Abstract
BACKGROUND AND OBJECTIVES:
The prevalence of chronic obstructive pulmonary disease (COPD) in Saudi Arabia is unknown. The aim of this study was to estimate the prevalence of COPD among smokers more than 40 years of age attending primary healthcare clinics in Saudi Arabia.
DESIGN AND SETTING:
A questionnaire was used in a cross-sectional collection of demographic data and other items related to diagnosis of COPD in patients visiting primary healthcare clinics.
METHODS:
Eligible subjects were current or ex-smokers and aged 40 years or above. Spirometry was performed according to American Thoracic Society criteria. Airflow obstruction was classified according to the 2003 update of the World Health Organization and Global Initiative for Chronic Obstructive Lung Disease criteria. COPD was defined as a ratio less than 0.70 of post-bronchodilator-predicted forced expiratory volume in the first second to forced vital capacity (FEV1/FVC <0.70).
RESULTS:
Because of incomplete data or poor performance on spirometry, of 1380 subjects eligible for the study, only 501 subjects were eligible for data analysis. Seventy-one patients had an FEV1/FVC ratio <0.70, comprising 14.2% of the study population, of which 95.8% were males. Current smokers comprised 57 (80.3%) subjects. Of the 71 subjects who fulfilled the criteria for COPD diagnosis, none were found to be in COPD stage I; 40 (56.3%) were in stage II and 31 (43.6%) were in stage III of the disease.
CONCLUSION:
Underdiagnosis of COPD in primary healthcare clinics in Saudi Arabia is common, but its extent is not different from the corresponding data available in the literature for other countries. Use of spirometry as a routine test for all patients older than 40 years of age and with a smoking history can help in early detection and proper diagnosis of COPD, which subsequently will help in implementation of preventive measures.
Chronic obstructive pulmonary disease (COPD) is defined as a preventable and treatable airflow limitation that is not fully reversible. The airflow limitation tends to be progressive and is linked to an abnormal inflammatory response due to exposure to noxious particles or gases.1 The diagnosis of COPD is based on symptoms, the presence of risk factors and spirometry. smoking is the main risk factor for development of COPD. Although COPD develops in only about 10% to 15% of smokers, studies have shown that 4 of 5 patients with COPD are either current or former smokers.1,2
COPD is now considered to be the fourth leading cause of death in the United States.3 This is at least in part because most COPD patients do not present to a physician until they are in the later stages of their disease or have significant impairment for which there are limited therapeutic options. In 2000, the national Lung Health Education Program (NLHEP) recommended that all smokers more than 45 years of age should undergo a screening pulmonary function test to identify undiagnosed COPD.4 The American College of Physicians (ACP) recommended that smokers with respiratory symptoms should have a spirometry to diagnose airflow obstruction.5,6
Despite the high rate of smoking in Saudi Arabia, there has not been a prevalence study on the magnitude of COPD among the Saudi population. Data are scarce with regards to the magnitude of this disease. The only available studies are hospital-based studies conducted on populations that are so small as to be not enough to extract significant conclusion on the extent of the problem.7,8 In this regard, there is a need for a more representative study to assess the prevalence of COPD in Saudi Arabia. Therefore, we conducted this survey to estimate the prevalence of COPD among smokers attending primary healthcare clinics in Saudi Arabia using spirometry as a screening tool.
METHODS
Our study was conducted in 60 private primary healthcare clinics throughout Saudi Arabia, including 35 clinics in Riyadh city (central region), 14 clinics in Jeddah city (western region) and 11 clinics in Dammam city (eastern region). The study was approved by the ethical committee of the Saudi Thoracic Society. All physicians involved in this survey underwent a training course on the methodology. All subjects aged 40 years and above who visited the primary healthcare clinics were asked if they were current or ex-smokers of more than 5 years′ duration. All current or ex-smokers who agreed to participate signed an informed consent document. Subjects with known lung disease, upper respiratory tract infection, or medical conditions that precluded use of spirometry were excluded. Clinically significant bronchiectasis was excluded by the clinical history and physical examination.
All eligible subjects completed a questionnaire used to collect demographic data and information on factors that are potentially associated with COPD (smoking history, smoking habits and respiratory symptoms). Spirometry was performed according to the American Thoracic Society (ATS) criteria9 by trained physicians using a spirometer with subjects in a seated position. Two separate measurements were made, one before and one at least 15 minutes after two puffs of salbutamol (400 μg) using a metered-dose inhaler with a spacer. Two investigators independently assessed the quality of the flow-volume curves and time-volume curves according to ATS criteria.
Airflow obstruction was defined according to the 2003 update of the World Health Organization (WHO) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.1 COPD was defined as a ratio of post-bronchodilator-predicted forced expiratory volume in the first second to forced vital capacity of less than 0.70 (FEV1/FVC<0.70), no history of atopy or asthma, and with a smoking history of at least 5 years. Stages of disease severity were classified according to GOLD guidelines. Stages of disease severity were classified as follows: mild (stage I) with FEV1>70%; moderate (stage II) with FEV1 50% to 70%; and severe (stage III) with FEV1<50%.1
Data were encoded in Microsoft Excel 2007 for Windows XP and exported to SPSS version 16 (SPSS, Chicago, USA). Continuous variables were expressed as mean, standard deviation and range; categorical variables were expressed as percentages. The t test, Mann-Whitney U test and/ or chi-square tests were performed to determine significance between variables. Statistical significance was reported when P values were <.05.
RESULTS
The number of eligible subjects who were current smokers or ex-smokers without known lung disease (other than COPD) was 1380. Of them, 879 were excluded due to poor performance on spirometry or incomplete data. The remaining 501 subjects were considered for the data analysis and constituted the study population. Table 1 shows the frequency distribution and mean spirometric values of the study population.
Table 1.
Frequency distribution and mean spirometric values for the study population (n=501).
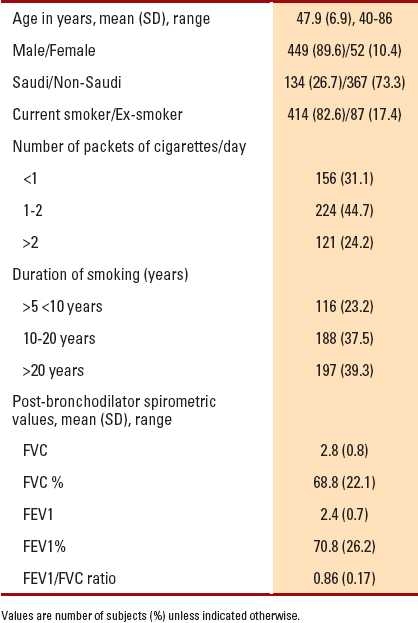
Seventy-one individuals had an FEV1/FVC ratio <0.70, comprising 14.2% of the study population, of which 95.8% were males. Saudi subjects constituted 40.8% (134 subjects) of the study population. Among Saudis, 29 (22%) subjects were diagnosed to have COPD. There were 57 (80.3%) subjects who were current smokers; 33 (46.5%) subjects smoked more than two packets of cigarettes per day; and 30 (42.3%) subjects were smokers for more than 20 years. The older the age, the longer the duration of smoking (P=.001), and the more severe the airflow obstruction (P=.001). Table 2shows the frequency distribution and mean spirometric values for patients with an FEV1/FVC ratio <0.70.
Table 2.
Frequency distribution and mean spirometric values for patients with FEV1/FVC ratio <0.70 (n=71).
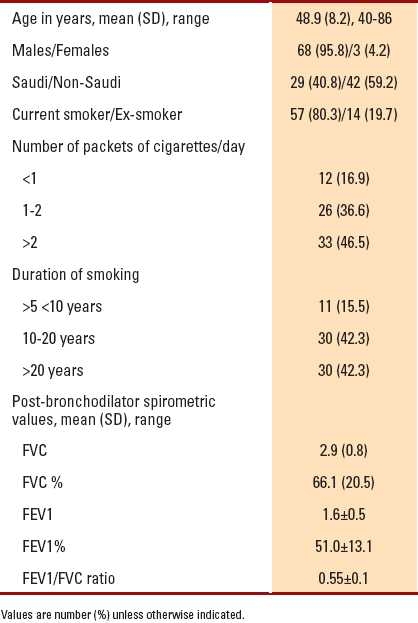
Of the 71 subjects, 50 (70.4%) subjects had cough, 23 (32.4%) subjects had dyspnea and 16 (22.5%) subjects had wheezes. All patients had at least one of the mentioned respiratory symptoms at presentation. None of the subjects were found to be in COPD stage I, whereas 40 (56.3%) subjects were in stage II and 31 (43.6%) subjects were in stage III of the disease (Table 3).
Table 3.
Stages of disease severity (n=71).

DISCUSSION
Our study showed that the prevalence rate of COPD among smokers (aged 40 years and above) attending private primary healthcare setting in Saudi Arabia is 14.2%. To our knowledge, this is the first study to address COPD prevalence in the Saudi primary healthcare setting. The 14.2% prevalence rate found in our study is comparable to that previously reported, i.e., about 10% to 15% of all smokers develop COPD.10 It is difficult to compare the prevalence observed in our study with findings of other studies as they used different population samples and criteria for the diagnosis. Nevertheless, the 14.2% prevalence rate reported in our study is quite comparable to what is previously reported by many studies worldwide. A large population-based study in the USA from 1988 to 1994 on a total of 20050 US adults reported COPD in 12.5% of current smokers and 9.4% in former smokers,11 prevalence rates that are slightly lower than our findings. A similar British study performed by Dickinson et al found results that were slightly lower than ours, with a prevalence of COPD in the general population of 9.9% in those aged 60 to 75 years.12 Interestingly, a large percentage of the patients with COPD (63.3% in the USA study and 52% in the British study) identified in these studies had not been previously diagnosed. Similar prevalence rates of COPD were reported in Denmark,13 Finland,14 Japan15,16 and Korea.17 On the other hand, a prevalence rate of COPD similar to or slightly higher than that found in our study was reported by other studies. The prevalence of chronic bronchitis in adults above the age of 55 years is 9.6% in rural areas and 17.1% in industrial areas of central Greece.18 Vrachnis et al reported 15.5% prevalence rate of COPD in Greece by using spirometry in 364 persons above the age of 55 years.19 Celli et al reported a prevalence rate of 16.8% in US residents aged 30 to 80 years,20 while in a multicenter survey in Spain, the prevalence rate of COPD was 13.1% for men and 10.5% for women.21 Menezes et al also reported a prevalence rate of COPD of 15.2% in Pelotas, Brazil.22
Based on our study, the prevalence of disease among Saudi smokers seems to be higher (22%) than that among non-Saudi smokers. This finding may not be a true representation of the disease prevalence among the Saudis, taking into account the small number of Saudi nationals participating in the study. A bigger population-based study is required to validate this finding. However, if this is proven to be a true estimate, then this high prevalence of COPD among Saudis could reflect either biological or genetic differences in the risk of obstructive lung disease, or it could be attributed to different smoking habits or types of cigarettes used in this country. It would be of interest to study whether using a different source of nicotine such as the hubbly bubbly (water pipes or Shisha), which is common in Saudi Arabia, would have a worsening effect on the prevalence of COPD compared to cigarette smoking. Unlike other studies, our data showed that most of the affected subjects were males and there was a very low prevalence of COPD among females. This may be related to sample selection or may reflect sociocultural differences with regard to smoking habits in Saudi Arabia. Another reason is that some females who smoked may not have admitted to their smoking habit for sociocultural reasons and therefore could not be included in this study.
Our study has limitations that need to be addressed and considered in the interpretation of the results. The use of a fixed ratio of FEV1/FVC, viz., <0.70, as the cut off point for airflow obstruction, according to the GOLD criteria, has the potential to misclassify older ages, since the ratio has a small but significant age-related regression.23 However, this was not a problem in our study as only 3 subjects with FEV1/FVC <0.70 were above the age of 60 years. The study subjects were selected from a private primary healthcare setting rather than a population-based selection, which may not provide a true picture of prevalence of COPD in the general population. The high rate among non-Saudi subjects is because our study was conducted in private primary healthcare clinics, which are mostly attended by non-Saudis. Additionally, a large number of eligible subjects were excluded from the study on the basis of inability to perform spirometry or incomplete data, which were beyond our control. In particular, we were careful not to manipulate the information provided by the subjects so as to avoid biased results.
This is the first study of its kind in Saudi Arabia, and the results obtained are comparable to those observed in other studies worldwide. There was no public advertisement of the study; therefore, no patient-selection bias could take place. However, this study does not eliminate the need for a large population-based study in Saudi Arabia. To make our results readily comparable to other studies and to similar or larger surveys that may be performed in Saudi Arabia in the future, we used the GOLD criteria for diagnosis and determined staging depending on spirometry as a validated tool that is currently used for screening and staging of COPD.
COPD is now considered a preventable and potentially treatable disease if detected in the early stages. Avoidance of exposure to harmful particles, mainly smoking, can prevent progression to a clinically significant stage of the disease. Primary healthcare professionals are on the frontline practitioners in the early diagnosis of the disease, especially in asymptomatic smoking subjects. Our study clearly demonstrates that COPD can be detected in its early stage simply by screening asymptomatic smokers with spirometric testing. It is an opportunity and a responsibility of general practitioners to have a high index of suspicion, adopt a more active management approach and, above all, take more aggressive steps towards reduction of smoking. We highly recommend that spirometry testing be made available and adopted routinely for all smokers above the age of 40 years in primary healthcare settings in Saudi Arabia. This can help in the diagnosis of COPD, allowing early preventive measures and subsequently reducing the prevalence of this chronic debilitating disease.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [Last accessed on 2009 Jul 21]. Available at: http://www.goldcopd.com.
- 2.Vestbo J. Chronic cough and phlegm in young adults: Should we worry? Am J Respir Crit Care Med. 2007;175:2–3. doi: 10.1164/rccm.200610-1458ED. [DOI] [PubMed] [Google Scholar]
- 3.Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. Screening for chronic obstructive pulmonary disease using spirometry: Summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2008;148:535–43. doi: 10.7326/0003-4819-148-7-200804010-00213. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: A consensus statement for the National Lung Health Education Program. Chest. 2000;117:1146–61. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Preventive Services Task Force. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force. Ann Intern Med. 2008;148:529–34. doi: 10.7326/0003-4819-148-7-200804010-00212. [DOI] [PubMed] [Google Scholar]
- 6.Enright P. Does screening for COPD by primary care physicians have the potential to cause more harm than good? Chest. 2006;129:833–5. doi: 10.1378/chest.129.4.833. [DOI] [PubMed] [Google Scholar]
- 7.M Døssing, J Khan, al-Rabiah F. Risk factors for chronic obstructive lung disease in Saudi Arabia. Respir Med. 1994;88:519–22. doi: 10.1016/s0954-6111(05)80334-8. [DOI] [PubMed] [Google Scholar]
- 8.Alamoudi OS. Prevalence of respiratory diseases in hospitalized patients in Saudi Arabia: A 5 years study 1996-2000. Ann Thorac Med. 2006;1:76–80. [Google Scholar]
- 9.Standardization of spirometry, 1994 update. American Thoracic Society Statement. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 10.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 11.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson JA, Meaker M, Searle M, Ratcliffe G. Screening older patients for obstructive airways disease in a semi-rural practice. Thorax. 1999;54:501–5. doi: 10.1136/thx.54.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange P, Groth S, Nyboe J, Appleyard M, Mortensen J, Jensen G, et al. Chronic obstructive lung disease in Copenhagen: Cross-sectional epidemiological aspects. J Intern Med. 1989;226:25–32. doi: 10.1111/j.1365-2796.1989.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 14.Isoaho R, Puolijoki H, Huhti E, Kivelä SL, Laippala P, Tala E. Prevalence of chronic obstructive pulmonary disease in elderly Finns. Respir Med. 1994;88:571–80. doi: 10.1016/s0954-6111(05)80004-6. [DOI] [PubMed] [Google Scholar]
- 15.Izumi T. Chronic obstructive pulmonary disease in Japan. Curr Opin Pulm Med. 2002;8:102–5. doi: 10.1097/00063198-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, Kuriyama T, et al. COPD in Japan: The Nippon COPD Epidemiology study. Respirology. 2004;9:458–65. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim DS, Kim YS, Jung KS, Chang JH, Lim CM, Lee JH, et al. Korean Academy of Tuberculosis and Respiratory Diseases. Prevalence of chronic obstructive pulmonary disease in Korea: A population-based spirometry survey. Am J Respir Crit Care Med. 2005;172:842–7. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
- 18.Gourgoulianis KI, Katikos P, Moraitis M, Argiriou N, Molyvdas PA. Chronic bronchitis in rural and industrial areas. Ann Agric Environ Med. 2000;7:29–31. [PubMed] [Google Scholar]
- 19.Vrachnis N, Iliodromitou Z, Hamos B. The prevalence of chronic obstructive pulmonary disease in Greeks over 55 years. Arch Hellenic Med. 1998;15:565–70. [Google Scholar]
- 20.Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22:268–73. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 21.Peña VS, Miravitlles M, Gabriel R, Jiménez-Ruiz CA, Villasante C, Masa JF, et al. Geographic variations in prevalence and underdiagnosis of COPD: Results of the IBERPOC multicentre epidemiological study. Chest. 2000;118:981–9. doi: 10.1378/chest.118.4.981. [DOI] [PubMed] [Google Scholar]
- 22.Menezes AM, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, Valdivia G, et al. Chronic obstructive pulmonary disease in five Latin American cities (the Platino study): A prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 23.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Mørkve O. Risk of overdiagnosis of COPD in asymptomatic elderly never-smoker. Eur Respir J. 2002;20:1117–22. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]


