Abstract
The purpose of the study was to identify subgroups of patients presenting with acute coronary syndromes based on symptom clusters. Two hundred fifty-six patients completed a symptom assessment in their hospital rooms. Latent class cluster analysis and analysis of variance were used to classify subgroups of patients according to selected clinical characteristics. Four subgroups were identified and labeled as Heavy Symptom Burden, Chest Pain Only, Sweating and Weak, and Short of Breath and Weak (model fit χ2 [130,891, n = 256] = 867.5, p = 1.00). The largest group of patients experienced classic symptoms of chest pain and shortness of breath but not sweating. Younger patients were more likely to cluster in the Heavy Symptom Burden group (F = 5.08, p = .002). Interpretation of the clinical significance of these groupings requires further study.
Keywords: symptom clusters, symptoms, acute coronary syndromes, latent class analysis
THE SYMPTOM EXPERIENCE DURING ACUTE CORONARY SYNDROMES
How patients perceive and interpret their symptoms serves as the impetus for treatment seeking during acute coronary syndromes (ACS). ACS include the diagnoses of ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), and unstable angina. Over five million patients present to emergency departments (ED) annually in the United States and are evaluated for chest pain and related symptoms (McCaig & Nawar, 2006). Symptoms and behavioral responses to symptoms, directly affect the efficacy of treatments, long-term morbidity, mortality, and quality of life for patients with ACS (Gorelik et al., 2007; Miller et al., 2008; Shaw et al., 2006). Additionally, interventions to improve symptom knowledge, symptom identification, symptom management, and care-seeking behaviors depend on empirically derived data from symptom research (Dodd et al., 2001).
The Concept of Symptom Clusters
Clustering of symptoms associated with a disease or treatment was first described in the cancer literature (Dodd, Miaskowski, & Lee, 2004; Miaskowski et al., 2006; Yeh et al., 2008). Dodd et al. (2001) define a symptom cluster as three or more concurrent symptoms that are related to each other. The definition was expanded by Kim, McGuire, Tulman, and Barsevick (2005) to include symptoms that occur together, are stable, and are relatively independent of other clusters. However, this definition does not address the clustering of symptoms in acute illness or how to identify pathophysiological mechanisms that may link symptoms.
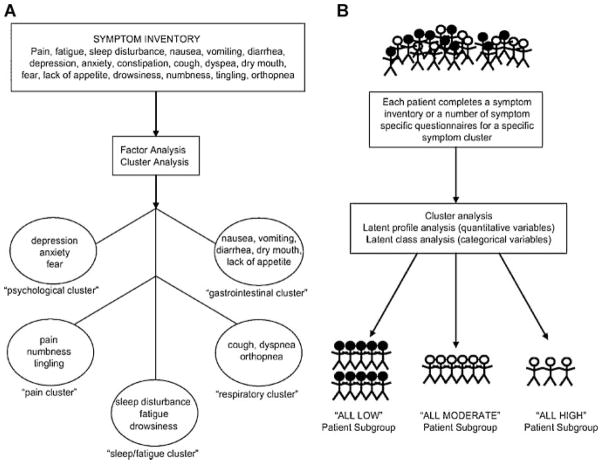
Clarifying the concept and current nomenclature of symptom clusters is useful in background for the aims of this study. Previously, two conceptual approaches have been used in symptom cluster research. These approaches are distinguished by their analytic techniques. In the first approach, factor analysis is used to identify clusters of symptoms in patients with a common disease. Exploratory factor analysis is a mainstay for discovering sets of items that are highly correlated and may be useful in constructing symptom “subscales” from a larger set of symptom items. In the second approach, two methods are used to identify clusters of individuals who are similar to one another because they share similar symptom profiles. The first method is a “scale development” approach and the second method is a “diagnostic classification” approach. With the second approach, clinical characteristics can be analyzed to further understand how the clusters differ among individuals. This second approach is accomplished through classic cluster analysis using a non-metric agglomerative technique. More recent cluster analytic techniques include latent profile analysis and latent class cluster analysis. These are typically achieved through maximum likelihood or weighted least-squares estimation. Miaskowski, Aouizerat, Dodd, and Cooper (2007) provided excellent models of these two approaches (see Fig. 1).
FIGURE 1.
Two conceptual approaches to symptom cluster research. A: The identification of symptom clusters “de novo.” B: The identification of subgroups of patients based on their experiences with a specific symptom cluster. Reprinted with permission from Miaskowski et al. (2007).
Symptom Clusters in Acute Coronary Syndromes
Patients rarely present with a single symptom during an episode of ACS. The mean number of symptoms reported during ACS has ranged from 6.6 to 8.6 (DeVon, Ryan, Ochs & Shapiro, 2008; DeVon & Zerwic, 2003; Horne, James, Petrie, Weinman, & Vincent, 2000). Most investigators have reported multiple symptoms in a checklist format. Frequently they have differentiated between typical and atypical symptoms (Noureddine, Arevian, Adra, & Puzantian, 2008; Stephen, Darney, & Rosenfeld, 2008; Milner, Vaccarino, Arnold, Funk & Goldberg, 2004). Researchers using checklists have confirmed that symptoms do not occur in isolation and may be related or cluster (Ryan et al., 2007).
Few investigators have studied symptom clusters during ACS (Fukuoka, Lindgren, Rankin, Cooper, & Carroll, 2007; Lindgren et al., 2008; Ryan et al., 2007). Further, these researchers clustered individuals according to symptoms rather than clustering symptoms, suggesting it may be possible to stratify patients according to their probability of experiencing specific clusters of symptoms. This would support developing tailored interventions for prompt recognition of and response to symptoms for at risk patients.
Ryan et al. (2007) conducted a secondary data analysis using nine studies from the United States and the United Kingdom. A total of 1,073 patients with acute myocardial infarction ACS were sampled. Patients with unstable angina were not included. Symptoms were clustered, and demographic data were used to characterize individuals who were likely to experience the symptoms in the identified clusters. Five clusters were detected; age, sex, and race were significant predictors of cluster membership. No cluster contained all typical symptoms of ACS—chest discomfort, sweating, shortness of breath, nausea, and light-headedness (Ryan et al., 2007). In one cluster, the symptoms measured had only a moderate to low probability of occurring, and therefore those individuals experienced very few symptoms. The cluster that contained the highest number of symptoms also included a report of classic symptoms of chest discomfort, shoulder discomfort, sweating, and fatigue. Individuals who experienced these symptoms were more likely to be younger or African-American. The cluster of symptoms that included the experience of chest and shoulder/arm/hand discomfort in the absence of other symptoms was more likely to be experienced by men.
Carroll and Rankin (2006) and Carroll, Rankin, and Cooper (2007) published two analyses of symptoms clusters from a clinical trial designed to improve health outcomes in unpartnered elders with coronary heart disease (Fukuoka et al., 2007; Lindgren et al., 2008). In the first analysis, a sample of 247 patients was interviewed following acute myocardial infarction, ACS, or coronary artery bypass surgery to examine prehospital symptomatology (Lindgren et al., 2008). The occurrence and intensity of pain, shortness of breath, fatigue, palpitations, sleep disturbance, nausea, and vomiting were included in the analyses. Three groups of patients were identified through the use of hierarchical cluster analysis techniques (Everitt, Landau, & Leese, 2001). The clusters were labeled (a) Classic ACS, (b) Weary, and (c) Diffuse Symptoms. The Classic ACS group was characterized by severe ischemic pain and moderate fatigue. The Weary group experienced severe fatigue, sleep disturbance, and shortness of breath. The Diffuse Symptoms cluster included the largest (49%) and oldest group of patients; this group reported generally low symptom intensities (Lindgren et al., 2008).
Two hundred-six patients from the Lindgren et al. (2008) cohort were interviewed 1 year after their cardiac event to determine those at risk for decreased quality of life (Fukuoka et al., 2007). These cardiac symptoms were also analyzed using hierarchical cluster analysis. The three clusters were similar to the clusters identified at baseline, 1 year earlier, and were labeled Weary, Diffuse Symptoms, and Breathless. The Classic ACS cluster was replaced by the Breathless cluster, suggesting a difference between acute and chronic symptoms or between acute and chronic symptoms associated with decreased functional status or heart failure. There were no differences between clusters of patients on the demographic factors of sex and age. The majority of individuals (68.4%) clustered in the Diffuse Symptom group.
Symptom clusters in patients with ACS have only been reported since 2007. Review of the existing literature reveals contradictory findings, including clustering on classic and less typical symptoms and both differences and lack of differences by age. The purpose of the current study was to investigate subgroups of patients admitted through the ED for ACS. Due to the limited empirical findings to date, the aims were exploratory. The specific aims were to determine if (a) subgroups of patients could be identified based on symptom clusters; (b) subgroups could be categorized according to demographic or clinical characteristics; and (c) there was a subgroup of patients with classic ACS symptoms based on classic heart attack symptoms published by the American Heart Association and the National Heart Lung and Blood Institute. For this study, classic symptoms were defined as chest pain, shortness of breath, sweating, nausea, and light-headedness (American Heart Association, 2010a).
In addition, we use the term classifying subgroups of patients on symptoms rather than the more commonly used term symptom clusters as it is a more accurate representation of the latent class statistical procedures used in the analyses. The terms and analogy represent concept B in Miaskowski et al.’s (2007) model (Fig. 1). Data were collected as part of a larger study designed to examine symptoms of ACS (DeVon et al., 2008). The study aims address gaps in the emerging science of symptom clusters in patients with ACS by including an extensive number of symptoms and enrolling a cohort of women and men.
METHODS
Sample and Setting
Two hundred eighty-two patients, hospitalized with a diagnosis of ACS, were invited to participate. All patients were recruited from the cardiac step-down units of two large non-academic medical centers in the Midwest. Both institutions serve as referral centers for cardiac patients. Ten patients (six women and four men) declined to participate. The ages of those who chose not to participate ranged from 40 to 85, and 6 of the 10 were Black. The remaining 272 patients gave written consent and completed the study. Sixteen patients had a primary discharge diagnoses other than ACS and were excluded from analyses, resulting in a final sample of 256. Approvals from the Institutional Review Boards at both hospitals and the sponsoring institution were obtained prior to the start of the study.
Procedures
All participants were recruited after being identified by nursing or medical staff as qualifying for the study. Eligibility criteria included an admitting diagnosis of ACS, 21 years or older, fluent in English, admission through the ED at least 12 hours prior to being interviewed, pain free, in stable condition, and adequate cognitive capacity. Cognitive capacity was deemed acceptable if the patient was able to understand the purpose of the study and provide informed consent. Patients were excluded if they had prior heart failure, evidenced by either elevated serum brain natriuretic peptide or documentation of heart failure as a diagnosis in the medical record. Patients with a history of cocaine use were also excluded. It was hypothesized that the symptom experience might vary for these patients because the chronic pathophysiological processes associated with obstructive coronary artery disease are different than the phenomena of vasoconstriction, tachycardia, systemic hypertension, and increased myocardial oxygen consumption associated with cocaine ingestion (Hollander, 2003). Patients with a history of heart failure were excluded, as many of the symptoms, including dyspnea and unusual fatigue, are similar to the acute symptoms of ACS and could confound the measurement of ACS symptoms. All data collection were completed in the patients’ private rooms to support confidentiality. Self-report instruments were chosen for the study, but in pilot testing we found that many patients did not have reading glasses or were on bedrest following angioplasty; therefore, all instruments were read to patients and answers were recorded by research staff.
Instruments
The Symptoms of Acute Coronary Syndromes Inventory (SACSI), developed by the first author, was used to collect symptom data. The SACSI was designed based on a review of the literature. The instrument includes 20 different symptoms that have been associated with ACS (Dempsey, Dracup, & Moser, 1995; McSweeney & Crane, 2000; Zerwic, 1998). Symptoms are measured on a 5-point scale. Patients indicate that they either did not experience the symptom (0), or they rate the severity of each symptom as mild (1), moderate (2), severe (3), or very severe (4). The SACSI was pilot tested in studies examining gender differences in the symptoms of unstable angina and ACS (DeVon & Zerwic, 2003).
Content validity using Lynn’s (1986) formula was established by cardiovascular experts in two prior studies. In an unstable angina study, the content validity index (CVI) for the entire instrument was .88 (p < .05; DeVon & Zerwic, 2003). The instrument was again reviewed by five content experts prior to the start of the current study and the computed CVI was .94 (p < .05). Although participants in the study reported here were asked if they experienced any other symptoms not contained on the SACSI, they did not provide any additional items, adding support for the construct validity of the instrument as a comprehensive measure of the symptoms of ACS. Cronbach’s alpha for the instrument in this study was .81.
Data Analyses
Power analyses and level of significance
All descriptive statistics and analysis of variance (ANOVA) tests were two-tailed using a .05 level of significance. Power for the study was computed based on the primary aims related to sex and symptoms of ACS. For chi-square tests, 160 subjects were needed to achieve power at the .85 level with a medium effect size (w = .30). For independent sample t-tests, 168 subjects were needed to achieve power at the .80 level for a medium effect size (d = .30). For ANOVA, 210 subjects were needed to achieve power at the .80 level with a medium effect size (f = .25). Consequently, the sample of 256 in this study allowed for the detection of small effect sizes for a number of symptoms. Actual effect sizes for this study ranged from a low of d = .15 for sweating to a high of d = .42 for unusual fatigue. Power analysis was not performed for the exploratory aims because the examination of symptom clusters is not a statistical test.
Latent class analysis
Latent class analysis, sometimes called latent class cluster analysis, is a type of finite mixture model (Hagenaars & McCutcheon, 2002). It is used to identify patient groups (latent classes) with similar symptom profiles. Latent class analysis is conceptually similar to cluster analysis (Everitt et al., 2001). It is used to identify latent classes based on an observed response pattern (Collins & Wugalter, 1992; Nyland, Bellmore, Nishina, & Graham, 2007). As an analytic approach, latent class analysis has several advantages over cluster analysis. Latent class analysis is model-based, generating probabilities for group membership. It is also possible to use statistical fit indices to assess model fit and help decide on the number of classes.
With latent class models, the final number of latent classes is not determined prior to analysis. Classes are identified by evaluating five tests including the (a) chi-squared test of model fit, (b) Bayesian information criterion (BIC), (c) Vuong–Lo–Mendel–Rubin likelihood ratio test (VLMR), (d) parametric bootstrapped likelihood ratio test (BLRT), and (e) entropy (the consistency between model-based latent classes and the classes to which observations are assigned). The model that fits the data best has a non-significant chi-squared test of model fit, the lowest BIC, and a VLMR and/ or BLRT that shows the estimated model to be better than the model with one fewer class. It is desirable for entropy to be .80 or greater. In addition, well-fitting models have log-likelihood values that are replicated in analyses with multiple “random starts,” indicating that the solution is not based on a local maximum for the log-likelihood. Finally, well-fitting models make sense conceptually, and the estimated classes differ as might be expected for variables that are not part of the generating model (Nyland, Asparouhoy, & Muthén, 2007).
Latent class analyses were conducted with Mplus Version 5.1 (Muthén & Muthén, 1998–2009a, 1998–2009b). Subsequent analyses of differences in clinical and demographic characteristics among the identified classes were carried out with SPSS for Windows. Symptoms measured on an ordinal scale were recoded to dichotomous variables and entered into MPlus. Often, latent class models use categorical, commonly dichotomous, variables (Collins & Wugalter, 1992; Lanza, Flaherty, & Collins, 2003). Binary variables were analyzed because the ordinal scales had only a 5-point range (with 0 representing not present and 4 representing very severe) and the distributions were highly skewed. Very little information in the item distributions was lost through dichotomization in this sample. Estimation was accomplished for these dichotomous items with robust maximum likelihood. The MPlus program provides results of variables in probabilities. We defined high probabilities as .60–1.0 and low probabilities as <.60. The cut points for high and low probabilities, although somewhat arbitrary, provided the most salient information on classes and clinical usefulness of the data. Clinical variables that have been previously shown to identify subgroups of patients by latent class were analyzed individually using analysis of variance (Ryan et al., 2007).
RESULTS
Characteristics of the Sample
Patients ranged in age from 24 to 97 years (M = 64.4 ± 13.6). The convenience sample was evenly divided within the three ACS diagnostic categories of unstable angina, NSTEMI, and STEMI. The majority of patients had a high school diploma or higher and were married. Characteristics of the sample appear in Table 1.
Table 1.
Sample Characteristics
| Variables | n | % |
|---|---|---|
| Age in years | ||
| Mean (SD): 64.4 years (13.6) | 256 | 100 |
| Range: 24–97 years | ||
| Type of acute coronary syndrome | ||
| Unstable angina | 88 | 34.4 |
| NSTEMI | 84 | 32.8 |
| STEMI | 84 | 32.8 |
| Race/ethnicity | ||
| Black | 51 | 19.9 |
| White (non-Hispanic) | 191 | 74.6 |
| Hispanic | 8 | 3.1 |
| Asian/Pacific Islander | 3 | 1.2 |
| Native American | 3 | 1.2 |
| Education | ||
| <High school | 67 | 26.2 |
| High school diploma | 86 | 33.6 |
| >More than high school | 103 | 40.2 |
| Annual household incomea | ||
| ≤$20,000 | 84 | 32.8 |
| $20,001–$50,000 | 91 | 35.5 |
| >$50,000 | 47 | 18.4 |
| Marital status | ||
| Single | 34 | 13.3 |
| Married | 136 | 53.1 |
| Divorced | 29 | 11.3 |
| Widowed | 57 | 22.3 |
Note: NSTEMI denotes non-ST elevation myocardial infarction and STEMI denotes ST elevation myocardial infarction.
Annual household income data were missing for 13.3% of participants.
Subgroups of Patients by Symptom Clusters
The items “new cough,” “fainting,” and “vomiting” were excluded from the final analysis because they were rarely reported, and there was too little variation in those items to be distributed across even two classes. The analyses of the remaining 17 symptoms resulted in a 4-class solution (model fit, χ2 [130,891, n = 256] = 867.5, p = 1.00, entropy = .835). Further review of the fit indices, symptom profiles for the latent classes, and examination of other variables with the latent class assignments suggested that a 4-class solution fit the data best. See Table 2 for the fit indices for the 2- through 5-class solutions. Although the BIC for the 3-class solution was smaller than for the 4-class solution, entropy was better for the 4-class solution. Further, the BIC for the 5-class solution was larger than for the 4-class solution, and VLMR was not significant for the 5-class solution. The VLMR is liberal in extracting classes (Nyland, Asparouhoy, et al., 2007); when it is not significant, too many classes have been extracted. Therefore, the 5-class solution was rejected in favor of the 4-class solution. A comparison of the symptom profiles for the latent classes for the 3 and 4-class solutions was made, and the 4-class solution made more sense from both a clinical and conceptual perspective (Nyland, Bellmore, et al., 2007). Despite the larger BIC for the 4-class solution, we believe it provides a better fit to the data than the 3-class solution.
Table 2.
Latent Class Solutions and Fit Indices for 2- Through 5-Class Solutions
| Model | LL | BIC | VLMR | BLRT | Entropy |
|---|---|---|---|---|---|
| 2-Class | −2602.43 | 5398.80 | 288.45ns | 291.34 | .72 |
| 3-Class | −2545.47 | 5384.62 | 104.18* | 113.92** | .81 |
| 4-Class | −2502.68 | 5398.79 | 85.58* | 85.58** | .84 |
| 5-Class | −2468.74 | 5430.65 | 67.88ns | 67.88** | .84 |
LL, log-likelihood; BIC, Bayesian information criterion; VLMR, the Vuong–Lo–Mendel–Rubin likelihood ratio test; BLRT, parametric bootstrapped likelihood ratio test for the K versus K − 1 model.
p < .05.
p < .01.
Classifying Subgroups of Patients by Symptoms
The Heavy Symptom Burden group (Class 1), contained the greatest number of high probability symptoms (13) and included the classic ACS symptoms of chest pain, shortness of breath, sweating, nausea, and lightheadedness. This symptom group contained the fewest patients, 37. As the label indicates, the Chest Pain Only group (Class 2) included only one symptom with a high probability of occurrence (chest pain); there were 58 patients in this group. The Sweating and Weak group (Class 3) also included 58 patients. These individuals had a high probability of four symptoms; sweating, chest pain, weakness, and unusual fatigue. The Short of Breath and Weak group (Class 4) contained the largest number of patients (102). There were five symptoms experienced by people in this group: shortness of breath, difficulty breathing, chest pain, weakness, and unusual fatigue. See Table 3 for individual class counts and probabilities of occurrence. A summary of high and low probabilities of symptoms by group is shown in Table 4.
Table 3.
Class Counts and Probability of Symptom Occurrence
| Symptoms | Class 1 (n = 37) | Class 2 (n = 58) | Class 3 (n = 58) | Class 4 (n = 102) |
|---|---|---|---|---|
| Sweating | .838 | .268 | .639 | .064 |
| Heartburn | .476 | .188 | .400 | .448 |
| Lightheaded | 1.0 | .074 | .493 | .516 |
| Indigestion | .487 | .260 | .411 | .160 |
| Shortness of breath | 1.0 | .368 | .084 | .898 |
| Chest pain | .949 | .912 | .743 | .842 |
| Palpitations | .525 | .089 | .204 | .271 |
| Nausea | .858 | .090 | .541 | .265 |
| Difficulty breathing | 1.0 | .130 | .000 | .816 |
| Dizziness | .913 | .000 | .475 | .343 |
| Loss of appetite | .612 | .062 | .433 | .303 |
| Weakness | .801 | .084 | .675 | .700 |
| Numbness in hands | .758 | .269 | .291 | .262 |
| Heat sensation | .715 | .177 | .412 | .378 |
| Unusually scared | .714 | .323 | .589 | .547 |
| Hyperventilate | .555 | .047 | .140 | .320 |
| Unusual fatigue | .919 | .271 | .607 | .697 |
| Total number of high probability symptoms ( >.60) | 13 | 1 | 4 | 5 |
| Total number of low probability symptoms (<.40) | 0 | 16 | 5 | 8 |
Note: High probability symptom percents (.60–1.0) appear in bold.
Table 4.
Summary of High and Low Symptom Probabilities by Class
| Group | High Probability | Low Probability |
|---|---|---|
| 1. Heavy Symptom Burden | Sweating | |
| Lightheaded | ||
| Shortness of breath | ||
| Chest pain | ||
| Nausea | ||
| Difficulty breathing | ||
| Dizziness | ||
| Loss of appetite | ||
| Weakness | ||
| Numbness in hands | ||
| Heat sensation | ||
| Unusually scared | ||
| Unusual fatigue | ||
| 2. Chest Pain Only | Chest pain | Sweating |
| Heartburn | ||
| Lightheaded | ||
| Indigestion | ||
| Shortness of breath | ||
| Palpitations | ||
| Nausea | ||
| Difficulty breathing | ||
| Dizziness | ||
| Loss of appetite | ||
| Weakness | ||
| Numbness in hands | ||
| Heat sensation | ||
| Unusually scared | ||
| Hyperventilate | ||
| Unusual fatigue | ||
| 3. Sweating and Weak | Sweating | Shortness of breath |
| Chest pain | Palpitations | |
| Weakness | Difficulty breathing | |
| Unusual fatigue | Numbness in hands | |
| Hyperventilate | ||
| 4. Short of Breath and Weak | Shortness of breath | Sweating |
| Chest pain | Indigestion | |
| Difficulty breathing | Palpitations | |
| Weakness | Nausea | |
| Unusual fatigue | Dizziness | |
| Loss of appetite | ||
| Numbness in hands | ||
| Heat sensation | ||
| Hyperventilate |
Subgroups of Patients and Clinical Characteristics
Three demographic and one clinical variable were chosen for analysis because of prior reports of differences in ACS symptoms across age, sex, race, and diabetes status (DeVon et al., 2008; Ryan et al., 2007; Zerwic, Ryan, DeVon, & Drell, 2003). Two additional variables were entered into analyses based on our hypotheses that groups of patients might vary according to their diagnosis, a proxy measure for degree of coronary artery occlusion, and time to presentation in the ED following onset of symptoms. There were no statistical differences in sex, race, diabetes status, diagnosis, and time from symptom onset to presentation in the ED (see Table 5). However, patients did vary by age. Results of post hoc analyses revealed that the youngest patients (M = 56.97 years) clustered in the Heavy Symptom Burden group.
Table 5.
Classes of Individuals by Clinical Characteristics
| Variable | Test Statistic | p-Value | Partial η2 |
|---|---|---|---|
| Class 1 | M = 56.97 ± 14.89, N = 37 | ||
| Class 2 | M = 67.53 ± 13.46, N = 58 | ||
| Class 3 | M = 64.14 ± 12.64, N = 58 | ||
| Class 4 | M = 65.27 ± 12.95, N = 102 | ||
| Age (range = 24–97 years) | F = 5.08 | .002 | .057 |
| Sex (female/male) | Chi-square = 4.32 | .222 | |
| Race (white/other) | Chi-square = 2.88 | .410 | |
| Diabetes (yes/no) | Chi-square = 2.65 | .449 | |
| Diagnosis (UA/NSTEMI/STEMI) | Chi-square = 10.71 | .098 | |
| Time to presentationa (hours) | Chi-square = 1.74b | .628 |
UA, unstable angina; NSTEMI, non-ST elevation myocardial infarction; STEMI, ST elevation myocardial infarction.
Time to presentation in emergency department after symptom onset.
Non-parametric analysis (Kruskal–Wallis Test) was performed since data were not normally distributed.
Classic ACS Symptom Cluster
There were no clusters that contained only the classic symptoms of ACS: chest pain, shortness of breath, sweating, nausea, and lightheadedness (American Heart Association, 2010a). Although the Heavy Symptom Burden group contained all of the classic symptoms, it also included eight symptoms considered to be less typical of ACS. This finding is extremely important because the “classic” picture of symptoms used to inform clinicians, patients, and the public have not been validated in any prior research. This includes research using quantitative and qualitative methods, medical record reviews, direct patient interviews, and large heterogeneous samples.
DISCUSSION
Symptom Clusters
As expected, the probability of experiencing chest pain was high in all four classes (Class 1 = 94.9%, Class 2 = 91.2%, Class 3 = 74.3%, and Class 4 = 84.2%), although it was highest in the smallest group (Class 1). This is reassuring for the majority of patients experiencing ACS, although 44 (17%) patients in this study did not experience chest pain. Extrapolating to the population expected to experience a new or repeat episode of ACS this year (1.255 million; American Heart Association, 2010b) means that over 213,000 Americans are at risk for delayed treatment or no treatment at all if signs or symptoms go unrecognized. The fact that Class 2, the Chest Pain Only group, contained only 58 patients is a concern. This represents only 22.6% of the sample. One may expect that these patients would be more likely to seek emergency care quickly because their decision-making is not complicated by multiple symptoms; however, the literature does not support this notion (Dracup et al., 2006, 2008; Eagle et al., 2002). Further study is required to determine if classes are predictive of time to treatment or short and long-term patient outcomes.
Subgroups of Individuals by Clinical Characteristics
It was hypothesized that patients would cluster on symptoms according to sex, age, race, diabetes status, diagnosis, and time to presentation in the ED following symptom onset. This hypothesis was not supported but it was consistent with a study of symptom clusters in patients with breast cancer in which Gwede, Small, Munster, Andrykowski, and Jacobsen (2008) found that no demographic variables including age, race, education, marital status, employment, or household income classified high and low symptom burden groups. Our findings varied from the findings of Ryan et al. (2007) in which cluster membership was predicted by sex, age, and race. The finding that the youngest patients clustered in the Heavy Symptom Burden group is concerning because the high probability of a very large number of symptoms may make it harder for patients to determine the significance of symptoms and may contribute to decision and treatment delay. However, this potential threat is mitigated by the fact that only 14.5% of patients comprised Class 1. A related concern is that older patients do not experience a heavy burden of symptoms, which may delay their decision to seek immediate care.
Class 2, the Chest Pain Only group, comprised the oldest patients (M = 67.53 years). This is regarded as a clinically positive finding because findings from prior research indicate that older persons are likely to experience less pain during ACS (DeVon et al., 2008). Ryan et al. (2007) also found that patients who clustered in a group that did not have a high probability for any symptom were significantly older. Prior studies have shown that symptoms of ACS change with age and may present an obstacle to symptom recognition for elders (Canto et al., 2000; Ĉulić, Eterović, Mirić, & Silić, 2002).
Future research is warranted to determine if age is related to time to presentation and outcomes following treatment as has been reported in prior research on individual symptoms (Ryan & Zerwic, 2003). Recently, Riegel et al. (2010) found that elders ( ≥73 years) were less likely to recognize symptoms of heart failure compared to patients who were <73 years. The authors concluded that failure to recognize symptoms may be attributable to poor interoception, the manner by which sensory nerves process stimuli originating within the body.
Finally, groups 3 and 4 were differentiated by only two symptoms; sweating and shortness of breath. The presence of sweating may be of particular importance because shortness of breath accompanies other conditions that may mimic symptoms of ACS such as heart failure, pulmonary embolism, or anxiety. Ryan et al. (2007) also noted that sweating may identify a subgroup of vulnerable patients.
Limitations
There are limitations associated with exploratory research. We were unable to formulate hypotheses for grouping patients based on symptoms or clinical characteristics from the literature, which would have guided the design of the study and the choice of variables to measure. We examined a number of possible demographic and clinical confounders including age, sex, race, diabetes status, diagnosis, and time to presentation in the ED for symptoms identified from the ACS symptom literature. Additionally, possible patient or clinical characteristics that may aid in classifying subgroups of patients with symptom clusters during ACS remain unknown because of the paucity of ACS symptom cluster literature.
Use of a convenience sample could have led to bias because only those patients who presented to the ED were eligible for the study. Consequently, patients with ACS who experienced silent ischemia or did not seek care were not represented in this study. However, strategies such as recruiting 7 days a week over a 12-hour period (8 a.m. to 8 p.m.) may have contributed to a more representative sample of the population than would otherwise occur. Because of HIPAA guidelines, the participants had to be identified by nursing personnel or attending physicians. In most cases, patients were approached by their primary care nurse who asked permission for their names to be released to the researchers. Because all potential participants were referred by hospital staff, there is no way of knowing if selection bias occurred. It is possible that the investigators did not receive names of patients who met inclusion criteria.
Implications
Building knowledge in the science of symptom clusters is important for several reasons. Basic scientists can work to identify mechanisms underlying symptom clusters. Clinical investigators can study physiological and behavioral explanations for clusters, design, and test interventions to improve knowledge and symptom management, and examine outcome measures such as disease progression or major cardiovascular events. Healthcare providers can implement interventions and provide ongoing support to patients experiencing anginal symptoms who are at risk for ACS. Knowledge of symptoms and symptom clusters is important for patients experiencing ACS because the symptoms serve as a cue to action. Finally, knowledge and understanding of symptom clusters are also important for bystanders, first responders, and triage nurses because they are all key players in the path to appropriate and expeditious diagnostic testing.
Gaps in knowledge of symptom clusters in acute illness, including ACS, remain. Relationships between symptom triggers and symptom clusters remain largely unknown. Future researchers should include an examination of symptom clusters in population cohorts and a comparison of symptom clusters in patients who have confirmed ACS to those in whom ACS has been ruled out.
Acknowledgments
Contract grant sponsor: National Institute for Nursing Research (NINR); Contract grant number: R15 NR08870.
References
- American Heart Association. Heart attack, stroke, and cardiac arrest warning signs. 2010a Retrieved 4/30/10, from http://www.americanheart.org/presenter.jhtml?identifier=3053Heart_Attack.
- American Heart Association. Heart disease & stroke statistics: 2010 update at-A-glance. 2010b Retrieved 4/30/10, from http://www.americanheart.org/downloadable/heart/1265665152970DS-3241%20Heart-StrokeUpdate_2010.pdf.
- Canto JG, Shlipak MG, Rogers WJ, Malmgren JA, Frederick PD, Lambrew CT, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. Journal of the American Medical Association. 2000;283:3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- Carroll DL, Rankin SH. Comparing interventions in older unpartnered adults after myocardial infarction. European Journal of Cardiovascular Nursing. 2006;5:83–89. doi: 10.1016/j.ejcnurse.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Carroll DL, Rankin SH, Cooper BA. The effects of a collaborative peer advisor/advanced practice nurse intervention: Cardiac rehabilitation participation and rehospitalization in older adults after a cardiac event. Journal of Cardiovascular Nursing. 2007;22:313–319. doi: 10.1097/01.JCN.0000278955.44759.73. [DOI] [PubMed] [Google Scholar]
- Collins LM, Wugalter SE. Latent class models for stage-sequential dynamic latent variables. Multivariate Behavioral Research. 1992;27:131–157. [Google Scholar]
- Ĉulić V, Eterović D, Mirić D, Silić N. Symptom presentation of acute myocardial infarction: Influence of sex, age, and risk factors. American Heart Journal. 2002;144:1012–1017. doi: 10.1067/mhj.2002. 125625. [DOI] [PubMed] [Google Scholar]
- Dempsey SJ, Dracup K, Moser DK. Women’s decision to seek care for symptoms of acute myocardial infarction. Heart & Lung. 1995;24:444–456. doi: 10.1016/s0147-9563(95)80022-0. [DOI] [PubMed] [Google Scholar]
- DeVon HA, Ryan CJ, Ochs AL, Shapiro M. Symptoms across the continuum of acute coronary syndromes: Differences between women and men. American Journal of Critical Care. 2008;17:14–25. [PMC free article] [PubMed] [Google Scholar]
- DeVon HA, Zerwic JJ. The symptoms of unstable angina: Do women and men differ? Nursing Research. 2003;52:108–118. doi: 10.1097/00006199-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Fawcett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. Journal of Advanced Nursing. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. Journal of the National Cancer Institute. Monographs. 2004;(32):76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- Dracup K, McKinley S, Doering LV, Riegel B, Meischke H, Moser DK, et al. Acute coronary syndrome: What do patients know? Archives of Internal Medicine. 2008;168:1049–1054. doi: 10.1001/archinte.168.10.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracup K, McKinley S, Riegel B, Mieschke H, Doering LV, Moser DK. A nursing intervention to reduce prehospital delay in acute coronary syndrome: A randomized clinical trial. Journal of Cardiovascular Nursing. 2006;21:186–193. doi: 10.1097/00005082-200605000-00006. [DOI] [PubMed] [Google Scholar]
- Eagle KA, Goodman SG, Avezum A, Budaj A, Sullivan CM, Lopez-Sendon J, et al. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: Findings from the global registry of acute coronary events (GRACE) Lancet. 2002;359(9304):373–377. doi: 10.1016/S0140-6736(02)07595-5. [see comment] [DOI] [PubMed] [Google Scholar]
- Everitt B, Landau S, Leese M. Cluster analysis. 4. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Fukuoka Y, Lindgren TG, Rankin SH, Cooper BA, Carroll DL. Cluster analysis: A useful technique to identify elderly cardiac patients at risk for poor quality of life. Quality of Life Research. 2007;16:1655–1663. doi: 10.1007/s11136-007-9272-7. [DOI] [PubMed] [Google Scholar]
- Gorelik O, Almoznino-Sarafian D, Yarovoi I, Alon I, Shteinshnaider M, Tzur I, et al. Patient-dependent variables affecting treatment and prediction of acute coronary syndrome are age-related. A study performed in Israel. International Journal of Cardiology. 2007;121:163–170. doi: 10.1016/j.ijcard.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Gwede CK, Small BJ, Munster PN, Andrykowski MA, Jacobsen PB. Exploring the differential experience of breast cancer treatment-related symptoms: A cluster analytic approach. Supportive Care in Cancer. 2008;16:925–933. doi: 10.1007/s00520-007-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars JA, McCutcheon AL. Applied latent class analysis. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- Hollander JE. Acute coronary syndrome in the emergency department: Diagnosis, risk stratification, and management. In: Theroux P, editor. Acute coronary syndromes: A companion to Braunwald’s Heart Disease. 7. Philadelphia, PA: Saunders; 2003. pp. 152–167. [Google Scholar]
- Horne R, James D, Petrie K, Weinman J, Vincent R. Patients’ interpretation of symptoms as a cause of delay in reaching hospital during acute myocardial infarction. Heart. 2000;83:388–393. doi: 10.1136/heart.83.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing. 2005;28:270–282. doi: 10.1097/00002820-200507000-00005. [erratum appears in Cancer Nursing 2005 September–October; 28(5):table of contents] [DOI] [PubMed] [Google Scholar]
- Lanza ST, Flaherty BP, Collins LM. Latent class and latent transition analysis. In: Schinka JA, Velicer WF, editors. Handbook of psychology: Research methods in psychology. Vol. 2. Hoboken, NJ: John Wiley & Sons; 2003. pp. 663–685. [Google Scholar]
- Lindgren TG, Fukuoka Y, Rankin SH, Cooper BA, Carroll D, Munn YL. Cluster analysis of elderly cardiac patients’ prehospital symptomatology. Nursing Research. 2008;57:14–23. doi: 10.1097/01.NNR.0000280654.50642.1a. [DOI] [PubMed] [Google Scholar]
- Lynn MR. Determination and quantification of content validity. Nursing Research. 1986;35:382–385. [PubMed] [Google Scholar]
- McCaig LF, Nawar EW. National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary. Advance Data. 2006;(372):1–29. [PubMed] [Google Scholar]
- McSweeney JC, Crane PB. Challenging the rules: Women’s prodromal and acute symptoms of myocardial infarction. Research in Nursing & Health. 2000;23:135–146. doi: 10.1002/(sici)1098-240x(200004)23:2<135::aid-nur6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Aouizerat BE, Dodd M, Cooper B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. Journal of the National Cancer Institute. Monographs. 2007;(37):39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncology Nursing Forum. 2006;33(5):E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- Miller CD, Fermann GJ, Lindsell CJ, Mahaffey KW, Peacock WF, Pollack CV, et al. Initial risk stratification and presenting characteristics of patients with evolving myocardial infarctions. Emergency Medicine Journal. 2008;25:492–497. doi: 10.1136/emj.2007.052183. [DOI] [PubMed] [Google Scholar]
- Milner KA, Vaccarino V, Arnold AL, Funk M, Goldberg RJ. Gender and age differences in chief complaints of acute myocardial infarction (Worcester Heart Attack Study) American Journal of Cardiology. 2004;93:606–608. doi: 10.1016/j.amjcard.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus software. Los Angeles, CA: Muthén & Muthén; 1998–2009a. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5. Los Angeles, CA: Muthén & Muthén; 1998–2009b. [Google Scholar]
- Noureddine S, Arevian M, Adra M, Puzantian H. Response to signs and symptoms of acute coronary syndromes: Differences between Lebanese men and women. American Journal of Critical Care. 2008;17:26–35. [PubMed] [Google Scholar]
- Nyland KL, Asparouhoy T, Muthén BO. Deciding of the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Nyland KL, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: What does latent class analysis say? Child Development. 2007;78:1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Riegel B, Dickson VV, Cameron J, Johnson JC, Bunker S, Page K, et al. Symptom recognition in elders with heart failure. Journal of Nursing Scholarship. 2010;42:92–100. doi: 10.111/j.1547-5069.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, DeVon HA, Horne R, King KB, Milner K, Moser DK, et al. Symptom clusters in acute myocardial infarction: Secondary data analysis. Nursing Research. 2007;56:72–81. doi: 10.1097/01.NNR.0000263968.01254.d6. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Zerwic JJ. Perceptions of symptoms of myocardial infarction related to health care seeking behaviors in the elderly. Journal of Cardiovascular Nursing. 2003;18:184–196. doi: 10.1097/00005082-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease: Results from the National Institutes of Health—National Heart, Lung, and Blood Institute—sponsored women’s ischemia syndrome evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- Stephen SA, Darney BG, Rosenfeld AG. Symptoms of acute coronary syndrome in women with diabetes: An integrative review of the literature. Heart & Lung. 2008;37:179–189. doi: 10.1016/j.hrtlng.2007. 05.006. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Chiang YC, Chien LC, Lin L, Yang CP, Chuang HL. Symptom clustering in older Taiwanese children with cancer. Oncology Nursing Forum. 2008;35:273–281. doi: 10.1188/08.ONF.273-281. [DOI] [PubMed] [Google Scholar]
- Zerwic JJ. Symptoms of acute myocardial infarction: Expectations of a community sample. Heart & Lung. 1998;27:75–81. doi: 10.1016/s0147-9563(98)90015-2. [DOI] [PubMed] [Google Scholar]
- Zerwic JJ, Ryan CJ, DeVon HA, Drell MJ. Treatment seeking for acute myocardial infarction symptoms: Differences in delay across sex and race. Nursing Research. 2003;52:159–167. doi: 10.1097/00006199-200305000-00005. [DOI] [PubMed] [Google Scholar]