Abstract
Background & Objectives:
Prevalence of metabolic syndrome is high among Asians including Indians. Scarce information is available about the magnitude of metabolic syndrome in rural areas and hence present study in rural area of Wardha district, central India.
Methods:
In 300 randomly selected subjects, blood pressure and anthropometric measurements such as height, weight, waist circumference and hip circumference were noted. Blood sample was collected after overnight fasting and was subjected to biochemical quantification such as fasting blood sugar, total cholesterol, triglycerides, HDL-C, VLDL-C and LDL-C. Data were analyzed using ATP-III definition as well as by modifying the waist circumference cut-offs as per Asia-Pacific guidelines.
Results:
Overall metabolic syndrome as per ATP-III criteria was observed in 5.0 per cent adult rural population. When ATP-III criteria were modified using waist circumference cut-offs recommended by Asia-Pacific guidelines, metabolic syndrome was seen in 9.3 per cent. It was 10.7 per cent among females and 8.2 per cent among males. Receiver operating characteristic curve was plotted to find out the best cut-off of BMI to identify the individuals with metabolic syndrome. The best cut-off for BMI came out to be 23.32 kg/m2.
Interpretation & conclusions:
The magnitude of metabolic syndrome was low among rural adults of Wardha as compared to reported values in urban areas. BMI of 23.32 kg/m2 and higher was found to predict significant risk of metabolic syndrome in these study subjects. However, studies with larger sample need to be conducted to confirm these findings.
Keywords: ATP-III, dyslipidaemia, metabolic syndrome, modified ATP-III
Metabolic syndrome is a constellation of risk factors such as central obesity, increased blood pressure, impaired glucose tolerance, altered lipid profile mainly low high density lipoproteins (HDL) and high triglycerides which predispose the individual to increased risk for development of diabetes mellitus and cardiovascular diseases1,2. Its pathogenesis is complex, but interaction of obesity, sedentary lifestyle and dietary and genetic factors are known for their contribution2,3. It is a useful tool to identify individuals at risk for diabetes and coronary heart disease. Epidemiological studies have reported a high prevalence of metabolic syndrome and cardiovascular mortality among non-resident Indians settled abroad4–6. Lifestyle factors appear to play an important role as obesity especially abdominal obesity, and dyslipidaemia worsen with urbanization and migration7,8.
Prevalence of metabolic syndrome is high among Asians including Indians, and is rising, particularly with the adoption of modernized lifestyle. Many studies in India have reported high prevalence of metabolic syndrome9–11. But these studies were carried out mainly in urban areas. Scanty information is available about the magnitude of metabolic syndrome in rural areas. Hence the present study was undertaken in rural area of Wardha district, central India, to find out the magnitude of metabolic syndrome.
Material & Methods
Study setting and study subjects:
The present cross-sectional study was carried out in all the villages of Primary Health Center (PHC), Anji from February 2007 to February 2008. The study site was located in rural Wardha district, about 758 km east from State capital Mumbai. Anji PHC covers 23 villages with a population of 33000 and is a field practice area of Mahatma Gandhi Institute of Medical Sciences, Sewagram. All adult residents of these 23 villages (above the age of 18 yr) were included in sampling frame. The protocol was approved by the institutional ethical committee.
Sampling method and sample size:
Considering 10 per cent prevalence of metabolic syndrome 3 per cent absolute error, alpha=0.1 and 10 per cent non-response; sample size required was 3008. The study subjects were selected by simple random sampling using computer generated numbers. The written informed consent was obtained from each participant.
Anthropometric measurements:
Body weight, height, waist and hip circumference were measured12. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meter.
Laboratory analysis:
The previous evening, the participants were visited and written informed consent was obtained after explaining the objectives and procedures of the study. After overnight fast blood samples (10 ml) were collected. The fasting blood sugar was analyzed by glucose oxidase peroxidase (GOP-PAP) method13; total cholesterol was analyzed by CHOD-PAP method14; triglycerides by GPO-PAP Trinder method15; and HDL-C by phosphotugstic acid method16. VLDL-C was calculated by indirect method as VLDL cholesterol is one-fifth of triglyceride level17. LDL-C was calculated by subtracting VLDL-C and HDL-C from total cholesterol. ERBA kits supplied by Transia Biochemicals Ltd., Mumbai, were used.
Statistical analysis:
According to the National Cholesterol Education Program Adult Treatment Panel (ATP) III,18the diagnosis of metabolic syndrome was made when three or more of the following risk factors were present: a waist circumference >102 cm in men and >88 cm in women, fasting glucose ≥110 mg/dl (6.1 mmol/l), systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥ 85 mmHg, fasting triglycerides ≥150 mg/dl (1.7 mmol/l), and HDL cholesterol < 40 mg/dl (1.0 mmol/l) in men and < 50 mg/dl (1.3 mmol/l) in women. ATP III definitions were based on the association of factors with subsequent coronary heart disease in Caucasian cohorts. As Indians have higher body fat content than their western counterparts for the same BMI, lower cut-offs of waist circumference were used as suggested by Asia-Pacific guidelines19. Waist circumference (WC) cut-offs were taken as >90 cm for males and >80 cm for females to define overweight19.
Chi-square test was used to test the null hypothesis. All P values were two-tailed and statistical significance was defined as P<0.05. Receiver operating characteristic (ROC) analysis was carried out to identify the optimal cut-off for the BMI to identify the individuals with metabolic syndrome.
Results
Of the 300 subjects selected, 101 (33.7%) were in the age group of 18-30 yr, 119 (39.6%) were in the age group of 31-50 yr while 80 (26.7%) were above 50 yr. Majority of the study subjects (56.3%) were males (Table I).
Table I.
Age and sex distribution of the study subjects
| Age (yr) | Sex |
Total Number (%) | |
|---|---|---|---|
| Male Number (%) | Female Number (%) | ||
| 18-30 | 51 (50.5) | 50 (49.5) | 101 (33.7) |
| 31-50 | 64 (53.8) | 55 (46.2) | 119 (39.6) |
| 51 and above | 54 (67.5) | 26 (32.5) | 80 (26.7) |
| Total | 169 (56.3) | 131 (43.7) | 300 (100.0) |
The levels of biochemical parameters (triglyceride, total cholesterol, VLDL-C, LDL-C, HDL-C and fasting blood sugar level) and the anthropometric parameters (waist circumference, BMI) did not differ significantly between the two sexes except for the waist-hip ratio. Also, systolic and diastolic blood pressure levels did not differ significantly (Table II).
Table II.
Clinical biochemistry, blood pressure and anthropometric parameters by sex
| Parameters | Male N=169 | Female N=131 | Total N=300 |
|---|---|---|---|
| Triglycerides (mg/dl) | 133.91 ± 6.31 | 125.17 ± 6.81 | 130.11 ± 4.66 |
| Total cholesterol (mg/dl) | 167.24 ± 3.14 | 169.71 ± 3.28 | 168.31 ± 2.27 |
| VLDL-C (mg/dl) | 26.79 ± 1.27 | 24.81 ± 1.36 | 25.93 ± 0.93 |
| LDL-C (mg/dl) | 99.58 ± 2.86 | 101.84 ± 3.06 | 100.56 ± 2.09 |
| HDL-C (mg/dl) | 40.86 ± 1.08 | 42.98 ± 1.06 | 41.78 ± 0.76 |
| Fasting glucose (mg/dl) | 89.89 ± 1.47 | 90.63 ± 2.29 | 90.21 ± 1.29 |
| Waist circumference (cm) | 76.82 ± 0.87 | 74.91 ± 1.04 | 75.99 ± 0.67 |
| Systolic BP (mm/Hg) | 130.04 ± 1.20 | 130.20 ± 1.49 | 130.11 ± 0.94 |
| Diastolic BP (mm/Hg) | 82.42 ± 0.83 | 83.21 ± 0.96 | 82.76 ± 0.63 |
| Body mass index (kg/m2) | 22.84 ± 0.27 | 22.66 ± 0.28 | 22.76 ± 0.19 |
| Waist-hip ratio* | 0.83 ± 0.01 | 0.80 ± 0.01 | 0.82 ± 0.01 |
| Values are mean ± SE; *P <0.05 |
The overall presence of metabolic syndrome as per ATP-III criteria was 5.0 per cent. It was significantly higher among females (7.6%) as compared to males (2.9%). When ATP-III criteria was modified using waist circumference cut-offs recommended by Asia-Pacific guidelines, metabolic syndrome was observed in 9.3 per cent. It was 10.7 per cent among females and 8.2 per cent among males. The difference was not statistically significant (Table III). The Figure shows the prevalence of metabolic syndrome by sex and age group. In youngest age group (18-30 yr), none of the male subject had metabolic syndrome while 4 per cent females had it. In the age group of 31-50 yr, more females than males were affected (16.4% in females vs. 10.9% in males) while in older age group (> 50 yr), more males were affected than females (13.0% in males vs. 11.5% in females).
Table III.
Proportion of adults with metabolic syndrome and individual parameters above threshold levels
| Parameters(yr) | Sex |
Total N = 300 | |
|---|---|---|---|
| Male N = 169 | Female N = 131 | ||
| Triglycerides (≥ 150 mg/dl) | 57 (33.5) | 34 (26.0) | 91 (30.2) |
| HDL-C (mg/dl) (M ≥ 40; F≥50)* | 85 (50) | 92 (70.2) | 177 (58.8) |
| Fasting glucose (≥ 110 mg/dl) | 23 (13.5) | 14 (10.7) | 37 (12.3) |
| Blood pressure (SBP ≥ 130 and/or DBP ≥ 85 mm/Hg) | 91 (53.5) | 71 (54.2) | 162 (53.8) |
| Waist circumference Int (M ≥ 102 cm; F ≥ 88 cm)* | 6 (3.5) | 23 (17.6) | 29 (9.6) |
| Waist circumference Asia (M ≥ 90 cm; F ≥ 80 cm)* | 28 (16.5) | 37 (28.2) | 65 (21.6) |
| Metabolic syndrome (ATP III criteria) | 5 (2.9) | 10 (7.6) | 15 (5.0) |
| Metabolic syndrome Asia (ATP III criteria modified as per Asia-Pacific guidelines) | 14 (8.2) | 14 (10.7) | 28 (9.3) |
| Figures in parentheses are percentages; *P<0.05 | |||
Fig.
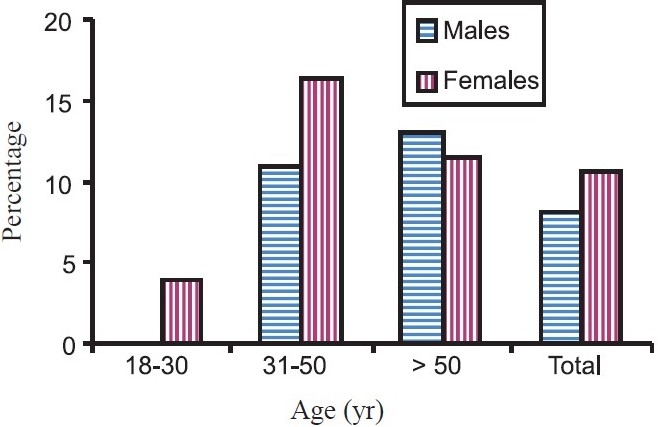
Prevalence of metabolic syndrome by age and sex.
ROC curve was plotted to find out the best cut-off of BMI to identify the individuals with metabolic syndrome. The best cut-off for BMI came out to be 23.32. Sensitivity of BMI at this cut-off was 0.75 (95% CI: 0.566-0.873), specificity was 0.685 (95% CI: 0.627-0.737). Likelihood ratio for BMI > 23.32 was 2.38 (95% CI: 1.8-3.1) and likelihood ratio for BMI ≤ 23.32 was 0.36 (95% CI: 0.19-0.69). The diagnostic odds ratio was 6.52 (95% CI: 2.67-15.9).
Discussion
In the present study, metabolic syndrome was found in 9.3 per cent rural population by modified criteria as per Asia-Pacific guidelines. Traditional societies and population residing in and around rural areas are expected to have low prevalence as these are not exposed to modernization8. The ICMR task force collaborative study reported the prevalence of metabolic syndrome to be 30 per cent in urban areas of Delhi and 11 per cent in rural Haryana using ATP-3 criteria20. Mishra et al9reported 30 per cent prevalence among the urban slum population in Delhi. Ramchandran et al21, reported a prevalence of 41 per cent in urban area of Chennai using modified ATP-3 criteria among adults aged 20 to 75 yr21. They also reported that prevalence was higher in women than men (46.5 vs 36.4%) and in older people. Sarkar et al8reported 30-50 per cent prevalence in Bhutia tribe, with no rural-urban difference. Among the Toto tribe, the rural community prevalence was low 4-9 per cent8. The differences may be attributed to the difference in study areas, and the different definitions of metabolic syndrome used.
Higher abdominal fat (android obesity) is known to be a risk factor for hypertension, hypertriglyceridaemia, hyperinsulinaemia and diabetes22–24. The BMI though does not account the distribution of body fat, is recommended by the World Health Organization as the most useful epidemiological measure of obesity and is most commonly used to tract obesity and related diseases25,26. In Caucasian populations, the association between BMI and mortality is “J-shaped” and nadir of the curve between 18.5 - 25.0 kg/m2 is taken as healthy BMI27. Cut-off values of 25.0 and 30.0 kg/m2are taken for overweight and obesity respectively and also for identifying the risk of associated morbidities28. In an effort to map the epidemic of obesity and associated risk of co-morbidities, it has become common practice to use these cut-off values in different populations with the assumption that different ethnic groups have similar morbidity/mortality risk for the specific BMI level in absence of any such evidence29. Same is true for other anthropometric indicators. As evidence emerged from many studies about the higher susceptibility of Asians at lower BMI, international task force suggested lower cut-off values for them (23 and 25 kg/m2 for overweight and obesity respectively)28. Studies from urban India also suggested lower cut-off values of these anthropometric indicators30–33. Deshmukh et al34 also reported lower cut-offs of BMI (21.7 kg/m2for males and 21.2 kg/m2 for females) and waist circumference (72.5 cm for males and 65.5 cm for females) for detection of hypertension in rural Wardha. In the present study, BMI of 23.32 kg/m2 emerged out to be the best cut-off for prediction of risk of metabolic syndrome. Asia-Pacific guidelines also advocate the use of BMI > 23 kg/m2 as a definition of overweight in Asian populations.
To conclude, the presence of metabolic syndrome was seen in 5.0 per cent rural adults of Wardha by ATP-III criteria. It was 9.2 per cent when the cut-off for waist circumference was modified as per Asia-Pacific guidelines. BMI of 23.32 kg/m2 and higher predicts significant risk of metabolic syndrome in these study subjects. As the higher degree of error was considered for the calculation of sample size which has led to decrease in the power of the study, studies with larger sample size need to be conducted to validate the present findings.
References
- 1.Balkau B, Valensi P, Eschwège E, Slamad G. A review of the metabolic syndrome. Diabetes Metab. 2007;33:405–13. doi: 10.1016/j.diabet.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Misra A, Vikram NK. Factors, definitions, predictive value & Asian Indian ethnicity : complexities of the metabolic syndrome. Indian J Med Res. 2008;127:293–6. [PubMed] [Google Scholar]
- 3.Sudha V, Radhika G, Mohan V. Current dietary trends in the management of diabetes. Indian J Med Res. 2004;120:4–8. [PubMed] [Google Scholar]
- 4.Kamath SK, Hussain EA, Amin D, Mortillaro E, West B, Peterson CT, et al. Cardiovascular disease risk factors in 2 distinct ethnic groups: Indian and Pakistani compared with American premenopausal women. Am J Clin Nutr. 1999;69:621–31. doi: 10.1093/ajcn/69.4.621. [DOI] [PubMed] [Google Scholar]
- 5.McKeigue P, Ferrie J, Pierpoint T, Marmot MG. Association of early-onset coronary heart disease in South Asian men with glucose intolerance and hyperinsulinemia. Circulation. 1993;87:152–61. doi: 10.1161/01.cir.87.1.152. [DOI] [PubMed] [Google Scholar]
- 6.Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, Yusuf S. Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J. 1996;48:343–53. [PubMed] [Google Scholar]
- 7.Misra A, Vikram N. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians : evidence and implications. Nutrition. 2004;20:482–91. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Das M, Mukhopadhyay B, Chakrabarti CS, Majumder PP. High prevalence of metabolic syndrome and its correlates in two tribal populations of India and the impact of urbanization. Indian J Med Res. 2006;123:679–86. [PubMed] [Google Scholar]
- 9.Mishra A, Pandey RM, Devi JR, Sharma R, Vikram NK, Khanna N. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25:1722–9. doi: 10.1038/sj.ijo.0801748. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Gupta R, Sarna M, Rastogi S, Gupta VP, Kothari K. Prevalence of diabetes, impaired fasting glucose and insulin resistance syndrome in an urban Indian population. Diabetes Res Clin Pract. 2003;61:69–76. doi: 10.1016/s0168-8227(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 11.Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATP III and IDF definitions in Asian Indians : the Chennai Urban Rural Epidemiology Study (CURES-34) Diabetes Metab Res Rev. 2007;23:127–34. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 12.Luepker RV, Evans A, McKeigue P, Reddy KS. Cardiovascular survey methods. 3rd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 13.Trinder P. Quantitative determination of glucose using GOP-PAP method. Clin Biochem. 1969;6:24–7. [Google Scholar]
- 14.Allain CC, Poo LS, Chan CSG, Richmand WA, Fu P. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 15.Trinder P. Triglyceride estimation by GPD-PAP method. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 16.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoprotein from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–7. [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Biochem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.The Asia-Pacific perspective: Redefining obesity and its treatment. Sydney, Australia: Health Communications Australia Pty Limit; 2000. WHO Western Pacific Region, International Association for the study of Obesity (IASO), International Obesity Task Force (IOTF) [Google Scholar]
- 20.IC Health. National Cardiovascular Disease Database. Available from: http: //www.whoindia.org/LinkFiles/NMH_Resources_National_CVD_database-Final_Report.pdf, accessed on February 10, 2008.
- 21.Ramchandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome in urban Asian Indian adults - a population study using modified ATP-III criteria. Diabetes Res Clin Pract. 2003;60:199–204. doi: 10.1016/s0168-8227(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 22.Ghafoornissa, Krishnaswamy K. Diet and heart disease. Hyderabad, India: National Institute of Nutrition; 2000. [Google Scholar]
- 23.Tanaka S, Togashi K, Rankinen T, Perusse L, Leon AS, Rao DC, et al. Sex differences in the relationships of abdominal fat to cardiovascular disease risk among normal-weight white subjects. Int J Obes Relat Metab Disord. 2004;28:320–3. doi: 10.1038/sj.ijo.0802545. [DOI] [PubMed] [Google Scholar]
- 24.Webb GP. Nutrition: a health promotion approach. 2nd ed. London: Arnold; 2002. [Google Scholar]
- 25.- The World health report 2002- Reducing risks, promoting healthy life. Geneva: World Health Organization; 2002. World Health Organization [Google Scholar]
- 26.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–5. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 28.The Asia-Pacific perspective: Redefining obesity and its treatment. Geneva: Switzerland: World Health Organization; 2000. World Health Organization. [Google Scholar]
- 29.de Onis M, Habicht JP. Anthropometric reference data for international use : Recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64:650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 30.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care. 2003;26:1380–4. doi: 10.2337/diacare.26.5.1380. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran A, Snehalatha C, Dharmaraj D, Vishwanathan M. Prevalence of glucose intolerance in Asian Indians : urban-rural difference and significance of upper body adiposity. Diabetes Care. 1992;15:1348–55. doi: 10.2337/diacare.15.10.1348. [DOI] [PubMed] [Google Scholar]
- 32.Dudeja V, Misra A, Pandey RM, Devina G, Kumar G, Vikram NK. BMI does not accurately predict overweight in Asian Indians in Northern India. Br J Nutr. 2001;86:105–12. doi: 10.1079/bjn2001382. [DOI] [PubMed] [Google Scholar]
- 33.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47) Eur J Clin Nutr. 2009;63:259–67. doi: 10.1038/sj.ejcn.1602920. [DOI] [PubMed] [Google Scholar]
- 34.Deshmukh PR, Gupta SS, Dongre AR, Bharambe MS, Maliye C, Kaur S, et al. Relationship of anthropometric indicators with blood pressure levels in rural Wardha. Indian J Med Res. 2006;123:657–64. [PubMed] [Google Scholar]


