Abstract
Background
Although family history of cancer is widely ascertained in research and clinical care, little is known about assessment methods, accuracy, or other quality measures. Given its widespread use in cancer screening and surveillance, better information is needed regarding the clarity and accuracy of family history information reported in the general population.
Methods
This telephone survey in Connecticut examined coherence and completeness of reports from 1,019 respondents about 20,504 biological relatives.
Results
Of 2,657 cancer reports, 97.7% were judged consistent with malignancy (vs. benign or indeterminate conditions); 79% were site-specific, 10.8% had unspecified cancer sites and 8.6% had “ill-defined” sites. Only 6.1% of relatives had unknown histories. Unknown histories and ambiguous sites were significantly higher for second-degree relatives. The adjusted percentage of first-degree relative reports with ambiguous sites increased with decreasing education and African-American race of survey respondents, and with deceased vital status of relatives. Ambiguous second-degree relative reports were also associated with deceased vital status, and with male gender of respondents.
Conclusions
These findings suggest that family history of cancer reports from the general population are generally complete and coherent.
Impact
Strategies are needed to improve site specificity and thus maximize the utility of such information in primary care settings.
Keywords: family history of cancer, quality, methods, assessment, population-based, survey research
Introduction
Family history of cancer is a major risk factor for many malignancies (1), functioning as a surrogate for genetic susceptibility to disease, or high risk behaviors and environmental exposures that cluster within families. Family history is obtained in widely varying degrees of detail in both clinical and population-based cancer research. In clinical care, family history of cancer is used to make recommendations for screening (2,3,4), genetic counseling referrals, genetic testing, and risk-reducing interventions (5,6,7,8).
Routine family history assessment has been proposed as a public health tool for risk stratification of primary care populations in order to tailor clinical interventions such as cancer screening and genetic counseling referrals, and to motivate risk-reducing behaviors (9,10,11,12). In 2004, the U.S. Surgeon General’s Family History Initiative was launched to promote awareness and ascertainment of family history information (13). In public health surveillance, family history information is periodically collected in population-based surveys, such as the National Health Interview Survey (NHIS) in 2000 and 2005 (14), the California Health Interview Survey in 2001 and 2005 (15), and various state surveys in the Behavioral Risk Factor Surveillance System (16). Family history variables can be used for estimating population prevalence of familial risk, stratifying health and behavior outcomes, and planning for allocation of preventive resources. For example, Ramsey et al. (17) used 2000 NHIS data to estimate the U.S. population prevalence of family history of breast, colorectal, lung, prostate, and ovarian cancer.
Methodologic research suggests that the quality and utility of family history of cancer are highly variable (18,19,20). Overall, validation of data collection instruments has been limited (21). Studies comparing self-reports against medical records indicate that accuracy varies by cancer site and the characteristics of the reporting family member (17,22,23). Because little is known about the qualitative characteristics of self-reported family histories and the types of reporting error, particularly in general population samples, methodologic research in this area may help improve family history assessment in primary care.
In 2001, we conducted a random digit dial survey in Connecticut (CT) to ascertain reports of family history of cancer in a general population household survey. This paper describes the coherence and completeness of such reports in a unique, population-based sample of 1,019 survey respondents who reported cancer histories for 20,504 biological relatives. Multivariate models are presented that identify demographic, sociologic, and medical characteristics associated with coherent reporting of specific cancer sites and general awareness of relatives’ cancer histories. The implications for clinicians and researchers are discussed. Validity of the family histories will be reported elsewhere.
Methods
Survey and Sampling Methods
The state of CT was selected as the survey site because it has the oldest population-based cancer registry in the U.S. (dating back to 1935), thus facilitating validation of cancer histories among older generations of relatives. The study protocol was approved by IRBs at the National Cancer Institute and Westat, Inc, the company that conducted the fieldwork. Trained interviewers with prior experience using family history questionnaires screened 2,418 households by random digit dial (RDD) methods to identify households with eligible members, as previously described (24). If corresponding addresses were available through reverse directories, an informational letter and pamphlet were initially sent by courier to each household; if the address was unavailable, first contact was by telephone. In the pamphlet, potential respondents were told, “In the first interview, we will ask questions about your family tree, the health of your blood relatives, and some information about yourself. You do not need to prepare ahead of time.”
To be eligible, subjects had to be between ages 25–64 years, raised by at least one biological relative, and have parents, or at least one parent and sibling, born or raised in the U.S. or Puerto Rico. The latter criteria were used to enrich the sample with participants whose relatives’ medical records could be obtained for a separate validation study. Respondents with the most recent birthday were selected in households with multiple eligible subjects. A total of 1,380 individuals completed the first of two sequential computer-assisted telephone interviews (CATI), which had an average duration of 20 minutes.
In the first interview, respondents provided a pedigree of all first-degree relatives (FDR) and second-degree relatives (SDR) except grandchildren, who were excluded due to low cancer prevalence. They were asked if each relative ever had cancer (response categories=yes, no, don’t know), and if yes: “What type of cancer did he/she have, or in what part of the body did the cancer start”? The latter question had an open-ended format in order to evaluate response patterns in the analysis. If a distinct recognizable body part was not provided, the interviewers were instructed to probe by asking: “Specifically, where in the body did the cancer start?” To reduce observer bias, interviewers were instructed to record verbatim cancer descriptions, including incidentally reported details such as metastases, co-morbidities, and medical procedures. Up to three primary cancers were ascertained per relative, along with age or year of diagnosis, vital status, date of birth, and date of death, if applicable. Personal cancer history and age at diagnosis were also ascertained from each respondent. This questionnaire is available for public use (25).
A second telephone interview with respondents to the first questionnaire was conducted within one month to allow time for random sampling of a subset of relatives whose cancer histories would be validated. In the second interview, respondent race/ethnicity, education, and income were obtained, along with additional information about the sampled relatives and permission to contact them for study recruitment. Of the 1,380 respondents who completed the first interview, 1,019 subsequently completed the second interview, whereas 216 could not be re-contacted and 145 refused. A thank-you letter and $20 compensation were sent to respondents completing the second interview. The CASRO (Council of American Survey Research Organizations) response rates (26) for the first and second interviews were 70% and 74%, respectively; the combined response rate (70% × 74%) was 51.8%.
Respondents were assigned sampling weights for each interview to adjust for differential selection probabilities and rates of non-response, and for post-stratification, which constrained the weighted sample to approximate the age and sex distribution of 2000 Census values for CT. When the responders and non-responders to the second interview were compared, there were no significant differences in the unadjusted percentages of males vs. females, or of those who did or did not have a personal or family history of cancer. Responders were significantly older, but the survey weights adjusted for this age difference.
Cancer coding and classification
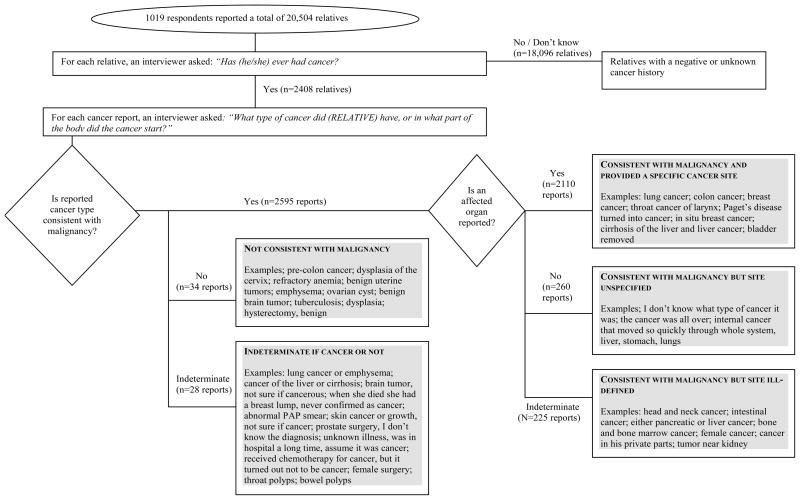
ICD-9 codes were assigned to the reported medical information about respondents and relatives. This system provides a three-digit disease code for cancers in a specific organ, and a fourth digit to further specify tumor location or some histologies (27). There are also codes for disseminated cancer of unspecified origin, cancers in “ill-defined” body regions, and medical procedures for cancer screening and treatment. Two nosologists independently coded up to four primary cancers per relative, as well as additional medical conditions or procedures if reported. Computer algorithms identified disparate coding and ambiguous reports for further review. The codes were independently reviewed for accuracy (AG, LW), and resolution of disparate coding and ambiguous reports was achieved by consensus with oncologists (MM, MHG). Reviewers also classified each report by its “coherence”, namely, whether the primary medical condition was judged to be consistent with malignancy (vs. a non-malignant or indeterminate condition), and whether the description was specific enough to identify an affected organ (vs. an ill-defined or unspecified cancer site). Figure 1 describes the ascertainment of verbatim reports and their classification into the following five categories for the analysis: 1) consistent with malignancy/a specific primary cancer site was reported; 2) consistent with malignancy/the cancer site was unspecified; 3) consistent with malignancy/the cancer site was ill-defined (e.g., a body region or several possible primary cancer sites were reported); 4) not consistent with malignancy, or; 5) indeterminate if cancer or not.
Figure 1.
Classification of 2,657 Family History of Cancer Self-Reports by Consistency and Site-Specificity (i.e., Coherence)
Statistical Methods
The analyses were performed using SAS v.9.1 (28) callable SUDAAN v.9.0 statistical software (29), and accounted for the sample weighting. Logistic regression analysis was conducted to identify respondent and relative characteristics associated with ill-defined or unspecified reports (vs. well-defined reports). A separate analysis examined characteristics associated with unknown cancer history (vs. a known positive or negative history) among relatives. For relatives with a positive cancer history, the completeness of information about age at diagnosis was assessed.
Final logistic regression models were obtained through backwards stepwise elimination of independent variables. As a criterion for elimination at each step, variables with the largest p-values above 0.05 in the two-sided Wald test of global significance were removed. Respondent sex, age and race/ethnicity were included in the final models regardless of their significance levels, to control for variability in the reporting of family history. The final models provided predicted margins (i.e., percentages that were adjusted for covariates) and 95% confidence intervals (C.I.) (30). Pair-wise differences in the referent vs. other adjusted percentages were evaluated using two-sided t-tests.
The standard error estimation in SUDAAN used a replicate weight approach based on the delete-one jackknife method (31). This approach accounts for extra variation from intra-familial correlations due to respondents reporting about multiple relatives, and the sample weighting used in the analysis. However, for the models of unknown cancer history in FDR, we used the linearization method of standard error estimation (30) because the small number of observations in the unknown category made the estimation of jackknife variances unstable. The linearization method also takes into account sample design and weighting, but tends to estimate slightly larger variances.
A total of 28,629 relatives, including 8,596 FDR and 20,033 SDR, were ascertained in the first interview. However, because respondent demographic characteristics were obtained only in the second interview, the current analysis was restricted to relatives of responders to the second interview, which included 20,504 total, 6,242 FDR, and 14,262 SDR (after excluding 74 with missing gender or cancer history information). When the relatives of responders and non-responders were compared, there was no significant difference in gender; however the relatives of responders were slightly more likely to have a positive or unknown cancer history (vs. none), and be deceased or a FDR. Mean family size was lower, and mean relative age at interview or time of death was higher. Although these differences were small in absolute terms, ranging from 0.5–4.0 percent, they were statistically significant (p≤0.05) due to the large sample size.
Results
Of the 1,019 respondents in the study, 8.3% [6.7–10.3%] reported a personal history of cancer, and 82.8% [79.7–85.6%] reported at least one relative in the family ever diagnosed with cancer; 51.9% [48.4–55.5%] reported at least one FDR and 71.6% [68.1–74.8%] reported at least one SDR. A slightly higher percentage of respondents with a personal history than without also reported a family history, but the difference was not statistically significant (p=.54).
Consistency and specificity of family cancer reports
Respondents reported 2,657 cancers among the 2,408 total relatives with a positive cancer history. As shown in Table 1, 97.7% of all reports were judged to be consistent with malignancy. However, when the reports were classified into the five analytic categories, only 79.0% were both consistent and site-specific, whereas 10.1% were consistent/unspecified site, and 8.6% were consistent/ill-defined site. A nominal 1.3% of reports were classified as not consistent with malignancy and 1.1% were indeterminate. As described below, these percentages varied significantly (p<.0001) by kinship when the 918 cancer reports about FDR (n=781) were compared with the 1,739 reports about SDR (n=1,627).
Table 1.
Adjusted weighted percentages of cancer reports with varying levels of consistency and site-specificity in relatives with a positive cancer history*
| Type of report** | Reports About All Relatives (N=2,657 reports) | Reports About First-degree Relatives (N=918 reports) | Reports About Second-degree Relatives (N=1,739 reports) |
|---|---|---|---|
| % (95% C.I.) | % (95% C.I.) | % (95% C.I.) | |
| Consistent with malignancy | 97.7 (96.9–98.3) | 95.3 (93.3–96.7) | 98.9 (98.2–99.3) |
| well-defined site | 79.0 (77.0–80.8) | 86.6 (83.6–89.2) | 75.0 (72.3–77.6) |
| unspecified site | 10.1 (8.5–11.9) | 2.1 (1.2–3.8) | 14.2 (11.8–16.9) |
| ill-defined site | 8.6 (7.5–9.9) | 6.5 (4.8–8.7) | 9.7 (8.2–11.4) |
| Not consistent with malignancy | 1.3 (0.9–1.9) | 2.9 (1.9–4.3) | 0.4 (0.2–1.0) |
| Indeterminate | 1.1 (0.7–1.7) | 1.8 (0.9–3.8) | 0.7 (0.4–1.2) |
After identifying 2,408 relatives (781 first-degree and 1,627 second-degree) with a cancer history, the 1,019 respondents were asked to report the cancer type or where in the body the cancer started. All percentages of cancer reports are weighted to the Connecticut population and adjusted for differential selection probabilities, rates of non-response, and post-stratification.
See Figure 1 for examples of reports in the different categories.
Consistent/site-specific reports
Overall, 86.6% of FDR reports were consistent/site-specific, compared with only 75.0% of SDR reports. Among the 2,110 total reports, the ten most commonly-reported sites were breast, lung, melanoma, prostate, colon/rectum, stomach, non-melanoma skin cancer, leukemia, lymphoma, and brain, in decreasing order (data not shown).
Consistent/ill-defined or unspecified reports
As shown in Table 1, 8.6% of FDR reports were consistent with malignancy but had an ill-defined (6.5%) or unspecified cancer site (2.1%). In contrast, a much larger percentage (23.9%) of SDR reports were ill-defined (9.7%) or unspecified (14.2%). Among the 225 ill-defined reports, the most common ambiguities were body regions or multiple organs mentioned instead of a single primary site. Forty percent of ill-defined reports were suggestive of digestive system cancers, including 13.3% that mentioned “abdomen” or “intestines” and 27.1% that mentioned other regions (“mouth, “GI tract”), multiple organs with at least one in the digestive system (e.g., “either liver or kidney cancer”), or mixed organs and regions (e.g., “stomach or intestinal cancer”). Furthermore, 38% were suggestive of respiratory system cancers, including 25.3% that mentioned “throat cancer” and 12.9% that mentioned other regions (“chest”), multiple organs with at least one in the respiratory system (“larynx or esophageal”), or mixed organs and regions (“lung or throat”). The remaining ill-defined reports were diverse with regard to possible site of origin. In contrast to ill-defined reports, the 260 unspecified reports were too ambiguous to even suggest a body region or organ (e.g., “the cancer was all over by the time they found it”; “I don’t know what kind of cancer it was”).
Table 2 shows combined percentages of ill-defined or unspecified reports, by respondent and relative characteristics. After adjustment for covariates in the table, the percentage of FDR reports decreased with increasing educational level of the respondent (p=0.005, test for trend). It also varied by respondent race/ethnicity (p=0.008) and by relatives’ vital status (p=0.03), with pair-wise comparisons showing higher percentages of ambiguous reports if respondents were African-American (vs. white) or relatives were deceased (vs. living). Percentages of ill-defined or unspecified reports about SDR varied significantly by respondent gender (p=0.001) and relatives’ vital status (p<0.0001). In pair-wise comparisons, higher percentages were observed if respondents were male or the relative’s vital status was deceased or unknown.
Table 2.
Unadjusted and adjusted weighted percentages of ill-defined or unspecified (versus well-defined) cancer reports, by relative and respondent characteristics*
| Characteristics | First-degree Relatives | Second-degree Relatives | ||
|---|---|---|---|---|
| Unadjusted % (95% C.I.) | Adjusted % (95% C.I.) | Unadjusted % (95% C.I.) | Adjusted % (95% C.I.) | |
| Respondent characteristics | ||||
| Sex | ||||
| female | 9.0 (5.7–13.9) | 19.7 (16.9–22.9) | 19.7 (16.7–22.6) | |
| male | 9.2 (6.3–13.1) | 30.6 (25.6–36.1) | 30.7 (25.3–36.0) | |
| p=0.001 | ||||
| Age | ||||
| 25–34 | 3.1 (1.0–9.6) | 21.4 (14.7–30.1) | ||
| 35–44 | 7.3 (3.9–13.1) | 21.6 (17.1–26.9) | ||
| 45–54 | 7.0 (4.7–10.5) | 27.0 (22.5–32.1) | ||
| 55–64 | 16.6 (10.3–25.7) | 27.0 (21.7–33.1) | ||
| Race/ethnicity | ||||
| White | 7.3 (5.7–9.3) | 7.8 (5.7–9.8) | 24.2 (21.5–27.0) | |
| African-American | 46.2 (18.3–76.6) | 32.6 (12.1–53.0) | 29.4 (14.2–51.3) | |
| Hispanic | 21.4 (9.0–43.1) | 16.8 (0.0–34.8) | 15.9 (8.3–28.4) | |
| other | 8.3 (2.3–25.7) | 5.9 (0.0–12.9) | 24.3 (11.9–43.1) | |
| p=0.008 | ||||
| Education | ||||
| < high school, vocational, or other | 13.8 (8.3–21.9) | 12.1 (5.9–18.3) | 24.9 (17.5–34.1) | |
| high school | 19.9 (11.2–32.8) | 15.2 (8.8–21.6) | 30.4 (23.3–38.6) | |
| 1–4 years college | 7.3 (4.9–10.9) | 8.5 (4.9–12.1) | 21.2 (17.3–25.8) | |
| > college | 2.5 (1.0–6.1) | 2.9 (0.1–5.7) | 25.6 (20.7–31.1) | |
| p=0.005, test for trend | ||||
| Main parental living arrangement during childhood | ||||
| Lived apart | 9.0 (4.3–17.9) | 20.7 (15.7–26.8) | ||
| Lived together | 9.1 (6.7–12.2) | 24.7 (21.9–27.7) | ||
| Ever had cancer | ||||
| no or unknown | 9.3 (6.8–12.6) | 24.8 (22.1–27.8) | ||
| yes | 7.6 (3.6–15.5) | 17.1 (10.5–26.6) | ||
| Household income | ||||
| ≤$20,000 | 17.1 (10.2–27.3) | 27.8 (20.0–37.3) | ||
| $20,001–40,000 | 7.9 (4.3–14.2) | 26.2 (18.7–35.5) | ||
| $40,001–60,000 | 14.3 (6.6–28.1) | 30.3 (22.0–40.0) | ||
| $60,001–80,000 | 7.6 (3.8–14.5) | 21.0 (16.3–26.5) | ||
| >$80,000 | 5.5 (3.2–9.5) | 21.9 (17.9–26.6) | ||
| Don’t know/refused | 10.3 (4.0–23.8) | 25.0 (13.6–41.4) | ||
| Total number of relatives | ||||
| ≤15 | 8.0 (5.0–12.6) | 21.9 (16.5–28.5) | ||
| 16–19 | 7.0 (3.6–13.3) | 21.1 (15.5–28.1) | ||
| 20–25 | 9.0 (5.7–14.0) | 28.6 (24.0–33.7) | ||
| ≥26 | 11.5 (5.9–21.1) | 23.5 (18.7–29.2) | ||
| Relative characteristics | ||||
| Sex | ||||
| female | 6.5 (4.2–10.1) | 24.8 (21.6–28.4) | ||
| male | 11.4 (7.6–16.7) | 23.4 (19.9–27.2) | ||
| Vital status | ||||
| living | 5.2 (3.3–8.3) | 6.1 (3.4–8.8) | 16.9 (12.0–23.3) | 17.5 (11.8–23.2) |
| deceased | 12.8 (9.0–17.8) | 11.3 (7.3–15.3) | 26.1 (23.1–29.3) | 25.9 (22.6–29.1) |
| unknown | NA | NA | 61.3 (0.4–99.9) | 70.4 (61.8–78.9) |
| P=0.03 | P<.00001 | |||
| Generation** | ||||
| same or younger | 12.6 (5.6–25.9) | 10.7 (4.2–24.7) | ||
| next older | 8.1 (6.1–10.6) | 26.0 (22.7–29.6) | ||
| oldest | NA | 22.8 (18.8–27.4) | ||
All percentages are weighted to the Connecticut population and adjusted for differential selection probabilities, rates of non-response, and post-stratification. Adjusted percentages control for respondent age and race/ethnicity, and for the other variables shown. Cells for variables that were not applicable to a particular model are labeled NA; cells for variables that were eliminated through backwards stepwise regression are blank.
Same or younger generation as the respondent includes siblings, children, and/or nieces and nephews; next older includes parents, aunts, and uncles; oldest includes grandparents.
Unknown cancer histories
The distribution of cancer history among the 20,504 relatives was based on answers to the question “Did he/she ever have cancer?” As expected in a general population sample (9), the large majority, 82.7%, had no reported history of cancer and 11.3% had a positive history. Only 6.1% had an unknown cancer history, although this proportion was substantially higher among SDR (8.5%) than in FDR (0.6%) (p=.0005). In logistic regression analysis of all relatives combined, SDR were significantly more likely to have an unknown cancer history than FDR (7.0% [6.0–7.9] vs. 1.5% [1.0–1.9%], respectively), after adjustment for the characteristics in Table 3.
Table 3.
Unadjusted and adjusted weighted percentages of relatives with unknown cancer history, by respondent and relative characteristics*
| Characteristics | First-degree Relatives (N=6,242) | Second-degree Relatives (N=14,262) | ||
|---|---|---|---|---|
| Unadjusted % (95% C.I.) | Adjusted % (95% C.I.) | Unadjusted % (95% C.I.) | Adjusted % (95% C.I.) | |
| Respondents | ||||
| Sex | ||||
| female | 0.6 (0.4–0.9) | 7.0 (5.8–8.3) | 7.0 (5.7–8.2) | |
| male | 0.7 (0.4–1.2) | 10.2 (8.5–12.3) | 10.3 (8.5–12.1) | |
| p=0.002 | ||||
| Age | ||||
| 25–34 | 0.4 (0.2–1.0) | 6.2 (4.4–8.7) | ||
| 35–44 | 0.6 (0.3–1.2) | 8.5 (6.8–10.6) | ||
| 45–54 | 0.8 (0.4–1.3) | 8.5 (6.8–10.5) | ||
| 55–64 | 0.7 (0.3–1.6) | 10.6 (8.2–13.6) | ||
| Race/ethnicity | ||||
| White | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 8.1 (6.9–9.4) | |
| African-American | 0.7 (0.2–2.0) | 0.5 (0.0–1.1) | 9.0 (5.1–15.5) | |
| Hispanic | 1.7 (0.6–5.0) | 1.6 (0.0–3.3) | 6.8 (3.6–12.4) | |
| other | 1.9 (0.8–4.5) | 2.4 (0.4–4.4) | 15.8 (9.5–25.1) | |
| p=0.01 | ||||
| Education | ||||
| < high school, vocational, or other | 1.6 (0.8–3.2) | 1.2 (0.5–1.8) | 12.2 (9.0–16.5) | 11.8 (8.3–15.3) |
| high school | 1.0 (0.5–2.0) | 0.8 (0.1–1.5) | 8.1 (6.0–10.9) | 8.1 (5.9–10.3) |
| 1–4 years college | 0.3 (0.1–0.5) | 0.3 (0.1–0.6) | 7.2 (5.9–8.9) | 7.3 (6.1–8.6) |
| > college | 0.4 (0.2–0.9) | 0.5 (0.1–0.9) | 8.4 (6.0–11.5) | 8.7 (6.4–11.0) |
| p=0.04, test for trend | p=0.05 | |||
| Main parental living arrangement during childhood | ||||
| lived together | 0.5 (0.3–0.7) | 7.5 (6.5–8.8) | 7.8 (6.6–9.0) | |
| lived apart | 1.8 (0.9–3.5) | 13.7 (10.9–17.1) | 12.4 (10.0–14.8) | |
| p=0.0004 | ||||
| Ever had cancer | ||||
| no or unknown | 0.6 (0.4–0.8) | 8.1 (7.0–9.3) | 8.0 (6.9–9.2) | |
| yes | 1.3 (0.5–3.2) | 12.4 (8.3–18.2) | 13.2 (9.1–17.3) | |
| Household income | p=0.006 | |||
| ≤$20,000 | 0.6 (0.1–2.9) | 9.3 (6.6–13.0) | ||
| $20,001–40,000 | 1.2 (0.6–2.3) | 11.4 (8.3–15.5) | ||
| $40,001–60,000 | 1.0 (0.5–2.0) | 7.2 (5.0–10.3) | ||
| $60,001–80,000 | 0.1 (0.0–0.7) | 7.8 (5.5–11.0) | ||
| >$80,000 | 0.5 (0.2–0.9) | 6.8 (5.4–8.5) | ||
| Don’t know/refused | 0.9 (0.3–2.8) | 13.4 (9.1–19.2) | ||
| Total number of relatives | ||||
| ≤15 | 1.0 (0.5–1.7) | 8.5 (6.7–10.7) | ||
| 16–19 | 0.4 (0.2–1.1) | 6.0 (4.3–8.3) | ||
| 20–25 | 0.3 (0.1–0.7) | 9.0 (7.3–11.1) | ||
| ≥26 | 0.8 (0.4–1.5) | 9.6 (7.4–12.3) | ||
| Relatives | ||||
| Sex | ||||
| female | 0.5 (0.3–0.9) | 7.5 (6.3–8.9) | ||
| male | 0.8 (0.5–1.2) | 9.4 (8.3–10.7) | ||
| Vital status | ||||
| living | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) | 3.0 (2.2–3.9) | 4.0 (2.8–5.1) |
| deceased | 1.0 (0.5–2.1) | 0.5 (0.1–0.8) | 12.4 (10.7–14.3) | 9.7 (8.1–11.2) |
| unknown | 49.0 (22.7–75.9) | 22.7 (1.6–43.8) | 73.9 (61.8–83.2) | 58.6 (44.9–72.3) |
| p<0.0001 | p<0.0001 | |||
| Generation** | ||||
| same or younger | 0.2 (0.1–0.4) | 0.3 (0.1–0.4) | 1.2 (0.6–2.2) | 2.3 (0.9–3.7) |
| next older | 1.4 (1.0–2.1) | 1.4 (0.7–2.0) | 10.2 (8.5–12.2) | 9.9 (8.2–11.6) |
| oldest | NA | NA | 12.9 (11.1–14.8) | 9.7 (8.2–11.2) |
| p=0.0001 | p<0.0001 | |||
All percentages are weighted to the Connecticut population and adjusted for differential selection probabilities, rates of non-response, and post-stratification. Adjusted percentages control for respondent age and race/ethnicity, and for other respondent and relative characteristics that were significantly associated with unknown cancer history in final logistic regression models. Cells for variables that were not applicable to a particular model are labeled NA; cells for variables that were eliminated through backwards stepwise regression are blank.
Same or younger generation as the respondent includes siblings, children, and/or nieces and nephews; older includes parents, aunts, and uncles; oldest includes grandparents.
Table 3 shows percentages of relatives with an unknown cancer history (vs. a reported positive or negative history), by respondent and relative characteristics. The percentage of FDR with unknown history decreased with increasing educational level of the respondents (p=0.04, test for trend). It also varied by respondent race/ethnicity (p=0.01), with respondents in the “other” category (i.e., Asians, Pacific Islanders, American Indians/Alaska Natives, mixed, unknown) reporting significantly more unknown histories than whites. Unknown history further varied by relative vital status (p<0.0001) and generation within the family structure (p=0.0001). Specifically, the percentage with an unknown history was higher if the vital status was unknown (vs. living), or the relative was one generation older (i.e., vs. the same or of a younger generation).
A larger set of respondent characteristics was associated with unknown cancer history among SDR, including gender (p=0.002), main living arrangement of parents during childhood (p=0.0004), personal history of cancer (p=0.006), and education (p=0.05), although no trends or significant pair-wise differences were observed across educational levels. As with FDR, unknown cancer history varied by SDR vital status (p<0.0001) and generation (p<0.0001). In pair-wise comparisons, the percentage of relatives with unknown cancer history was higher if respondents were male, had a personal history of cancer (vs. none or an unreported history), or the parents had lived apart for most of the respondents childhood (vs. together). It was also higher if relative’s vital status was unknown or deceased, or the relative was one or two generations older than the respondent.
Age at diagnosis
Respondents were asked to provide age or year of diagnosis for all relatives’ cancers except non-melanoma skin cancers. Year of diagnosis was used to calculate age at diagnosis if birth year or current age was also provided. If unable to provide that level of detail, respondents were next asked for ten-year age or year ranges and whether the diagnosis was early, mid, or late within that range. If range was unknown, the respondent was asked whether the relative was “under age 50” or “50 or older” when diagnosed. Age at diagnosis was then estimated. For example, a cancer reported early in the 60-year age range was assigned a diagnosis age of 62, whereas a cancer reported as under age 50 was assigned age 45. Of 2,488 reports that were judged consistent with cancer (excluding the non-melanoma skin cancers), 16% specified an age at diagnosis, 38% included a year, 42% provided a ten-year age or year range, 2% specified <50 or 50+ years old, and 2% had no information.
Discussion
This study characterized the completeness and coherence of family cancer history reports ascertained in a general population sample. Only 6.1% of relatives had a completely unknown cancer history and there was little evidence of over-reporting of other conditions as cancer. Eighty three percent of respondents reported a positive history of cancer in at least one relative. However, only 79% of reports describing types of cancer diagnosed in relatives were judged to be fully coherent, based on the dual criteria of consistency with malignancy and site-specificity. Coherence of reports varied significantly in association with a number of covariates, discussed in greater detail below. These findings suggest that family history data may be sufficiently detailed to provide useful risk assessment in the primary care setting or to monitor familial risk patterns in population surveys. However, risk assessment may be hampered by ambiguous cancer site information in approximately one fifth of reported diagnoses.
Since the study questionnaire used an open-ended format to ascertain type of cancer, the responses were distributed across a qualitative continuum. There were three intermediate categories of reports in between the 79% fully coherent reports and the 1% indicative of indeterminate disease. The ill-defined reports mentioned body regions or several distinct organs, reflecting respondent uncertainty about the exact diagnosis. The unspecified reports lacked information about any possible site of origin, suggesting wider gaps in respondent knowledge, or perhaps some cases with disseminated disease at diagnosis where site of origin was not determined. Given that family history reports must be interpretable in the context of disease risk in order to have clinical utility (32), such reports would generally have little use in the clinical setting, where familial risk patterns are evaluated based on the specific cancer sites involved. The third intermediate category contained reports of non-malignant diseases, indicating that some respondents held imprecise beliefs about what constitutes cancer. Although contradictory, these reports can still be useful for ruling out cancer.
Not surprisingly, both the coherence and completeness of family history reports, and respondent awareness that their relatives did or did not have cancer, varied by kinship and other factors that influence communication within families. A much greater percentage of ambiguous cancer reports concerned SDR, who also had more unknown cancer histories than FDR. Given that SDR outnumber FDR in almost all families (24) and information about them is generally of lower quality, the cost vs. benefit of collecting SDR data warrants consideration in settings where its value may be limited. One consequence of not seeking SDR information is that cancer patterns suggesting an underlying hereditary susceptibility may be missed in small families with few at-risk FDRs (33). For example, in some families, it may be difficult to recognize paternally transmitted breast-ovarian cancer syndrome without SDR information. Thus, the decision of whether or not to seek information about SDR may vary, depending on the cancer site of interest and what is known regarding its genetics.
Besides kinship, vital status of the relative was the only variable consistently associated with both ill-defined or unspecified cancer reports and unknown cancer history. After adjustment for vital status, unknown cancer history among SDR was further associated with older generations, and with parental separation during the respondent’s childhood. Encouraging individuals to communicate with “family gatekeepers” who possess detailed information about the larger pedigree may improve family history ascertainment for deceased or older generations of SDR. Our findings support the need for initiatives, such as the Surgeon General’s Family History Initiative (13) to encourage people to learn more about, and maintain records of, their family health history.
Demographic characteristics of respondents were another major source of variation in the coherence and completeness of family history of cancer reports. Lower educational attainment was positively associated with ill-defined or unspecified cancer reports in relatives, and with unknown cancer history in each kinship group, suggesting less awareness or perceived benefit of family history information among respondents from lower socioeconomic subgroups. Male gender of the respondent was also associated with ill-defined or unspecified cancer reports, and unknown cancer history, but only among SDR. Other studies have found underreporting of family history of common cancers by men (34, 35), possibly attributable to cultural practices favoring women as family gatekeepers. Our data suggest that men may provide coherent family history of cancer information for their closest relatives.
After adjustment for education and all other factors, African-Americans were more likely to report ill-defined or unspecified cancers than whites, although these estimates were unstable due to small sample size. This may partly reflect limited cancer information-sharing within families (34,36,37), fewer inquiries about family history from health care providers (38), or less awareness of paternal family history (39). Findings that coherence and completeness of reports vary significantly by demographic features of respondents suggest that interventions to promote family history-taking should be tailored to the educational and cultural characteristics of population subgroups (40,41).
In this study, 8.3% of respondents reported a personal history of any cancer, including non-melanoma skin cancer. This was higher than a published national estimate of 4.8% from the NHIS that excluded non-melanoma skin cancers (42). We used 2001 NHIS data (14) to obtain an estimate that, like our survey, restricted respondent ages to 25–64 years and included all skin cancers. The resulting estimate of 5.1% was still lower than 8.3%, perhaps reflecting true differences between CT and the U.S. overall, or reporting errors in both surveys. Unexpectedly, unknown cancer among SDR was significantly higher if respondents reported a personal history of cancer, and a non-significant increase was also observed among FDR. We would expect respondents with a personal history to have greater awareness of relatives’ diagnoses, or to have a similar awareness as those without a personal history if their diagnosis was simply non-melanoma skin cancer. This counter-intuitive finding may be partly explained by misclassification of respondents with regard to their personal cancer history. For example, family history knowledgeable- respondents may have underreported their personal history, and also, 7 of the 96 who did report a personal history actually mentioned non-malignant diagnoses. Awareness may have been further tempered by the fact that 25 of them only reported non-melanoma skin cancers.
Respondents were able to provide a year or age of diagnosis for relatives’ cancers in a slim majority of cancer reports (54%). By further probing, using 10 year age-ranges, and where appropriate, about a diagnosis < or ≥age 50, we were able to estimate age for an additional 44% of relatives. This type of probing offers an easy, low-cost strategy to obtain an estimate of age at diagnosis for the cancer reports.
Specific strengths of this study include the population-based sample design that likely approximates primary care populations in demographics and the high prevalence of average to moderate cancer risk. Therefore, our results can shed light on potential barriers to and facilitators of family history ascertainment in primary care practice settings, where such information is of increasing interest for risk assessment and personalized preventive care (19,42,43). The open-ended format of the family history questions in the survey instrument was an additional strength. Although this format is not necessarily cost-effective in studies seeking limited family history data, it enabled us to conduct a qualitative evaluation of the full range of verbatim responses. It is not well understood which if any kinds of family history questions or advance materials ultimately yield less ambiguous and more accurate responses.
There are several limitations to this study. First, the results may not be generalizable to ineligible subgroups of respondents, such as those under 25 or above 64 years old, or immigrants. In one national survey, the latter were one-third as likely to report any family history of cancer than U.S.-born respondents (44), suggesting that our prevalence estimate of any family cancer history might have been substantially lower had immigrants been included. Furthermore, because the Connecticut sampling frame and study sample had greater proportions of whites and people with higher income and education than the U.S. population (24), the coherence and completeness of family history reports may be overestimated relative to the U.S. overall or to populations with different demographic characteristics. The number of Hispanics and other minority groups was small, resulting in unstable estimates of ambiguous cancer reports and unknown cancer histories.” Pre-interview family history ascertainment by respondents whose households received advance recruitment materials, and inadvertent over-interpretation of verbatim reports by interviewers, may have also caused some overestimation. Also, this analysis used data from a follow-up interview (since respondent demographic data were not obtained in the baseline interview several weeks earlier), and it is possible that differential loss of respondents with less family history awareness introduced some bias.
An additional caveat is that study conclusions can only be drawn about the interpretive value of family history reports and not about their accuracy, as the latter was not evaluated in the present analysis and will be reported elsewhere. However, the distribution of reported cancers did show consistency with other studies: for example, four of the five most common cancers in family members (i.e., breast, lung, colorectal, and prostate) were also reported as the top four in a cancer screening trial of 149,332 participants in 10 U.S. locations (35), and in the 2000 NHIS (17). Although our survey was conducted in 2001, we are not aware of time trends that would render these results obsolete; in fact, our telephone response rates may have been higher than what could now be achieved (45). Finally, the ability to draw conclusions about childhood cancers is somewhat limited since respondents’ grandchildren were excluded from ascertainment (although childhood cancers were ascertained from included relatives).
In primary care settings, recording and utilization of family health information may be limited by time or other considerations (18,44,46). Therefore, data collection tools that enable patients to record family histories on their own time and transfer the information to their clinicians are potentially very useful. In recent years, a number of computerized tools have been developed to support family history ascertainment in broad populations (23). Public use software, such as My Family Health Portrait, CDC Family Healthware™, and others (47,48,49) aim to promote and facilitate patient and consumer-based collection of family history information for risk assessment and health promotion purposes. Recent progress has been made toward standardizing core family history information to integrate into electronic health records (50). The identification of five qualitative categories of cancer reports in this study provides a conceptual framework for designing such tools in ways that help shift the distribution of cancer responses towards consistency and site-specificity. For example, including definitions of cancer and site-specific descriptions (particularly for confusing sites like abdominal organs), or culturally sensitive suggestions regarding how to approach family members for information, may help reduce the percentage of ill-defined or inconsistent reports. However, additional research is needed to refine current family history data collection methods.
Conclusion
In conclusion, most family history of cancer reports obtained in this general population survey were coherent, as we have defined it, and surprisingly few relatives had clearly non-malignant disease misreported as cancer or totally unknown cancer histories. However, the percentage of coherent cancer site reports and known history of any cancers declined for second-degree and deceased relatives, and with decreasing educational levels of respondents. Strategies are needed to promote interest in, and increase knowledge of, family history of cancer, particularly in lower socioeconomic populations that are medically underserved, and for relatives whose information is harder to ascertain. In population surveys and general epidemiologic studies, the costs vs. benefits of including SDR must be carefully considered given the lower interpretive value of their cancer reports. It may be appropriate to exclude collection of SDR information depending on planned applications of the data.
Acknowledgments
This research was supported by contracts N01-PC-95039 and N02-PC-25001 of the Division of Cancer Control and Population Sciences, National Cancer Institute. Support for Drs. Greene, Mai, Martin, and Graubard was provided by the Intramural Research Program of the Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
References
- 1.Lindor NM, McMaster ML, Lindor CJ, Greene MH National Cancer Institute, Division of Cancer Prevention, Community Oncology and Prevention Trials Research Group. Concise handbook of familial cancer susceptibility syndromes – second edition. J Natl Cancer Inst Monogr. 2008;38:1–93. doi: 10.1093/jncimonographs/lgn001. [DOI] [PubMed] [Google Scholar]
- 2.Noe M, Schroy P, Demierre M, Babayan R, Geller AC. Increased cancer risk for individuals with a family history of prostate cancer, colorectal cancer, and melanoma and their associated screening recommendations and practices. Cancer Causes Control. 2008;19:1–12. doi: 10.1007/s10552-007-9064-y. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Guide to Clinical Preventive Services [Internet] Rockville (MD): Agency for Healthcare Research and Quality; [cited 2008 Jan 23]. Available from: http://www.ahrq.gov/clinic/cps3dix.htm#cancer. [Google Scholar]
- 4.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 6.Beery TA, Shooner KA. Family history: the first genetic screen. Nurse Pract. 2004;29:14–25. doi: 10.1097/00006205-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Trepanier A, Ahrens M, McKinnon W, et al. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns. 2004;13:83–114. doi: 10.1023/B:JOGC.0000018821.48330.77. [DOI] [PubMed] [Google Scholar]
- 8.Eberl MM, Sunga AY, Farrell CD, Mahoney MC. Patients with a family history of cancer: identification and management. J Am Board Fam Pract. 2005;18:211–217. doi: 10.3122/jabfm.18.3.211. [DOI] [PubMed] [Google Scholar]
- 9.Yoon PW, Scheuner MT, Peterson KL, Gwinn M, Faucett A, Khoury MJ. Can family health history be used as a tool for public health and preventive medicine? Genet Med. 2002;4:304–310. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Yoon PW, Scheuner MT, Khoury MJ. Research priorities for evaluating family history in the prevention of common chronic disease. Am J Prev Med. 2003;24:128–135. doi: 10.1016/s0749-3797(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 11.Guttmacher AE, Collins FS, Carmona RH. The family history -- more important than ever. N Engl J Med. 2004;351:2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Notice to reader: National Family History Day – Thanksgiving Day. MMWR. 2007;56:1192. [Google Scholar]
- 13.Surgeon General’s Family Health History Initiative [Internet] Washington: US Department of Health and Human Services; [cited 2008 Dec 15]. Available from: http://www.hhs.gov/familyhistory/ [Google Scholar]
- 14.National Health Interview Survey: questionnaires, datasets and related documentation 1997 to present [Internet] Atlanta: Centers for Disease Control and Prevention (US); [reviewed 2009 Jun19; updated 2009 Aug 18; cited 2009 Sep 2]. Available from: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm. [Google Scholar]
- 15.California Health Interview Survey [Internet] Los Angeles: UCLA Center for Health Policy Research;; [updated 2003 Aug 29; cited 2009 Sep 2].[2 p.] Available from: http://www.chis.ucla.edu/pdf/TopicAreasCHIS2001.pdf. [Google Scholar]
- 16.Genomics and Family History Survey Questions [Internet] Seattle: University of Washington Center for Genomics and Public Health; c2006–2007 [updated 2007 Mar; cited 2009 Sep 2]. [55 p.] Available from: http://depts.washington.edu/cgph/pdf/Compiled_Genomics_Questions_BRFSS.pdf. [Google Scholar]
- 17.Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med. 2006;8:571–575. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murff HJ, Greevy RA, Syngal S. The comprehensiveness of family cancer history assessments in primary care. Community Genet. 2007;10:174–180. doi: 10.1159/000101759. [DOI] [PubMed] [Google Scholar]
- 19.Rich E, Burke W, Heaton C, et al. Reconsidering the family history in primary care. J Gen Intern Med. 2004;19:273–280. doi: 10.1111/j.1525-1497.2004.30401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sifri RD, Wender R, Paynter N. Cancer risk assessment from family history: gaps in primary care practice. J Fam Pract. 2002 Oct;51(10):856. [PubMed] [Google Scholar]
- 21.Reid GT, Walter FM, Brisbane JM, Emery JD. Family history questionnaires designed for clinical use: a systematic review. Public Health Genomics. 2009;12:73–83. doi: 10.1159/000160667. [DOI] [PubMed] [Google Scholar]
- 22.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer?: An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi N, Wilson B, Santaguida P, et al. Collection and use of cancer family history in primary care. Evid Rep Technol Assess. 2007 Oct;(159):1–84. [PMC free article] [PubMed] [Google Scholar]
- 24.Garceau A, Wideroff L, McNeel T, Dunn M, Graubard B. Population estimates of extended family structure and size. Community Genet. 2008;11:331–42. doi: 10.1159/000133305. [DOI] [PubMed] [Google Scholar]
- 25.Family History Validation Questionnaires [Internet] Bethesda (MD): National Cancer Institute (US); [modified 2006 Nov 1; cited 2009 Sep 3]. Available from: http://riskfactor.cancer.gov/studies/family/questionnaires.html. [Google Scholar]
- 26.Council on American Survey Organizations. On the definition of response rates. New York: Council of American Survey Organizations; 1982. [Google Scholar]
- 27.U.S. Department of Health and Human Services. International Classification of Diseases, 9th Revision, Clinical Modification. 6. Los Angeles: Practice Management Information Corp; 2005. [Google Scholar]
- 28.SAS Institute Inc. SAS/STAT® User’s Guide, Version 9.0. Cary (NC): SAS Institute Inc; 2002. [Google Scholar]
- 29.Research Triangle Institute. SUDAAN Language Manual, Release 9.0. Research Triangle Park (NC): Research Triangle Institute; 2004. [Google Scholar]
- 30.Korn EL, Graubard BI. Analysis of Health Surveys. New York: John Wiley and Sons; 1999. p. 93. [Google Scholar]
- 31.Rust KF, Rao JN. Variance estimation for complex surveys using replication techniques. Stat Methods Med Res. 1996;5:283–310. doi: 10.1177/096228029600500305. [DOI] [PubMed] [Google Scholar]
- 32.Bowen DJ, Ludman E, Press N, Vu T, Burke W. Achieving utility with family history colorectal cancer risk. Am J Prev Med. 2003;24:177–182. doi: 10.1016/s0749-3797(02)00591-3. [DOI] [PubMed] [Google Scholar]
- 33.Khoury MJ, Flanders WD. Bias in using family history as a risk factor in case-control studies of disease. Epidemiology. 1995;6:511–9. doi: 10.1097/00001648-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Glanz K, Grove J, Le Marchand L, Gotay C. Underreporting of family history of colon cancer: correlates and implications. Cancer Epidemiol Biomarkers Prev. 1999;8:653–639. [PubMed] [Google Scholar]
- 35.Pinsky PF, Kramer BS, Reding D, Buys S. PLCO Project Team. Reported family history of cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2003;157:792–799. doi: 10.1093/aje/kwg043. [DOI] [PubMed] [Google Scholar]
- 36.Weinrich SP, Faison-Smith L, Hudson-Priest J, Royal C, Powell I. Stability of self-reported family history of prostate cancer among african-american men. J Nurs Meas. 2002;10:39–46. doi: 10.1891/jnum.10.1.39.52547. [DOI] [PubMed] [Google Scholar]
- 37.Hall M, Olopade OI. Confronting genetic testing disparities: knowledge is power. JAMA. 2005;293:1783–1785. doi: 10.1001/jama.293.14.1783. [DOI] [PubMed] [Google Scholar]
- 38.Murff JH, Byrne D, Haas JS, Puopolo AL, Brennan TA. Race and family history assessment for breast cancer. J Gen Intern Med. 2005;20:75–80. doi: 10.1111/j.1525-1497.2004.40112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupfer SS, McCaffrey S, Kim EE. Racial and gender disparities in hereditary colorectal cancer risk assessment: the role of family history. J Cancer Educ. 2006 Spring;21(1 Suppl):S32–6. doi: 10.1207/s15430154jce2101s_7. [DOI] [PubMed] [Google Scholar]
- 40.Petruccio C, Mills Shaw KR, Boughman J, et al. Healthy choices through family history: a community approach to family history awareness. Community Genet. 2008;11:343–51. doi: 10.1159/000133306. [DOI] [PubMed] [Google Scholar]
- 41.Maradiegue A, Edwards QT. An overview of ethnicity and assessment of family history in primary care settings. J Am Acad Nurse Pract. 2006;18:447–456. doi: 10.1111/j.1745-7599.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 42.Culler D, Grimes SJ, Acheson LS, Wiesner GL. Cancer genetics in primary care. Prim Care. 2004;31:649–83. doi: 10.1016/j.pop.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Murff JH, Byrne D, Syngal S. Cancer risk assessment: quality and impact of the family history interview. Am J Prev Med. 2004;27:239–245. doi: 10.1016/j.amepre.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Orom H, Coté ML, González HM, Underwood W, 3rd, Schwartz AG. Family history of cancer: is it an accurate indicator of cancer risk in the immigrant population? Cancer. 2008;112:399–406. doi: 10.1002/cncr.23173. [DOI] [PubMed] [Google Scholar]
- 45.Keeter S, Kennedy C, Dimock M, Best J, Craighill P. Gauging the impact of growing nonresponse on estimates from a national RDD telephone survey. Public Opin Q. 2006;70:759–779. doi: 10.1093/poq/nfl035. [DOI] [Google Scholar]
- 46.Tyler CV, Jr, Snyder CW. Cancer risk assessment: examining the family physician’s role. J Am Board Fam Med. 2006;19:468–77. doi: 10.3122/jabfm.19.5.468. [DOI] [PubMed] [Google Scholar]
- 47.Family history collection tools [Internet] Atlanta: Centers for Disease Control and Prevention;; [reviewed 2009 Jul 21; updated 2009 Jul 21; cited 2009 Sep 3]. Available from: http://www.cdc.gov/genomics/famhistory/resources/tools.htm. [Google Scholar]
- 48.Yoon PW, Scheuner MT, Jorgensen C, Khoury MJ. Developing Family Healthware, a family history screening tool to prevent common chronic diseases. Prev Chronic Dis. 2009;6:A33. [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill SM, Rubinstein WS, Yang C, et al. Familial risk for common diseases in primary care: the Family Healthware Impact Trial. Am J Prev Med. 2009;36:506–14. doi: 10.1016/j.amepre.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Feero WG, Bigley MB, Brinner KM. New standards and enhanced utility for family health history information in the electronic health record: an update from the American Health Information Community’s Family Health History Multi-Stakeholder Workgroup. J Am Med Inform Assoc. 2008;15:723–728. doi: 10.1197/jamia.M2793. [DOI] [PMC free article] [PubMed] [Google Scholar]