Meredith MA. In: Handbook of Multisensory Processes. Spence C, Calvert G, Stein B, editors. Cambridge, MA: MIT Press; 2004. pp. 343–355. [Google Scholar]
- 26.Wallace MT, Carriere BN, Perrault TJ, Jr, Vaughan JW, Stein BE. The development of cortical multisensory integration. J Neurosci. 2006;26:11844–11849. doi: 10.1523/JNEUROSCI.3295-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carriere BN, et al. Visual deprivation alters the development of cortical multisensory integration. J Neurophysiol. 2007;98:2858–2867. doi: 10.1152/jn.00587.2007. [DOI] [PubMed] [Google Scholar]
- 28.Rauschecker JP, Korte M. Auditory compensation for early blindness in cat cerebral cortex. J Neurosci. 1993;13:4538–4548. doi: 10.1523/JNEUROSCI.13-10-04538.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renier LA, et al. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68:138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson CR, Graybiel AM. Ectosylvian visual area of the cat: Location, retinotopic organization, and connections. J Comp Neurol. 1987;261:277–294. doi: 10.1002/cne.902610209. [DOI] [PubMed] [Google Scholar]
- 31.Lomber SG, Payne BR. Cerebral areas mediating visual redirection of gaze: Cooling deactivation of 15 loci in the cat. J Comp Neurol. 2004;474:190–208. doi: 10.1002/cne.20123. [DOI] [PubMed] [Google Scholar]
- 32.McIlwain JT. Distributed spatial coding in the superior colliculus: A review. Vis Neurosci. 1991;6:3–13. doi: 10.1017/s0952523800000857. [DOI] [PubMed] [Google Scholar]
- 33.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlen SJ, Kahn DM, Krubitzer L. Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience. 2006;142:843–858. doi: 10.1016/j.neuroscience.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 35.Allman BL, Keniston LP, Meredith MA. Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci USA. 2009;106:5925–5930. doi: 10.1073/pnas.0809483106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korte M, Rauschecker JP. Auditory spatial tuning of cortical neurons is sharpened in cats with early blindness. J Neurophysiol. 1993;70:1717–1721. doi: 10.1152/jn.1993.70.4.1717. [DOI] [PubMed] [Google Scholar]
- 37.Roe AW, Pallas SL, Kwon YH, Sur M. Visual projections routed to the auditory pathway in ferrets: Receptive fields of visual neurons in primary auditory cortex. J Neurosci. 1992;12:3651–3664. doi: 10.1523/JNEUROSCI.12-09-03651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauschecker JP, Kniepert U. Auditory localization behaviour in visually deprived cats. Eur J Neurosci. 1994;6:149–160. doi: 10.1111/j.1460-9568.1994.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 39.Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15:552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- 40.Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993;70:205–215. doi: 10.1016/0378-5955(93)90159-x. [DOI] [PubMed] [Google Scholar]
- 41.Mellott JG, et al. Areas of cat auditory cortex as defined by neurofilament proteins expressing SMI-32. Hear Res. 2010;267:119–136. doi: 10.1016/j.heares.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomber SG, Payne BR, Horel JA. The cryoloop: An adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods. 1999;86:179–194. doi: 10.1016/s0165-0270(98)00165-4. [DOI] [PubMed] [Google Scholar]
- 43.Lomber SG, Malhotra S, Hall AJ. Functional specialization in non-primary auditory cortex of the cat: Areal and laminar contributions to sound localization. Hear Res. 2007;229:31–45. doi: 10.1016/j.heares.2007.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.