Abstract
BACKGROUND AND OBJECTIVES:
Primary hypothyroidism may be associated with ovarian enlargement and/ or cyst formation. We evaluated the effect of thyroid hormone replacement therapy on hormonal changes, ovarian volume and sonographic appearance.
DESIGN AND SETTING:
Open, prospective study of women admitted to university gynecology clinic.
PATIENTS AND METHODS:
The study included 26 patients with untreated hypothyroidism who had polycystic (n=10) or normal-appearing (n=16) ovaries and 20 euthyroidic controls. Basal serum total testosterone, free testosterone, androstenedione, dehydroepiandosterone-sulfate, prolactin, estradiol, luteinizing hormone, follicle-stimulating hormone, free T3, free T4 and thyroid-stimulating horone, together with ovarian volumes, were determined and repeated after euthyroidism was achieved.
RESULTS:
Ovarian volumes of patients with hypothyroidism were significantly greater compared with controls, and their magnitudes diminished significantly during thyroid hormone replacement therapy. Hypothyroidic patients with polycystic ovaries had significantly higher serum free testosterone and dehydroepiandosterone-sulfate, but lower androstenodione levels compared with those who had normal-appearing ovaries. Serum total testosterone concentrations were significantly higher in hypothyroidic patients without polycystic ovaries, and thyroid hormone replacement therapy achieved a significant reduction in total as well as free testosterone.
CONCLUSION:
Severe longstanding hypothyroidism leads to increased ovarian volume and/or cyst formation. A decrease in ovarian volume, resolution of ovarian cysts and reversal of the polycystic ovary syndrome-like appearance, together with improvement in serum hormone levels, occurred after euthyroidism was achieved.
Thyroid hormones have various effects on the reproductive system of the human female. Alteration in thyroid function, particularly hypothyroidism, can cause ovulatory dysfunction, the latter being the leading cause of impaired female fertility.1–3 Although the underlying causes of hypothyroidism and polycystic ovary syndrome (PCOS) are completely different, these two entities have many features in common, including oligo- or anovulation; decreased serum sex hormone–binding globulin; increased serum free testosterone, luteinizing hormone (LH) and cholesterol concentrations.4–7 Moreover, since ultrasonography became available, an increase in ovarian volume and the appearance of bilateral multicystic ovaries, sometimes mimicking polycystic ovaries, have been reported in various cases with primary hypothyroidism.8–15
Consistent regression of the ovarian cysts after thyroid hormone replacement therapy supports a causal relationship between hypothyroidism and ovarian stimulation. In addition, the presence of ovarian cyst has been considered a diagnostic marker for hypothyroidism.16–18 Enlargement and cystic changes in ovaries of patients with hypothyroidism has been observed in numerous case reports after Silver et al19 raised this concern the first time.8–15 However, we are unaware of prospective case-controlled studies in the literature, showing whether there is any association between ovarian cysts and hypothyroidism, or whether treating these patients with thyroid hormones could decrease ovarian volume, reverse morphological changes and affect serum hormone levels. The aim of this study was to compare basal and post-treatment ovarian volumes of patients with primary hypothyroidism (with or without polycystic ovaries) and to determine whether there is any change in serum levels of ovarian and/ or adrenal hormones after thyroid hormone replacement therapy.
METHODS
Twenty-eight women with untreated primary hypothyroidism admitted to the Department of Gynecology, the Department of Endocrinology, or the Metabolism Polyclinics at Erciyes University between June 2002 and July 2004 were enrolled in this prospective study. As people living in rural areas around the capital of Kayseri were devoid of medical service, some patients presented with a full-blown clinical picture of hypothyroidism. Pregnancy occurred in two patients during the study, and these patients were excluded from the final analyses. The Ethics Committee of the Erciyes University School of Medicine approved the study, and informed consent was obtained from all patients.
All patients were in the reproductive age group, had no history of previous ovarian surgery and had not received any medication that could affect adrenal hormone metabolism. Primary hypothyroidism was diagnosed on the basis of low serum free thyroxine (FT4) (<9.0 pg/mL) and elevated thyroid-stimulating hormone (TSH) (>5 μIU/mL) levels together with the presence of signs and symptoms of hypothyroidism. In all cases, hypothyroidism was diagnosed for the first time. No patient had received any thyroid hormone replacement therapy prior to presentation. Exclusion criteria were the presence of secondary hypothyroidism, congenital adrenal hyperplasia, Cushing syndrome, androgen-secreting ovarian or adrenal tumor and polycystic ovary syndrome (PCOS). Patients who were excluded because of PCOS were evaluated according to the Rotterdam criteria.20 Congenital adrenal hyperplasia was excluded with an intravenous adrenocorticotropic hormone (ACTH) stimulation test. To identify those patients who might be heterozygous for 21-hydroxylase defect, serum 11-desoxycorticosterone levels below 13 ng/mL and serum 17-hydroxyprogesterone levels below 10 ng/mL were considered normal.21,22 Patients who had first- or second-degree relatives with established PCOS were also excluded. Twenty healthy women with regular menstrual cycles without any hormonal disturbances served as controls. Ovaries with 12 or more peripherally arranged antral follicles (2-9 mm in diameter) and/ or increased ovarian volume (>10 mL) were defined as polycystic.20 Then, patients were divided into 3 groups: (1) controls (with normal menstrual cycle, normal hormonal profile and without any endocrinologic diseases and ovarian morphologic disturbances; (2) hypothyroid patients with polycystic ovaries; and (3) hypothyroid patients without polycystic ovaries. The age, body weight and height of each patient were recorded, and a complete physical examination was performed. Ovarian volume was measured as described by Campbell et al.23 Sonographic examinations using 3.5 MHz abdominal or 6.5 MHz vaginal transducers (Hitachi, EMB 450, Japan) were carried out by the same operator (N.E.) to avoid inter-observer variability. Measurements were recorded before and after thyroid hormone therapy. The underlying cause of hypothyroidism was autoimmune thyroiditis (n=23), postpartum hypothyroidism (n=2) or treatment with radioactive iodine (n=1). Most of the patients had autoimmune thyroiditis and were, for the first time in their lives, found to be hypothyroid. Therefore, the mean duration of hypothyroidism in the study population could not be determined in retrospect.
Blood was taken at baseline and after euthyroidism was achieved for measuring serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), 17-hydroxyprogesterone (17OHP), dehydroepiandosterone-sulfate (DHEAS), total testosterone (T), free T, cortisol, 11-desoxycorticosterone (11DOC), androstenedione (A), free thyroxine (FT4), free triiodothyronine (FT3), thyroid-stimulating hormone (TSH) and thyroid peroxidase antibody (anti-TPO). Samples were obtained in the fasting state and at the early follicular phase (in women with regular menstrual cycles) or on a convenient day for those who were amenorrheic. Patients with hypothyroidism were treated with appropriate doses of L-thyroxine (Tefor, Organon, Holland) until a euthyroid state was attained, at which time serum hormone levels were re-evaluated. The size and appearance of the ovaries were checked at least 3 months after euthyroidism was achieved. Depending on the age of the patient, the severity of hypothyroidism and compliance, the mean time to achieve euthyroidism was 7.8 (4) months. The median duration of follow-up was 12 months (range, 9-26).
FSH, LH, total T, free T, A, estradiol (E2) (DSL-4900, Webster, Texas) and DHEAS (Immunotech, Marseilles, France) were measured by radioimmunoassay. The intra-assay and inter-assay coefficients of variation were, respectively, 3.2% and 8.4% for FSH, 6.8% and 7.9% for LH, 8.1% and 9.1% for total T, 3.7% and 7.9% for free T, 4.3% and 6.0% for A, 5.2% and 5.5% for E2, and 5.6% and 4.1% for DHEAS.
Mean values and standard deviation for continuous variables were calculated using standard methods. All variables were tested for normality using the Kolmogorov-Smirnov test, and appropriate logarithmic transformations were applied to the variables that were not normally distributed. Baseline data between the three groups (controls, hypothyroid patients with polycystic ovaries and hypothyroid patients without polycystic ovaries) were compared using one-way analysis of variance and post hoc Tukey test. Pretreatment comparisons between controls and all hypothyroid patients (those with and without polycystic ovaries) were performed by the independent samples t test. Differences within the groups after treatment were compared with the independent samples t test. The pretreatment and post-treatment data of patients with hypothyroidism were compared using the paired t test. All analyses were performed using SPSS, 13.0 version (Chicago, Illinois). Statistical significance was set at P<.05.
RESULTS
Of the 26 women with hypothyroidism who fulfilled the inclusion criteria, 10 had sonographical evidence of polycystic ovaries and 16 did not. Twenty healthy euthyroidic women served as controls. Table 1 summarizes the serum hormone levels and ovarian volumes of the study population and controls before and after hormone replacement therapy. As patients with severe (TSH ≥ 100 IU/mL; n=4) and mild (TSH <100 IU/mL; n=22) hypothyroidism were included, baseline serum TSH levels showed great variation (range, 7.48-163 μIU/mL).
Table 1.
Baseline serum hormone levels and ovarian volume of the study population and controls before and after replacement therapy with T4.
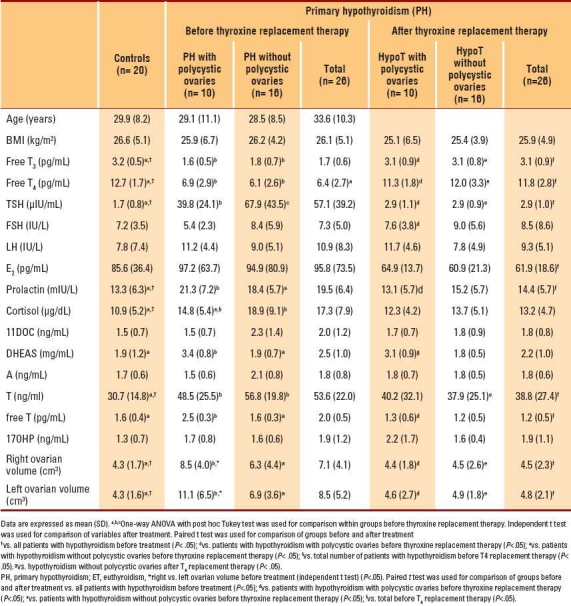
The mean age and mean body mass index (BMI) of hypothyroid patients with PCOS, hypothyroid patients without PCOS and controls were comparable, as were basal serum FSH, LH, E2, 11DOC, DHEAS, A, free T and 17OHP concentrations (P>.05). Hypothyroid patients with PCOS had significantly higher pretreatment serum prolactin, DHEAS and free T levels but lower TSH levels than hypothyroid patients without PCOS. When compared with controls, pretreatment serum free T3, free T4, TSH, prolactin, cortisol and total T concentrations were significantly different in patients with hypothyroidism (P<.05).
A significant improvement in serum hormone levels occurred after thyroid hormone replacement therapy. Serum FT3 and FT4 levels increased whereas serum TSH, prolactin, E2, free T and total T levels decreased. The serum DHEAS levels of patients with polycystic ovaries remained high, and there was no overall change in serum cortisol, 17OHP and 11DOC levels after achievement of euthyroidism. Before the initiation of therapy, women with hypothyroidism and polycystic ovaries had complaints of excessive hair growth (n=2) and/or oligomenorrhea (n=6), whereas the corresponding numbers of women with hypothyroidism and normal-appearing ovaries who had these complaints were 5 and 14, respectively. Improvement in the menstrual cycle occurred in 18 women (5 cases with polycystic ovaries and 13 cases with normal-appearing ovaries) after achieving euthyroidism.
Patients with hypothyroidism and polycystic ovaries had significantly higher basal DHEAS and free T but lower A levels when compared with those with normal-appearing ovaries (P<.05). Serum total T concentrations were significantly higher in hypothyroid patients without polycystic ovaries, and thyroid hormone replacement therapy achieved a significant reduction in total and free T levels. The ovarian volumes of patients with hypothyroidism (with or without polycystic ovaries) were significantly greater when compared with controls (P<.05, Figure 1). Basal ovarian volumes within the study population were similar, although the slightly larger size of the left ovary in those with polycystic ovaries was statistically significant (P<.05). There was no association between serum pretreatment TSH levels and ovarian volumes (P>.05; the Pearson correlation coefficient for the left and right ovary was 0.256 and 0.07, respectively). Ovarian volumes of hypothyroid patients with or without polycystic ovaries decreased significantly during hormone replacement therapy and became comparable with those of controls (P<.05, Figure 1). Of the 22 patients with mild hypothyroidism, 10 had polycystic ovaries before thyroxine therapy. In all these patients, the polycystic appearance of the ovaries regressed after euthyroidism was achieved for at least 3 months.
Figure 1.
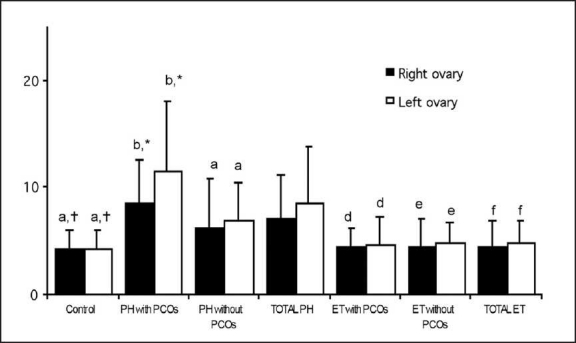
Ovarian volume (mean [SD]) in patients with primary hypothyroidism before and after treatment with L-thyroxine compared with that in controls.
DISCUSSION
Primary hypothyroidism is the most common pathological hormone deficiency, the prevalence of overt and subclinical disease being 0.3% and 4.3%, respectively.24 Deficiency of thyroid hormones has many profound end-organ effects, which also include those in the reproductive system of the human female. Longstanding hypothyroidism can interfere with gonadotropin secretion by increasing serum prolactin levels.4 Clinical manifestations, including menstrual irregularities and impaired fertility, are the results of anovulation and/or luteal phase defect.1
This study showed that basal ovarian size of patients with hypothyroidism (with or without polycystic ovaries) was significantly larger when compared with controls. In other words, hypothyroidism had profound effects on ovarian size, other than simply producing ovarian cysts. A plausible explanation for this finding can be had from animal studies, which have shown that hypothyroidism causes collagen deposition within the ovarian intercellular matrix.25 In humans, hypothyroidism is characterized by deposition of mucopolysaccharides (hyaluronic acid and chondroitin sulfate) within the connective tissue ground substance of various organs. Moreover, similar myxedemetous changes of the ovarian stroma together with an increase in collagen content and sclerosis have been reported in a 16-year-old patient with hypothyroidism and bilaterally enlarged cystic ovaries.26 Alteration of ovarian stroma may alter paracrine and autocrine mechanisms of communication within the ovary, resulting in disrupted follicular development and altered steroidogenesis. Cyst formation may also cause ovarian enlargement. Animal studies have shown that perinatal exposure to TSH in vivo and in vitro causes a "hormonal imprinting effect" which leads to amplification of the response to subsequent FSH receptor binding.27 Elevated TSH in combination with normal serum FSH levels may get activated with FSH receptors, causing profound stimulation of ovarian follicles. Although it is not clear whether a similar action is operative in humans, the occurrence of spontaneous ovarian hyperstimulation syndrome in pregnant15,28–30 and nonpregnant patients12,29,30 with hypothyroidism supports this theory.
Ghosh et al31 summarized the plausible underlying pathophysiologic mechanisms of ovarian cyst formation in patients with subclinical and overt hypothyroidism. They stated that the formation of ovarian cysts, similar to those occurring in polycystic ovary syndrome, may be restricted only to individuals with subclinical hypothyroidism, and that patients with severe and longstanding hypothyroidism should not show ovarian cyst formation. Our results support this theory, as approximately half of the patients with mild hypothyroidism showed polycystic ovaries. In contrast, other researchers have claimed that severe hypothyroidism is required for the cysts to form and that they disappear in several months, even after inadequate thyroid hormone replacement.10
In the present study, basal ovarian volumes of hypothyroid patients with polycystic ovaries were slightly different from those of hypothyroid patients without polycystic ovaries, the left being larger than the right — a finding for which no reasonable explanation could be determined. As the number of cases was limited, this may also represent a type I error. A significant decrease in ovarian volume after initiating thyroid hormone replacement therapy and achieving euthyroidism was found. The decrease in ovarian volume was comparable in hypothyroid patients with and without polycystic ovaries. The ovarian size of euthyroid patients did not differ from that of the controls at the end of therapy. Although numerous case reports have emphasized a beneficial effect,8–15,19 this study shows prospectively for the first time in the literature that thyroid hormone replacement therapy reverses ovarian size in patients with hypothyroidism.
Ghosh et al31 reported that hormonal changes associated with overt hypothyroidism include a decrease in sex hormone–binding globulin (SHBG), together with a concomitant increase in TSH, prolactin, estradiol, total and free T. They found a decreased LH-FSH ratio in severe hypothyroidism but an increased ratio in subclinical hypothyroidism. Furthermore, the increase in serum androgen levels (T, A and DHEAS) was more prominent in patients with subclinical hypothyroidism who also had polycystic ovaries on ultrasound, compared with those who had severe hypothyroidism without polycystic ovaries.31 We found similar hormonal changes in the present study population before starting hormone replacement therapy when compared with controls. Although the overall basal serum androgen level of patients with hypothyroidism tended to be higher, only the difference in total T was significant. Hypothyroid patients with polycystic ovaries had significantly higher serum DHEAS and free T but lower A levels. Achieving euthyroidism decreased overall serum prolactin, E2, total and free T levels (but not A and DHEAS values) significantly.
Resumption of regular menses occurred in 50% of PCOS and 81% of non-PCOS patients after euthyroidism had been achieved. Additionally, the polycystic appearance of the ovaries disappeared in all patients after thyroxine treatment. These findings further support the connotation that the PCOS-like appearance of the ovaries can be caused by primary hypothyroidism.
One drawback of this study is that serum SHBG levels were not evaluated. Previous studies have found that irrespective of the severity of thyroid dysfunction, hypothyroidism is associated with low serum SHBG levels.5,31 As SHBG is one of the major proteins to which T is bound in plasma (the other is albumin), a decrease in SHBG is associated with increase in free T concentrations. However, in the present study, only patients with hypothyroidism and polycystic ovaries exhibited high pretreatment serum free T concentrations, whereas those without polycystic ovaries had free T levels within the normal range. Thyroid hormone replacement restored plasma free T levels in the former group, confirming that hypothyroidism has profound effects on SHBG levels. As hypothyroidism can indirectly affect serum free T levels, these patients may show signs of hirsutism. However, hirsutism was not a prominent finding in our patients, so evaluation of hirsutism scores was not focused on.
It has been claimed that changes in SHBG levels play a central role in the underlying pathophysiological mechanisms of hormonal as well as ovarian morphological changes in hypothyroidism. Low SHBG and concomitant high free T levels trigger a vicious circle which results in ovarian enlargement and/or cyst formation.31 Thyroid hormone replacement restores serum SHBG levels and, with decreasing serum TSH, diminishes the FSH-like stimulation of ovarian follicles, thereby reversing ovarian size and initiating ovulation. The time required for this restoration to occur is not known, but it is believed to take several months. As we have not followed up the patients in this study after euthyroidism was achieved, we are not able to make a statement on the time required for restoration.
The relationship between thyroid hormones and ovarian size was shown in a rat model study.32 Thyroid function might affect the size of the gland and also the number of LH-/hCG-binding sites in rat ovaries. The ovaries of the hyperthyroid group were diminished in size, and the number of receptors per ovary also was reduced when compared with the control group. In contrast, the ovaries of the hypothyroid group were higher in size, and the number of receptors per ovary was increased. In our study, the ovarian volume was increased in patients with hypothyroidism. In another study, simultaneous insulin sensitivity could be correlated to hypothyroidism and PCOS.33 Additionally, hypothyroidism may cause insulin resistance, which may lead to PCOS. In our study, hypothyroidism might have affected the ovaries and this situation might have been altered with the treatment of hypothyroidism. Our study supports the finding of various studies found in literature — that there is a close connection between ovarian and thyroid functions.
We conclude that severe longstanding hypothyroidism leads to an increase in ovarian volume, which can also be accompanied by cyst formation. Although several mechanisms have been implicated, the exact cause is still unknown. We do not know whether the hormonal change that is triggered by hypothyroidism leads to ovarian enlargement, or whether myxedemal infiltration is primarily implicated. Changes in plasma hormone levels, as well as the sonographical appearance of the ovaries, may have many similarities with those of classical PCOS. Therefore, patients with suspected PCOS (both based on clinical and hormonal changes) should be reevaluated after a possible underlying hypothyroidism has been appropriately treated. A decrease in ovarian volume and resolution of ovarian cysts should be expected after euthyroidism has been achieved with thyroid hormone replacement therapy. However, the clinical pictures of overt hypothyroidism and PCOS are easy to confuse, and therefore the present results should only be applied to patients in whom overt hypothyroidism and PCOS might coexist. Consequently, further studies might be needed to confirm the relationship between ovary and thyroid functions; there might be an interaction between them.
REFERENCES
- 1.Krassas GE. Thyroid disease and female reproduction. Fertil Steril. 2000;74:1063–70. doi: 10.1016/s0015-0282(00)01589-2. [DOI] [PubMed] [Google Scholar]
- 2.Poppe K, Velkeniers B. Female infertility and the thyroid. Best Pract Res Clin Endocrinol Metab. 2004;18:153–65. doi: 10.1016/j.beem.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Raber W, Nowotny P, Vytiska-Binstorfer E, Vierhapper H. Thyroxine treatment modified in infertile women according to thyroxine-releasing hormone testing: 5 year follow-up of 283 women referred after exclusion of absolute causes of infertility. Hum Reprod. 2003;18:707–14. doi: 10.1093/humrep/deg142. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell RE, Chang RJ. Report of the National Symposium on the Clinical Management of Prolactin-Related Reproductive Disorders. Fertil Steril. 1986;45:607–10. doi: 10.1016/s0015-0282(16)49329-5. [DOI] [PubMed] [Google Scholar]
- 5.Yen SS. The polycystic ovary syndrome. Clin Endocrinol. 1980;12:177–207. doi: 10.1111/j.1365-2265.1980.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathur RS, Moody LO, Landgrebe SC, Peress MR, Rust PF, Williamson HO. Sex-hormone binding globulin in clinically hyperandrogenic women: Association of plasma concentrations with body weight. Fertil Steril. 1982;38:207–11. doi: 10.1016/s0015-0282(16)46461-7. [DOI] [PubMed] [Google Scholar]
- 7.Mahesh VB, Mills TM, Bagnell CA, Conway BA. Animal models for study of polycystic ovaries and ovarian atresia. Adv Exp Med Biol. 1987;219:237–57. doi: 10.1007/978-1-4684-5395-9_12. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita Y, Kawamura T, Fujikawa R, Mochizuki H, Okubo M, Arita K. Regression of both pituitary and ovarian cysts after administration of thyroid hormone in a case of primary hypothyroidism. Intern Med. 2001;40:751–5. doi: 10.2169/internalmedicine.40.751. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Tiu CM, Chou YH, Chen WY, Hwang B, Niu DM. Congenital hypothyroidism with multiple ovarian cysts. Eur J Pediatr. 1999;158:851–2. doi: 10.1007/s004310051221. [DOI] [PubMed] [Google Scholar]
- 10.Van Voorhis BJ, Neff TW, Syrop CH, Chapler FK. Primary hypothyroidism associated with multicystic ovaries and ovarian torsion in an adult. Obstet Gynecol. 1994;83:885–7. [PubMed] [Google Scholar]
- 11.Fernandez-Real JM, Ricart-Engel W, Maroto-Genover A, Macia F. Primary hypothyroidism and concomitant bilateral ovarian masses. J Pediatr Endocrinol Metab. 1995;8:263–6. doi: 10.1515/jpem.1995.8.4.263. [DOI] [PubMed] [Google Scholar]
- 12.Chen CP, Chen CW, Wang KG. Spontaneous ovarian hyperstimulation syndrome and hyperprolactinemia in primary hypothyroidism. Acta Obstet Gynecol Scand. 1996;75:70–1. doi: 10.3109/00016349609033288. [DOI] [PubMed] [Google Scholar]
- 13.Nishi Y, Masuda H, Iwamori H, Urabe T, Sakoda K, Uozumi T. Primary hypothyroidism associated with pituitary enlargement, slipped capital femoral epiphysis and cystic ovaries. Eur J Pediatr. 1985;143:216–9. doi: 10.1007/BF00442143. [DOI] [PubMed] [Google Scholar]
- 14.Gregory JL, Wilson DM, Parker B, Wood BP. Radiological case of the month.The overlap syndrome: An unusual presentation. Am J Dis Child. 1992;146:421–2. [PubMed] [Google Scholar]
- 15.Cardoso CG, Graca LM, Dias T, Clode N, Soares L. Spontaneous ovarian hyperstimulation and primary hypothyroidism with a naturally conceived pregnancy. Obstet Gynecol. 1999;93:809–11. doi: 10.1016/s0029-7844(98)00435-9. [DOI] [PubMed] [Google Scholar]
- 16.Riddlesberger MM, Jr, Kuhn JP, Munschauer RW. The association of juvenile hypothyroidism and cystic ovaries. Radiology. 1981;139:77–80. doi: 10.1148/radiology.139.1.7208945. [DOI] [PubMed] [Google Scholar]
- 17.Jafri SZ, Bree RL, Silver TM, Ouimette M. Fetal ovarian cysts: Sonographic detection and association with hypothyroidism. Radiology. 1984;150:809–12. doi: 10.1148/radiology.150.3.6695083. [DOI] [PubMed] [Google Scholar]
- 18.Lyon AJ, De Bruyn R, Grant DB. Transient sexual precocity and ovarian cysts. Arch Dis Child. 1985;60:819–22. doi: 10.1136/adc.60.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver HK. Juvenile hypothyroidism with precocious sexual development. J Clin Endocrinol Metab. 1958;18:148–54. doi: 10.1210/jcem-18-8-886. [DOI] [PubMed] [Google Scholar]
- 20.Rotterdam ESHRE / ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Azziz R, Bradley EL, Jr, Potter HD, Boots LR. Adrenal androgen excess in women: Lack of a role for 17-hydroxylase and 17,20-lyase dysregulation. J Clin Endocrinol Metab. 1995;80:400–5. doi: 10.1210/jcem.80.2.7852496. [DOI] [PubMed] [Google Scholar]
- 22.Azziz R, Dewailly D, Owerbach D. Clinical review 56: Nonclassic adrenal hyperplasia: Current concepts. J Clin Endocrinol Metab. 1994;78:810–5. doi: 10.1210/jcem.78.4.8157702. [DOI] [PubMed] [Google Scholar]
- 23.Campbell S, Goessens L, Goswamy R, Whitehead M. Real-time ultrasonography for determination of ovarian morphology and volume.A possible early screening test for ovarian cancer? Lancet. 1982;1:425–6. doi: 10.1016/s0140-6736(82)91622-1. [DOI] [PubMed] [Google Scholar]
- 24.Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 25.Adams WC, Leathem JH. Influence of hypothyroidism and chronic gonadotrophin on ovarian collagen in the rat. Endocrinology. 1964;75:138–9. doi: 10.1210/endo-75-1-138. [DOI] [PubMed] [Google Scholar]
- 26.Hansen KA, Tho SP, Hanly M, Moretuzzo RW, McDonough PG. Massive ovarian enlargement in primary hypothyroidism. Fertil Steril. 1997;67:169–71. doi: 10.1016/s0015-0282(97)81876-6. [DOI] [PubMed] [Google Scholar]
- 27.Dobozy O, Csaba G, Hetenyi G, Shahin M. Investigation of gonadotropin-thyrotropin overlapping and hormonal imprinting in the rat testis. Acta Physiol Hung. 1985;66:169–75. [PubMed] [Google Scholar]
- 28.Chae HD, Park EJ, Kim SH, Kim CH, Kang BM, Chang YS. Ovarian hyperstimulation syndrome complicating a spontaneous singleton pregnancy: A case report. J Assist Reprod Genet. 2001;18:120–3. doi: 10.1023/A:1026543027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nappi RG, Di Naro E, D’Aries AP, Nappi L. Natural pregnancy in hypothyroid woman complicated by spontaneous ovarian hyperstimulation syndrome. Am J Obstet Gynecol. 1998;178:610–1. doi: 10.1016/s0002-9378(98)70448-x. [DOI] [PubMed] [Google Scholar]
- 30.Zalel Y, Katz Z, Caspi B, Ben-Hur H, Dgani R, Insler V. Spontaneous ovarian hyperstimulation syndrome concomitant with spontaneous pregnancy in a woman with polycystic ovary disease. Am J Obstet Gynecol. 1992;167:122–4. doi: 10.1016/s0002-9378(11)91642-1. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, Kabir SN, Pakrashi A, Chatterjee S, Chakravarty B. Subclinical hypothyroidism: A determinant of polycystic ovary syndrome. Horm Res. 1993;39:61–6. doi: 10.1159/000182697. [DOI] [PubMed] [Google Scholar]
- 32.Fitko R, Szlezyngier B. Role of thyroid hormone in controlling the concentration of luteinizing hormone/human chorionic gonadotropin receptors in rat ovaries. Eur J Endocrinol. 1994;130:378–80. doi: 10.1530/eje.0.1300378. [DOI] [PubMed] [Google Scholar]
- 33.Pallotti S, Gasbarrone A, Franzese IT. Relationship between insulin secretion, and thyroid and ovary function in patients suffering from polycystic ovary. Minerva Endocrinol. 2005;30:193–7. [PubMed] [Google Scholar]


