Abstract
Pseudomonas aeruginosa phosphorylcholine phosphatase (PchP) catalyzes the hydrolysis of phosphorylcholine (Pcho), is activated by Mg2+ or Zn2+, and is inhibited by high concentrations of substrate. This study has shown that PchP contains two sites for alkylammonium compounds (AACs): one in the catalytic site near the metal ion-phosphoester pocket, and the other in an inhibitory site responsible for the binding of the alkylammonium moiety. The catalytic mechanism for the entry of Pcho in both sites and Zn2+ or Mg2+ follows a random sequential mechanism. However, Zn2+ is more effective than Mg2+ at alleviating the inhibition produced by the entry of Pcho or different AACs in the inhibitory site. We postulate that Zn2+ induces a conformational change in the active center that is communicated to the inhibitory site, producing a compact or closed structure. In contrast, Mg2+ produces a relaxed or open conformation.
1. Introduction
Pseudomonas aeruginosa phosphorylcholine phosphatase (PchP) catalyzes the hydrolysis of phosphorylcholine (Pcho) [1]. Pcho is the product of the action of hemolytic phospholipase C (PlcH) on phosphatidylcholine or sphingomyelin and is hydrolyzed to choline and inorganic phosphate (Pi) by the action of PchP. Thus, both the PlcH and PchP enzymes are involved in the pathogenesis of P. aeruginosa [2]. PchP contains three motifs that are characteristic of the enzymes belonging to the haloacid dehalogenase (HAD) superfamily [3]. Moreover, all three motifs have an important role in the catalytic process of Pcho or p-nitrophenylphosphate (p-NPP) in the presence of Mg2+, Zn2+, or Cu2+ as activators of the enzyme [4]. Using Pcho as the substrate, we have shown that Mg2+ is an equal activator for the enzyme at pH 5.0 and at pH 7.4; however, Zn2+ is an activator at pH 5.0 but an inhibitor at pH 7.4. The inhibition produced by Zn2+ at pH 7.4 is reversible and occurs in the presence or absence of Mg2+. This activation or inhibition of PchP by Zn2+ is caused by the transition from octahedral to tetrahedral geometry in the coordination sphere of the metal ion [5]. These results, in combination with the fact that PchP is inhibited by high Pcho concentrations and previous observations that different AACs may act as inhibitors of PchP [1, 6, 7], led us to evaluate the catalytic mechanism of PchP with Pcho as the substrate, Mg2+ or Zn2+ as activators, and AACs as inhibitors.
2. Materials and Methods
2.1. Materials
Isopropyl-β-D-thiogalactopyranoside (IPTG) and HisLinkTM resin were purchased from Promega. Pcho and p-NPP were purchased from Sigma-Aldrich Co. Trimethylamine hydrochloride, tetramethylammonium chloride (T4MA), choline chloride, chlorocholine chloride, betaine hydrochloride, hexamethonium chloride, decamethonium bromide, tubocurarine hydrochloride pentahydrate, neostigmine bromide, 2-amino-2-methyl−1-propanol, L-histidinol dihydrochloride (Sigma-Aldrich), and all other chemicals were of analytical quality (Sigma-Aldrich or Merck).
2.2. Bacterial Strains, Growth Conditions, and Enzyme Purification
The production of the PchP His-tag fusion protein in E. coli BL21 CodonPlus (Stratagene) was performed as previously described [5]. In the enzyme's purification, 1.5 mM de EDTA was added to remove any residual metal. Thereafter, the water utilized was three times distilled in glass and checked by Atomic Absorption Spectrometry (AAS). Under this condition, without the addition of metal ion, phosphorylcholine phosphatase activity was not found when it was measured in optimal conditions with Pcho or p-NPP as substrates. The addition of very low Zn2+ concentration, 0.5 μM, or 0.25 mM Mg2+ produced effective enzyme activation. The protein yield was between 15 mg L−1 and 20 mg L−1. The specific activity, as measured with 10 mM p-NPP and 2 mM Mg2+, was 110 μmoL p-nitrophenol min−1 (mg protein)−1. The specific activity of the same preparation, as measured with 0.05 mM Pcho and 2 mM Mg2+, was 54.5 μmoL Pi min−1(mg protein)−1.
2.3. Protein Concentration
The protein concentration was determined by spectrophotometric measurement at 280 nm, using the theoretical molar extinction coefficient (ε = 69,915 M−1 cm−1) [8]. Additional details have been described previously [5].
2.4. Enzyme Activity and Kinetic Data Analysis
The standard assay to measure acid phosphatase activity was performed with p-NPP [4]. PchP activity with Pcho as the substrate was measured based on the release of Pi as described by Baykov et al. [9] and detailed by Otero et al. [5]. One unit of PchP was defined as the amount of enzyme that released 1 μmoL of p-nitrophenol or Pi from p-NPP or Pcho per minute at 37°C. Kinetic data were analyzed using the program DYNAFIT (http://www.biokin.com/dynafit) [10] to perform the global fits of the data to assess the best fit to activation and inhibition mechanisms (model discrimination analysis) and to calculate the corresponding constants. Results for Pcho inhibition of the enzyme activity, as measured with p-NPP, were analyzed utilizing the Hill equation: log v/V − v = K0.5 − nlog [I] as described previously [11].
2.5. Analysis of the Metal Ion-Ligand Distances
Distances between the metal ion and ligands found in the active site of PchP were calculated with the program MESPEUS (http://tanna.bch.ed.ac.uk/) for metal coordination groups in proteins based on distances in the Cambridge Structural Database (CSD) and other data taken from protein structures determined at or near atomic resolution [12].
3. Results
3.1. PchP Activity with Pcho as Substrate and Mg2+ or Zn2+ as Activators
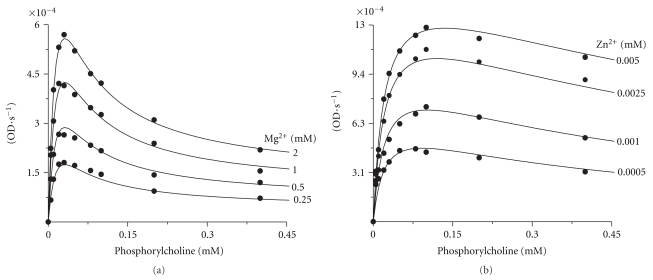
The saturation curves of PchP with different Pcho concentrations in the presence of variable concentrations of Mg2+ or Zn2+ are shown in Figures 1(a) and 1(b), respectively. The kinetic constants, KM and KA, indicated that the KM values for Pcho did not change in the presence of Mg2+ or Zn2+ despite the fact that Zn2+ has 1,000-fold stronger affinity for PchP compared to Mg2+ (Table 1). Comparison of the curves from Figures 1(a) and 1(b) demonstrates that the inhibition in the presence of Zn2+ produced by high Pcho concentrations significantly decreased as compared to the curves obtained in the presence of Mg2+. The KSI values for Pcho in the presence of Zn2+ were nearly 33-fold higher than the values obtained in the presence of Mg2+ (Table 1). These results led us to conduct experiments with p-NPP or Pcho as substrates with either T4MA or Pcho as inhibitors in the presence of Mg2+ or Zn2+.
Figure 1.
Saturation curves for PchP with different Pcho concentrations (0.0005–0.4 mM) in the presence of 0.25 mM, 0.5 mM, 1 mM, and 2 mM Mg2+ (a) or 0.0005 mM, 0.001 mM, 0.0025 mM, and 0.005 mM Zn2+ (b). The enzyme activity was measured in 100 mM sodium acetate buffer, pH 5.0. The statistics of least-squares fit were as follows: mean square = 9.99254 × 10−11 (a) and 1.08357 × 10−9 (b); root-mean-square deviation = 9.99627 × 10−6 (a) and 3.29177 × 10−5 (b); number of datapoints = 40; optimized parameters = 6; degrees of freedom = 34; percentage confidence interval level = 99%.
Table 1.
Kinetic constants of PchP for substrate and inhibitors obtained in the presence of Mg2+ or Zn2+ as metal ion activators. Values ± SD of at least three experiments in duplicate were performed for each set of curves that are shown in each corresponding figure indicated in this table.
| Constants | Mg2+ | Zn2+ | Observations |
|---|---|---|---|
| KMPcho, mM ± SD | 0.021 ± 0.008 | 0.022 ± 0.007 | Data taken from Figure 1 and Scheme 1 |
| KA, mM ± SD | 0.91 ± 0.08 | 0.0009 ± 0.00005 | |
| kcat1, s−1± SD | 121 ± 8 | 143 ± 12 | |
| kcat2, s−1± SD | 12 ± 0.9 | 9 ± 0.7 | |
| KSI1, mM ± SD | ND | ND | S·E·S, Scheme 1, is a nonproductive complex |
| KSI2, mM ± SD | 0.037 ± 0.01 | 1.21 ± 0.09 | Data taken from Figure 1 and Scheme 1 |
| KI1, mM ± SD | 0.17 ± 0.04 | 0.83 ± 0.06 | Data taken from Figure 2 and Scheme 2 |
| KI2, mM ± SD | 0.035 ± 0.008 | 0.45 ± 0.07 | |
| K0.5, mM ± SD | 0.056 ± 0.007 | 0.11 ± 0.02 | Data taken from Figure 4 |
| n Hillvalue ± SD | 1.8 ± 0.03 | 1.2 ± 0.08 | |
3.2. Inhibition by T4MA with p-NPP or Pcho as Substrates and Mg2+ or Zn2+ as Activators
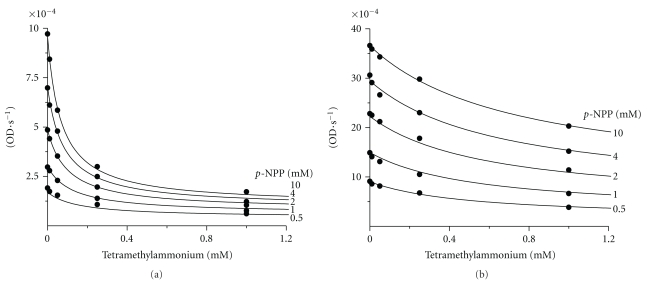
p-NPP was employed as the substrate to avoid interaction of the active site of PchP with the alkylammonium moiety, which is contained in the Pcho molecule. Consequently, the inhibition produced by T4MA was caused only by the NR4+ group of the AAC. The inhibition curves resulting from T4MA that were obtained in the presence of Mg2+ or Zn2 indicated that Zn2+ was more effective than Mg2+ in preventing the inhibition produced by increasing concentrations of the T4MA (Figure 2 and Table 1).
Figure 2.
Inhibition of PchP activity by T4MA in the presence of 2 mM Mg2+ (a) or 0.03 mM Zn2+ (b). The enzyme activity was measured with different p-NPP concentrations (0.5 mM, 1 mM, 2 mM, 4 mM, and 10 mM) in 100 mM sodium acetate buffer, pH 5.0. The statistics of least-squares fit were as follows: mean square = 1.26295 × 10−10 (a) and 2.63174 × 10−9 (b); root-mean-square deviation = 1.12381 × 10−5 (a) and 5.13005 × 10−5 (b); number of datapoints = 24; optimized parameters = 5; degrees of freedom = 20; percentage confidence interval level = 99%.
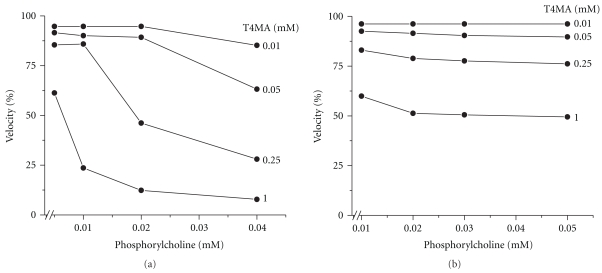
The differential effect of Mg2+ and Zn2+ on the inhibition caused by T4MA was also observed by measuring the enzyme activity with subinhibitory concentrations of Pcho as substrate (Figure 3). Under these conditions and in the presence of Mg2+, the inhibition produced by increasing concentrations of Pcho and variable T4MA concentrations had an additive effect (Figure 3(a)). In contrast, the inhibition produced by increasing concentrations of both AACs in the presence of Zn2+ was low or nearly negligible (Figure 3(b)).
Figure 3.
Effect of T4MA on the PchP activity measured at low Pcho concentrations. Pcho concentration varied between 0.005 mM and 0.04 mM when the activity was measured in the presence of 3 mM Mg2+. With 0.003 mM Zn2+ as the activator, the Pcho concentration varied between 0.01 mM and 0.05 mM. As was shown in Figure 1, in these assay conditions there was no enzyme inhibition by high substrate concentrations. 3 mM Mg2+ and 0.003 mM Zn2+ correspond to three times the KA values that are shown in Table 1. All determinations were performed in 100 mM sodium acetate buffer, pH 5.0. Because several parts of these experiments were performed with low Pcho concentrations, special care was taken to measure the enzyme activity under strict conditions of initial velocity.
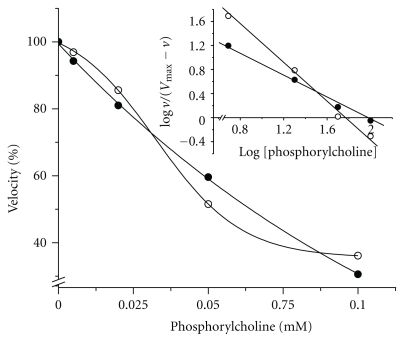
3.3. Inhibition of PchP by Pcho with p-NPP as Substrate and Mg2+ or Zn2+ as Activators
The inhibitory effect of Pcho on PchP activity when the activity was measured with p-NPP as the substrate and Mg2+ or Zn2+ as activators is shown in Figure 4. The “n” Hill coefficient value decreased from 1.8 in the presence of Mg2+ to 1.2 in the presence of Zn2+ (Table 1).
Figure 4.
Inhibition of PchP activity by Pcho measured with 10 mM p-NPP in the presence of 2 mM Mg2+ (○) or 0.03 mM Zn2+ (•). All determinations were performed in 100 mM sodium acetate buffer, pH 5.0.
3.4. AACs as Inhibitors of PchP
The PchP activity measured with variable concentrations of p-NPP and variable concentrations of AAC in the presence of Mg2+ indicated that all of the tested AACs were inhibitors of PchP with different degrees of efficiency (Table 2). At 1 mM final concentration, the highest percentages of inhibition were produced by trimethylamine, T4MA, choline, chlorocholine, hexamethonium, and decamethonium. This inhibition was also reflected in the low values of the KI1 and KI2 constants. To a lesser extent, 2-amino-2-methyl−1-propanol and L-histidinol, which are compounds with an N-positive charge but without methyl groups attached to the nitrogen atom, were also poor inhibitors of enzyme activity (Table 2). In addition to the presence of a charged group, such as the −COO− in betaine, the presence of large-R groups, such as in neostigmine, or the decrease of N-methyl groups, such as in tubocurarine, also significantly reduced the inhibition of PchP, as shown by the comparison of KI values or percentage of inhibition in Table 2.
Table 2.
Inhibition of PchP by AACs. Percentage inhibition was measured with 1 mM inhibitor, 10 mM p-NPP, and 2 mM Mg2+ at pH 5.0. Inhibition constants were calculated with the application of the program DYNAFIT from activity curves performed in the presence of 0.5, 1, 2, 4, and 10 mM p-NPP with 2 mM Mg2+ in 100 mM sodium acetate buffer, pH 5.0. The AAC concentrations were 0.01, 0.05, 0.25, and 1 mM. Values shown are averaged from three independent experiments ± SD. PU: partial uncompetitive and C: competitive inhibition.
| Inhibitor | Number of N-alkyl groups | % Inhibition | Inhibition constants | Type of inhibition | |
|---|---|---|---|---|---|
| KI1, mM ± SD | KI2, mM ± SD | ||||
| Trimethylamine | N,N,N-trimethyl | 81 | 0.21 ± 0.03 | 0.06 ± 0.01 | PU + C |
| Tetramethylammonium | N,N,N,N-tetramethyl | 91 | 0.17 ± 0.04 | 0.04 ± 0.01 | PU + C |
| Choline | 1 N,N,N-trimethyl | 85 | 0.89 ± 0.08 | 0.10 ± 0.01 | PU + C |
| Chlorocholine | 1 N,N,N-trimethyl | 82 | 0.44 ± 0.04 | 0.12 ± 0.01 | PU + C |
| Betaine | 1 N,N,N-trimethyl | 26 | 1.44 ± 0.09 | 7.75 ± 0.91 | PU + C |
| Hexamethonium | 2 N,N,N-trimethyl | 85 | 0.37 ± 0.05 | 0.08 ± 0.01 | PU + C |
| Decamethonium | 2 N,N,N-trimethyl | 83 | 0.21 ± 0.04 | 0.08 ± 0.01 | PU + C |
| Tubocurarine | 1 N-methyl + 1 N,N-dimethyl | 22 | 2.51 ± 0.21 | 4.10 ± 1.20 | PU + C |
| Neostigmine | 1 N,N-dimethyl + 1 N,N,N-trimethyl | 55 | 6.25 ± 0.34 | 0.57 ± 0.05 | PU + C |
| 2-amino-2-methyl-1-propanol | 1 N | 74 | 3.94 ± 0.16 | 0.21 ± 0.01 | PU + C |
| L-histidinol | 1 N | 56 | 2.11 ± 0.05 | 0.31 ± 0.08 | PU + C |
4. Discussion
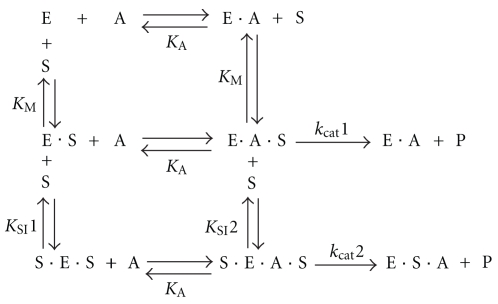
In this study, the experimental data treated with DYNAFIT resulted in two schemes for the PchP catalytic mechanism. Scheme 1 illustrates that (i) PchP binds two substrate molecules and one metal ion and (ii) the more probable mechanism for the catalytic action of PchP with Pcho is independent of the identity of the metal ion. Considering the first part of Scheme 1 (top), the kinetic parameters are consistent with a random sequential mechanism in which the metal ion (A) or the substrate (S) initially binds to PchP (E). The EA or ES complexes bind to S or A, respectively, to form the EAS productive complex before the enzyme releases the product (P). The second part of Scheme 1 (bottom) shows a random mechanism for the interaction of a second substrate molecule with the ES or EAS complexes to form SES or SEAS complexes (S before E in SES or in SEAS complexes indicates that the second Pcho molecule binds to a site different from the catalytic site of PchP). After SES binds, the metal ion also forms the SEAS complex, which is capable of forming P but does so with less efficiency than the EAS complex. An interesting comparison can be made between the catalytic mechanisms resulting from the use of the two different substrates, Pcho and p-NPP. Using p-NPP as the substrate, an ordered mechanism occurs in the presence of Mg2+ or Zn2+ [4]. The discrepancy between the catalytic mechanisms may be caused by the different affinity of PchP for the substrates; one of the them is positively charged with an N-trimethylammonium moiety (KM Pcho ≈ 0.020 mM), and the other contains a p-nitrophenol group (KM p−NPP ≈ 3 mM). This difference in affinities may lead to a more equal competition between the metal and substrate for the enzyme because the presence of metal is not required for Pcho binding.
Scheme 1.
Catalytic mechanism of PchP with Pcho as substrate. The data shown in Figure 1 were analyzed with the program DYNAFIT. E: enzyme; S: substrate, Pcho; A: divalent cation activator; P: product.
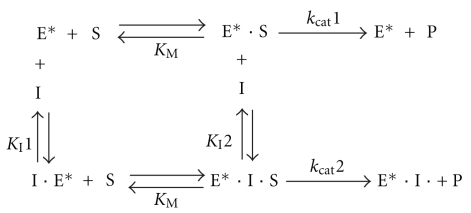
Experiments performed with Mg2+ or Zn2+ indicate that Zn2+ produced enzyme activation at concentrations 1,000-fold lower than Mg2+, and Zn2+ prevented inhibition due to the entry of the second Pcho molecule much more effectively than Mg2+. The experimental data obtained with T4MA showed that in the presence of either metal ion, Mg2+ or Zn2+, the inhibition produced by T4MA followed a mixed-type inhibition mechanism with competitive and partial uncompetitive components (Scheme 2). This type of inhibition was obtained with p-NPP, which is a substrate without the alkylammonium moiety, and it is confirmatory that the enzyme contains two binding sites for alkylammonium ion: one site, which is indicated by the competitive component, may be the site for the alkylammonium moiety of the Pcho substrate, and the second site may be responsible for inhibition due to high substrate concentration, which was shown with Pcho.
Scheme 2.
Mechanism of PchP inhibition produced by T4MA. The data shown in Figure 2 were analyzed with the program DYNAFIT. In this scheme, (E*) represents the enzyme bound to the metal ion for simplicity. S: substrate, I: inhibitor, and P: product. In this scheme, kcat1 ≫ kcat2.
Experiments with many AACs demonstrating competitive and uncompetitive inhibitory components and the clear differences found for the KI1 and KI2 values confirmed that PchP contains two binding sites for the alkylammonium moiety of Pcho. Among these compounds, the KI1 and KI2 values, as defined in Scheme 2, indicated that T4MA and trimethylamine were the compounds that possessed higher affinities for both alkylammonium sites of PchP. Based on the differences in the inhibitory capacity produced by the remaining AACs, the lowered inhibition and increased KI values were caused by one or more of the following reasons: hydrophilic interactions, repulsion or attraction of charges, length or size of the molecule's structure, and lack of hydrophobic interactions at the opposing end of the inhibitory molecule. The importance of the number of N-methyl groups and the presence of hydroxyl or carboxyl groups in the structure of the inhibitory molecule has been previously discussed [7]. Overall, the kinetic data suggest that P. aeruginosa PchP contains a catalytic site with an inhibitory site in its vicinity. The catalytic site is formed by two subsites: one subsite is for the phosphoester moiety of Pcho, which is closely associated with the metal ion site, and the second sub-site is responsible for the binding of the N-trimethyl moiety of Pcho. The inhibitory site also has the capacity to bind the N-trimethyl moiety of Pcho.
The most notable result from this study was the differential effects of Zn2+ and Mg2+ as activators of PchP when the enzyme was in the presence of high concentrations of Pcho or T4MA. According to Beassoni et al. [4] and Otero et al. [5], the octahedral coordination complex between Mg2+ or Zn2+ with different ligands is formed with the carboxylate groups of 31D and 262D, the carbonyl group of 33D, the oxygen from the phosphate and two water molecules. Therefore, to explain the differential effect of Zn2+ and Mg2+, we believe that it is necessary to consider the fact that Zn has a higher nuclear charge than Mg. The consequence of this different nuclear charge may be translated into changes in bond distances between the central metal ion and the different functional groups of the enzyme that are coordinately involved to form the octahedral complex with Mg2+ or Zn2+. This assumption may be supported by considering the distances that were calculated with the MESPEUS program when assigning the coordination index for Mg2+ and Zn2+ to be six. The average distances between Mg2+ and the –COO− and C=O groups were 2.07 Å and 2.26 Å, respectively. Shorter average distances were obtained for Zn2+, –COO− and C=O groups at 1.99 Å and 2.07 Å, respectively. If these average distances are present in the coordination compounds formed from the active site residues of PchP and the two metal ions, it is possible to assume that the coordination sphere of the octahedral complex forms an open or relaxed active center in the presence of Mg2+, whereas the presence of Zn2+ shifts the conformation of the active center toward a closed or compact form. This conformational change may not be a stochastic process but is a process directed by the interaction forces produced by the higher nuclear charge of the Zn2+ ions as compared to the nuclear charge of the Mg2+ ions. Therefore, it is reasonable to postulate that Zn2+ induces a conformational change as proposed by Weikl and Von Deuster [13], which may promote the hydrolysis of Pcho and decrease its inhibitory effect by preventing the entry of the second substrate molecule.
From a physiological standpoint, it is interesting to note that the activity of this enzyme is controlled by environmental factors, such as N-methylammonium compounds, Mg2+ and Zn2+ that are present or may be found in any ecological niche, where P. aeruginosa can grow and multiply. Considering that the infectious process takes place in an acidic medium, where P. aeruginosa can find free Pcho or esterified phosphatidylcholine or sphingomyelin, our present data in addition to those described previously [5] indicate that, at low concentrations, Zn2+ favors the action of PchP to release choline through two processes: (i) by increasing its catalytic activity at low concentrations of substrate and (ii) by its protective effect against enzyme inhibition that may be caused by high concentrations of Pcho or other AACs.
We have recently crystallized PchP and obtained preliminary X-ray diffraction data for the enzyme [14]. Thus, we will be able to investigate the interactions of the enzyme with substrate, metal ions, and other effectors in more detail based on the solved structure of PchP in the near future, and we will be able to gain very useful information on the relationship between the structure and function of this enzyme in greater depth.
Acknowledgments
A. T. Lisa and C. E. Domenech are Career Members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). L. H. Otero would like to acknowledge fellowship support from CONICET-Ministerio de Ciencia y Tecnología de Córdoba (MinCyT-Córdoba), P. R. Beassoni would like to acknowledge fellowship support from CONICET, and C. Boetsch, a student, would like to acknowledge fellowship support from MinCyT-Córdoba. This work was supported by grants from the Agencia Nacional de Promociones Científicas y Tecnológicas (FONCyT), the MinCyT-Córdoba, and SECyT-UNRC of Argentina.
References
- 1.Salvano MA, Domenech CE. Kinetic properties of purified Pseudomonas aeruginosa phosphorylcholine phosphatase indicated that this enzyme may be utilized by the bacteria to colonize in different environments. Current Microbiology. 1999;39(1):1–8. doi: 10.1007/pl00006819. [DOI] [PubMed] [Google Scholar]
- 2.Lisa AT, Beassoni PR, Masssimelli MJ, Otero LH, Domenech CE. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Badajoz, Spain: 2007. A glance on Pseudomonas aeruginosa phosphorylcholine phosphatase, an enzyme whose synthesis depends on the presence of choline in its environment; pp. 255–262. [Google Scholar]
- 3.Beassoni PR, Otero LH, Massimelli MJ, Lisa AT, Domenech CE. Critical active-site residues identified by site-directed mutagenesis in Pseudomonas aeruginosa phosphorylcholine phosphatase, a new member of the haloacid dehalogenases hydrolase superfamily. Current Microbiology. 2006;53(6):534–539. doi: 10.1007/s00284-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 4.Beassoni PR, Otero LH, Lisa AT, Domenech CE. Using a molecular model and kinetic experiments in the presence of divalent cations to study the active site and catalysis of Pseudomonas aeruginosa phosphorylcholine phosphatase. Biochimica et Biophysica Acta. 2008;1784(12):2038–2044. doi: 10.1016/j.bbapap.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Otero LH, Beassoni PR, Lisa AT, Domenech CE. Transition from octahedral to tetrahedral geometry causes the activation or inhibition by Zn2+ of Pseudomonas aeruginosa phosphorylcholine phosphatase. BioMetals. 2010;23(2):307–314. doi: 10.1007/s10534-010-9289-1. [DOI] [PubMed] [Google Scholar]
- 6.Lisa TA, Garrido MN, Domenech CE. Pseudomonas aeruginosa acid phosphatase and cholinesterase induced by choline and its metabolic derivatives may contain a similar anionic peripheral site. Molecular and Cellular Biochemistry. 1984;63(2):113–118. doi: 10.1007/BF00285217. [DOI] [PubMed] [Google Scholar]
- 7.Garrido MN, Lisa TA, Domenech CE. Pseudomonas aeruginosa acid phosphatase contains an anionic site with a trimethyl subsite—kinetic evidences obtained with alkylammonium ions. Molecular and Cellular Biochemistry. 1988;84(1):41–49. doi: 10.1007/BF00235191. [DOI] [PubMed] [Google Scholar]
- 8.Gasteiger E, Hoogland C, Gattiker A, et al. The Proteomics Protocols Handbook. Totowa, NJ, USA: Humana Press Inc.; 2005. Protein identification and analysis tools on the ExPASy server; pp. 571–607. [Google Scholar]
- 9.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Analytical Biochemistry. 1988;171(2):266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 10.Kuzmič P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Analytical Biochemistry. 1996;237(2):260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
- 11.Segel IH. Enzyme Kinetics. New York, NY, USA: John Wiley and Sons; 1975. Behavior and analysis of rapid equilibrium and steady-state enzyme systems. [Google Scholar]
- 12.Hsin K, Sheng Y, Harding MM, Taylor P, Walkinshaw MD. MESPEUS: a database of the geometry of metal sites in proteins. Journal of Applied Crystallography. 2008;41(5):963–968. [Google Scholar]
- 13.Weikl TR, Von Deuster C. Selected-fit versus induced-fit protein binding: kinetic differences and mutational analysis. Proteins. 2009;75(1):104–110. doi: 10.1002/prot.22223. [DOI] [PubMed] [Google Scholar]
- 14.Otero LH, Beassoni PR, Domenech CE, Lisa AT, Albert A. Crystallization and preliminary X-ray diffraction analysis of Pseudomonas aeruginosa phosphorylcholine phosphatase. Acta Crystallographica Section F. 2010;66(8):957–960. doi: 10.1107/S1744309110024061. [DOI] [PMC free article] [PubMed] [Google Scholar]