Abstract
How ciliary and flagellar motility is regulated is a challenging problem. The flagellar movement in Chlamydomonas reinhardtii is in part regulated by phosphorylation of a 138 kD intermediate chain (IC138) of inner arm dynein f (also called I1). In the present study, we found that the axoneme of mutants lacking dynein f lacks a novel protein having ankyrin repeat motifs, registered as FAP120 in the flagellar proteome database. FAP120 is also missing or decreased in the axonemes of bop5, a mutant that has a mutation in the structural gene of IC138 but assembles the dynein f complex. Intriguingly, the amounts of FAP120 in the axonemes of different alleles of bop5 and several dynein f-lacking mutants roughly parallel their contents of IC138. These results suggest a weak but stoichiometric interaction between FAP120 and IC138. We propose that FAP120 functions in the regulatory process as part of a protein complex involving IC138.
Keywords: cilia, IC138, IC97, dynein f(I1), FAP120
INTRODUCTION
Cilia and flagella of eukaryotes beat through coordinated force generation by multiple species of dyneins assembled in outer and inner dynein arms. Studies with Chlamydomonas flagella have shown that outer arm dynein and inner arm dynein have strikingly different properties, and the inner arm dynein is particularly important for producing proper flagellar waveforms (Brokaw and Kamiya, 1987). Chlamydomonas inner arm dyneins comprise at least seven different species designated species “a” to “g”, each of which has one or two distinct heavy chains and several intermediate and light chains (Kagami and Kamiya, 1992; Kamiya, 2002;). Of these species, species f (dynein f; also called dynein I1) is unique in that only this dynein contains two heavy chains and has a subunit composition entirely different from that of other inner-arm dyneins (reviewed in Wirschell et al., 2007; King and Kamiya, 2008). This dynein is considered to be important for the regulation of flagellar beating, because mutants that lack it are deficient in phototaxis (King and Dutcher, 1997; Okita et al., 2005). Several lines of evidence have indicated that phosphorylation of a subunit, a 138 kD intermediate chain, regulates the force generation of dynein f and cellular behavior, although how the phosphorylation is controlled is not established (Habermacher and Sale, 1997; King and Dutcher, 1997; Hendrickson et al., 2004).
Because of the functional importance of dynein f, its molecular composition has been extensively studied. It is composed of two heavy chains encoded by DHC1 (Myster et al., 1999) and DHC10 (Perrone et al., 2000), three intermediate chains of 140 kD (IC140), 138 kD (IC138), and 97 kD (IC97, also referred to as IC110; Porter et al., 1992), and several light chains including LC7a, LC7b, LC8, Tctex1, and Tctex2b (Yang and Sale, 1998; DiBella et al., 2004; Hendrickson et al., 2004; Pazour et al., 1998; Harrison et al., 1998; Dibella et al., 2004; Maureen Wirschell1, Chun Yang, Pinfen Yang, Laura Fox, Haru-aki Yanigasawa, Ritsu Kamiya, George B. Witman, Mary Porter, and Winfield S. Sale, in preparation). These subunits remain associated after dynein f is extracted from the axoneme with high-salt solutions and purified by density gradient centrifugation, ion-exchange chromatography or gel-filtration. Interestingly, despite the well-defined subunit structure, some of the dynein f subunits are dispensable for assembly; recent studies have shown that partial dynein f is formed in mutants lacking LC7b, IC97 and IC138 (Wirshell et al., in preparation; Rachel Bower, Kristyn VanderWall Mills, Eileen O'Toole, Cathy A. Perrone, Laura A. Fox, Maureen Wirschell, Winfield S. Sale and Mary E. Porter.., in preparation). In addition, we may expect that there may well be another class of proteins that weakly associate with this dynein, because it undergoes phosphorylation-dependent regulation, a process that must involve multiple proteins, and also because it must be transported and targeted to correct sites on the outer doublet through interaction with other proteins. In fact, several mutations are known that affect the phosphorylation level of IC138 (King and Dutcher, 1997; Yang and Sale, 2000).
In the present study, we found that a previously uncharacterized protein is decreased in the axonemes of four genetically independent mutants lacking dynein f. Our analyses indicate that it is a protein registered in the flagellar proteome database as FAP120, a protein with an ankyrin repeat motif. This protein is also missing or decreased in the axonemes of several alleles of bop5, a mutant deficient in the structural gene of IC138 (Hendrickson et al., 2004). These and other results prompted us to conclude that it is a novel subunit of inner arm dynein f that weakly associates with an IC138 complex that includes IC97 and LC7b. It may well regulate the dynein f function through interaction with the IC138 complex.
MATERIALS AND METHODS
Strains and Culture
The strains used in this study were Chlamydomonas reinhardtii wild-type 137c and the mutants listed in Table 1. The following mutants were produced in the Kamiya laboratory by UV-mutagenesis and used for the first time in this study: ida1-7, ida2-9, ida3-2, ida7-2, ida7-3, bop5-3, bop5-4, bop5-5, bop5-6. The new alleles of bop5, bop5-3, bop5-4, bop5-5, and bop5-6 were first isolated by mutagenizing oda1 mutant (deficient in outer arm dynein) and collecting paralyzed-flagella mutants. These mutants were backcrossed with wild type to remove the oda1 mutation; the mutants without the oda1 background displayed straight swimming with a slightly slower velocity than wild type. They were crossed with the S1D2 strain for AFLP mapping (Kathir et al., 2003). Tetrad analyses indicated that all mutants mapped near the bop5 locus. Finally, immuno-blot analysis of their axonemes with anti-IC138 antibody detected clear defects in the quantity of IC138, indicating that they are bop5 alleles. bop5-2 is an insertional allele described elsewhere (Bower et al., paper in preparation). All cells were grown in Tris-acetate/phosphate medium (Gorman and Levine, 1965) with aeration on a 12 h/12 h light/dark cycle.
Table 1.
Mutant strains used in this study
| Mutant | Affected protein |
Defects | Reference |
|---|---|---|---|
| ida1-1 | DHC1 | Loss of dynein f/I1 |
Kamiya et al. (1991), Myster et al. (1999) |
| ida1-7 | |||
| ida2-1 | DHC10 | Loss of dynein f/I1 |
Kamiya et al. (1991), Perrone et al. (2000) |
| ida2-9 | |||
| ida3-1 | unknown | Loss of dynein f/I1 | Kamiya et al. (1991) |
| ida3-2 | |||
| ida7-1 | IC140 | Loss of dynein f/I1 | Perrone et al. (1998) |
| ida7-3 | |||
| ida7-4 | |||
| bop5-1 | IC138 | Partial or complete loss of IC138. Loss of IC97 and LC7b. |
Dutcher et al. (1988), Hendrickson et al. (2004), Wirschell et al., in preparation Bower, et al., in preparation |
| bop5-2 (6F5) | |||
| bop5-3 | |||
| bop5-4 | |||
| bop5-5 | |||
| bop5-6 | |||
| mia1-1 | unknown | IC138 hyperphosphorylation |
King and Dutcher (1997) |
| mia2-1 | unknown | IC138 hyperphosphorylation |
King and Dutcher (1997) |
| oda1 | DC1 | Loss of outer arm dynein |
Kamiya (1988), Takada et al. (2002) |
| ida4 | p28 | Loss of dynein a, c, d |
Kamiya et al. (1991), LeDizet and Piperno (1995) |
| ida5 | actin | Loss of dynein a, c, d, e | Kato-Minoura et al. (1997) |
| sup-pf-5 | unknown | Loss of dynein regulatory complex |
Piperno et al. (1994) |
| pf18 | unknown | Loss of central pair | Harris (Sourcebook) |
| pf14 | RSP3 | Loss of radial spokes | Luck et al. (1977) |
| mbo1 | unknown | Backward swimming | Segal et al. (1984) |
Isolation of Axonemes and Preparation of Whole Cell Samples
Flagellar axonemes were isolated by the dibucaine method of Witman (1986). Flagella were suspended in HMDEK buffer (30 mM HEPES, 5 mM MgSO4, 1 mM dithiothreitol [DTT], 1 mM EGTA, 50 mM K-acetate, pH 7.4) and demembranated by extraction with 0.2% Nonidet P-40 in HMDEK buffer to yield axonemes. For SDS-PAGE of Chlamydomonas whole cells, cells were extracted with methanol and chloroform to remove the DNA and RNA fractions, centrifuged at 10,000 g for 5 min, and the resultant pellets were used as samples.
SDS-PAGE and Immunoblotting
One-dimensional SDS-PAGE of proteins was performed with 8–10% acrylamide gels by the method of Laemmli (1970). Two-dimensional electrophoresis was performed using Immobiline DryStrip gel (Pharmacia LKB Biotechnology, Uppsala, Sweden) covering a pH range of 3.0–10.5, as described previously (Nakamura et al., 1989; Kato-Minoura et al., 1997). Gels were stained with silver or used for immunoblot analysis. Immunoblot procedures were modified from those of Towbin et al. (1979). Proteins were transferred to PVDF membranes (Millipore Corp., Bedford, MA), and probed with primary antibodies. Immunoreactive bands were detected using alkaline phosphatase-conjugated second antibody and a BCIP-NBT solution kit (Nacalai Tesque, Kyoto, Japan).
Column Chromatography of Crude Dynein Extracts
Crude inner-arm dynein extracts were obtained by extraction of oda1 axonemes with 0.6 M KCl in HMDEK, followed by centrifugation. The crude dynein extract in the supernatant fraction was fractionated by anion-exchange chromatography using a 10-fold diluted sample on a Mono Q or a Uno Q column (Amersham Pharmacia Biotech, Uppsala, Sweden) as described previously (Kagami and Kamiya., 1992, Yamamoto et al., 2008). The sample was eluted with a linear KCl gradient in HMDEK. Gel-filtration column chromatography was performed using 3-fold diluted sample on a Superose 6 gel-filtration column (Amersham Pharmacia Biotech, Uppsala, Sweden). The sample was eluted with 0.2 M KCl in HMDEK at the flow rate of 0.075ml/minute.
Protein Identification
The protein band of FAP120 was separated by 2D-PAGE and was subsequently excised from the gels and digested with trypsin. The resultant peptide mixture was analyzed by mass spectrometry (Hitachi Science Systems, Tokyo). The obtained data were then used to search the C. reinhardtii genome database (JGI v. 2.0, http://genome.jgi-psf.org/chlre2/chlre2.home.html) to identify genomic sequences that encode each peptide.
Bacterial Expression of FAP120
The coding region of the cDNA of FAP120 was amplified by PCR with primers FAP120-1 (GCGGATCCATGGACGGGGAAGAGC) and FAP120-2 (CGGAATTCTCACTTCTTCTTCTTGGCTGCC), which contain the recognition sites for BamHI and EcoRI, respectively (underlined). The PCR product was ligated to the BamHI and EcoRI sites of the bacterial expression vector pCold I (Takara), resulting in a fusion protein that contained a His-tag sequence at its N-terminal. The expression and purification of the fusion protein was performed as described by the manufacturer. The expressed protein, after purification with a nickel affinity column, was subjected to factor Xa cleavage to remove the His-tag sequence.
Polyclonal Antibody Production
The recombinant FAP120 was dialyzed against PBS and used for production of polyclonal antibody. Two rabbits were immunized, and antisera were obtained using standard procedures. Antibody was blot-purified using recombinant FAP120 on PVDF membrane. The antibodies to IC140 and IC138 were described previously (Yang and Sale, 1998; Hendrickson et al., 2004), and the antibody to IC97 was produced to an N-terminal fusion protein (Wirschell et al., paper in preparation).
Immunofluorescence Microscopy
Immunofluorescence microscopy was performed according to Sanders and Salisbury (1995). Wild-type and ida1 cells were fixed with 3% formaldehyde for 10 min at room temperature, followed by treatment with cold methanol (−20°C) for 10 min. Specimens were stained with anti-FAP120 antibody in immunofluorescence blocking buffer and fluorescein isothiocyanate-labeled anti-rabbit IgG antibody (Sigma-Aldrich).
RESULTS
A Novel 42-kD Polypeptide is Absent or Reduced in Mutant Axonemes Lacking Inner-Arm Dynein f (I1)
We previously found that an antibody raised against the PACRG protein (Ikeda et al., 2007) also detected a novel ~42-kD band in addition to the ~30-kD PACRG band. Intriguingly, we observed that this 42-kD epitope was missing in ida1, a mutant that lacks inner arm dynein f (I1), while it was present in several other kinds of mutant axonemes lacking dyneins (oda1, ida5), radial spokes (pf14), central pair microtubules (pf18), or other axonemal structures (mbo1, sup-pf-5) (Fig. S1a). This epitope appeared to be similarly reduced in ida2, ida3 and ida7, mutants that also lack dynein f but are genetically distinct from ida1 (Fig. S1b)
Identification of the 42 kD Protein as FAP120
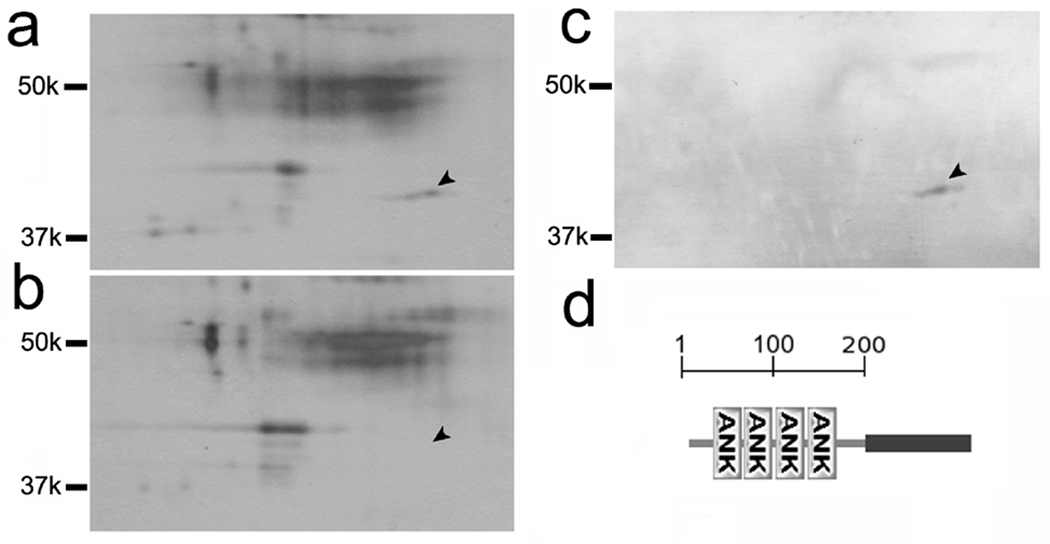
For identification of the 42-kD protein, we carried out mass spectrometry using protein samples separated by two-dimensional gel electrophoresis. Extraction of wild type axonemes with 0.1% Sarkosyl in TED buffer readily removed the 42-kD protein while leaving most of PACRG unextracted (not shown). Two-dimensional gel electrophoresis of the extract had a 42-kD protein spot, which was absent in the ida1 extract (Fig. 1a, b; arrowheads). This spot was recognized by the antibody (Fig. 1c). It was cut out, digested with trypsin, and analyzed by tandem mass spectrometry (MS/MS). Three partial amino acid sequences were determined. Search in the JGI database of C. reinhardtii genome (release 2.0) revealed that this 42-kDa protein is identical to a protein registered as FAP120 (Flagellar Associated Protein with Ankyrin Repeats) in the flagellar proteome database (Pazour et al., 2005)(JGI Linkout: Version 2: C_20103, Protein ID 162472). The gene of this protein is on linkage group II.
Fig. 1.
Identification of the 42 kD protein as FAP120. (a)–(c) Separation of the 42 kD protein by 2D electrophoresis. Silver-stained gel patterns of 0.1% Sarkosyl extracts from wild-type (a) and ida1 (b) axonemes. A region covering approximately 30–60 kD is shown. A spot at ~42 kD indicated by the arrowhead is missing in the ida1 pattern (b). Duplicate gel of wild-type gel pattern (a) was transferred to a PVDF membrane and analyzed by immunoblotting (c). The 42 kD spot reacted with the antibody. This band was cut out from the gel and analyzed by mass spectrometry. (d) The determined FAP120 cDNA sequence was analyzed using the programs SMART (http://smart.embl-heidelberg.de/) and Coils (http://www.ch.embnet.org/software/COILS_form.html). The analysis indicates that FAP120, a 294-amino acid protein with a molecular weight of 31861.12 and a pI of 4.94, contains four ankyrin repeat motifs in its N terminus half and a putative coiled-coil structure in its C terminus half.
The cDNA of FAP120 is predicted to encode a 294-amino acid protein with a molecular weight of 31861.12 and a pI of 4.94. This molecular weight is significantly lower than the apparent molecular mass (~42 kD). It may be due to its acidic pI. Sequence analysis using the programs SMART (http://smart.embl-heidelberg.de/) and Coils (http://www.ch.embnet.org/software/COILS_form.html) indicated that it contains four tandem ankyrin motif repeats in the N terminal half and a coiled-coil region in the C terminal half (Fig. 1d). The C-terminal coiled coil region has a potential to form a dimer, as predicted by Multicoil program (Wolf et al., 1997; http://groups.csail.mit.edu/cb/multicoil/cgi-bin/multicoil.cgi) (Fig. S2). The ankyrin repeat is composed of consecutive copies of approximately 33 residues, and is assumed to play an important role in protein-protein interactions (Li et al., 2006). BLAST search with FAP120 sequences identified many ankyrin repeat proteins in a wide range of organisms.
Amount of FAP120 in Wild-type and Mutants Lacking Dynein f
For further analysis of FAP120, we raised specific antibodies using bacterially expressed protein as the antigen. Affinity-purified antibody detected a major band corresponding to FAP120 and two weak bands slightly above and below the major band in wild type axonemes (Fig. 2a). A weak band is also present below the major band of recombinant FAP120, which is slightly larger than the native FAP120 due to the presence of a 6×His tag. We speculate that these bands represent covalently-modified or degraded forms of FAP120, although their true origins are currently unknown. FAP120 is present in the whole flagella and axoneme, but only in a negligibly small amount in the membrane/matrix fraction (Fig. 2b). The absolute amount of FAP120 in the axoneme was estimated by immunoblot analysis using known amounts of bacterially expressed recombinant FAP120 as standards (Fig. 2c). The percentage of FAP120 in the total axonemal proteins was estimated to be approximately 0.18%. Since the amount of tubulin in the total axoneme is ~60% (Yanagisawa and Kamiya, 2004), the weight ratio of FAP120 to tubulin should be ~1:330. This indicates that the molar ratio of FAP120 to tubulin dimer is about 1:110. Since the molar ratio of dynein f to tubulin in the axoneme is ~1:310, this leads to an estimate of dynein f: FAP120 of 1: ~2.8.
Fig. 2.
Immunoblot analysis of wild-type and mutant axonemes using the FAP120-specific antibody. (a) Western analysis of wild-type axonemes with an affinity purified FAP120 antibody. Only a single band of ~42 kDa is recognized. (b) Immunoblot analysis of isolated flagella (fla), the membrane and matrix fraction (M&M) and axonemes (axo) prepared from the same amount of flagella. FAP120 is barely detectable in the membrane and matrix fraction. (c) Estimation of the amount of FAP120 present in the wild-type axoneme by immunoblot analysis using a dilution series of recombinant FAP120 as a standard. Comparison of the immuno-blot signal intensities of FAP120 in 3 µg of the wild-type axoneme (axo) and the diluted series of recombinant FAP120 (diluted from 6 ng to 0 ng; correponding to 0.2% to 0% (weight/weight) of the total mass of the axoneme run on lane 1) yields an estimate of FAP120 to be ~0.18% of the total axonemal proteins. The number above each lane indicates the dilution factor expressed as the percentage of the total weight of the axoneme (axo.)(× 1/100). Because the recombinant FAP120 has a 6×His tag and an additional linker, its mobility on the western blot differs from that of the native FAP120 in the axoneme. (d) Quantification of FAP120 present in the mutant axonemes by immunoblot analysis. The amount of FAP120 in the axonemes of mutants lacking dynein f was estimated by densitometry using a series of diluted wild-type axonemes as a standard (lane 1 (wt), 100%; lane 2 (wt × 1/3), 33%; lane 3 (wt × 1/10), 10%; and lane 4 (wt × 1/30), 3.3%). FAP120 is present in mutant axonemes in small amounts ranging from < 1% to ~18% of the wild-type level.
The amount of FAP120 in different alleles of mutants lacking dynein f was estimated by comparing the band densities between the mutant axonemes and successively diluted wild type axonemes (Fig. 2d). Visual inspection indicates that FAP120 is present in dynein f-deficient axonemes in amounts reduced to < 1% to 18% of the wild-type level. The amount varies from one allele to another, but is independent of the mutated gene.
In contrast to the small content of FAP120 in the axonemes of ida1, almost wild-type levels of FAP120 were present in the cell bodies, although the contents were somewhat variable from one culture of the cells to another (Fig. S3).
Column Chromatography Analysis of FAP120
The above data suggest that FAP120 is present possibly in association with the dynein f complex. To determine whether FAP120 is in fact contained in the dynein f complex, we examined FAP120 distribution in inner arm dyneins separated by column chromatography. Extraction of the oda1 axoneme with 0.6 M KCl solubilized FAP120 as well as most of axonemal dyneins. The extract was diluted and separated by anion-exchange column chromatography into nearly discrete dynein species (Fig. 3a). SDS-PAGE and western blot with the FAP120 antibody (Fig. 3b) showed that FAP120 was eluted at lower salt concentration than was dynein f complex containing IC140, IC138, IC97 and several light chains (arrowhead in Fig. 3a). Thus, FAP120 does not appear to be firmly associated with dynein f. Gel-filtration column chromatography on a Superose 6 column also failed to detect association between FAP120 and dynein f complex (data not shown). Taken together, association between FAP120 and dynein f complex, if any, must be weak in axonemal extracts.
Fig. 3.
FAP120 does not co-elute with dynein f from ion-exchange column. (a) The elution pattern of the crude dynein extract from oda1 axonemes by anion-exchange chromatography on a Mono Q column (solid line, absorbance at 280 nm; broken line, KCl conc.). a–g, the peak fractions that contain the designated inner arm dynein complex as a major component (Kagami and Kamiya, 1992). The horizontal bar indicates the fractions for which immuno-blot analysis was carried out. (b) Immuno-blot detection of FAP120 in the indicated fractions. The peak of FAP120 distribution (marked by the arrowhead in a) does not correspond to any particular dynein peak.
Localization of FAP120
Indirect immunofluorescence microscopy using anti-FAP120 antibody showed that this protein was localized along the length of the wild type flagella (Fig. 4). In contrast, only very faint staining was detected in the ida1 axoneme. This faint staining may be derived from a trace amount of FAP120 present in the ida1 axoneme or due to non-specific binding of the FAP120 antibody to other proteins.
Fig. 4.
Immunofluorescence microscopy of wild-type and ida1 cells. Top, differential interference contrast images. Bottom, indirect immunofluorescence localization of FAP120. Fluorescent signal is observed along the length of the axonemes of wild-type, whereas it is only negligibly present in the ida1 axoneme.
The Amount of FAP120 in the Axoneme Correlates with That of IC138
We have isolated four alleles of bop5, a mutant of the structural gene of IC138 (Hendrickson et al., 2004) by screening mutants that give rise to a non-motile phenotype when combined with the background of oda1, a mutation that results in loss of outer dynein arms. Unlike the previously isolated bop5 allele, bop5-1, which expresses truncated IC138 (Hendrickson, 2004), the alleles we isolated lack IC138 almost completely or express full-length IC138 in greatly reduced amounts as assessed from immunoblot patterns (see Fig. 5). The difference in phenotype is most likely due to the difference in the mutated gene in these bop5 alleles. Despite the loss or extensive decrease of IC138, SDS-PAGE patterns did not show a significant reduction of the heavy chains (Fig. S4, Bower, et al., paper in preparation). An intermediate chain of dynein f, IC140, appears to be reduced in all bop5 alleles (Figs S4 and 6), but this reduction is not correlated with the amount of IC138 remaining in the axoneme (Fig. S4). We interpret this reduction to be due to an increased proteolytic susceptibility of IC140 in the absence of IC138. Immunoblot analysis using the FAP120 antibody showed that two alleles of bop5 (bop5-3, bop5-4) that completely lack IC138 have extremely low amounts of FAP120 and IC97. In contrast, another allele of bop5, bop5-6, and ida1(ida1-1), both of which retain small amounts of IC138, have comparably small amounts of FAP120 and IC97 in their axonemes (Fig. 5). Thus, the amounts of FAP120 and IC97 in the flagella roughly parallel that of IC138. These results suggest that FAP120 forms a stoichiometric complex with IC138. In addition, we confirmed that bop5-1, a previously described mutant expressing truncated IC138 (Hendrickson et al., 2004) and bop5-2, an independently isolated novel allele that completely lacks IC138 (Bower et al., paper in preparation), have very little amounts of detectable FAP120 in their axonemes (Fig. 6). The almost complete absence of FAP120 in the bop5-1 axoneme indicates that the short C-terminal segment of IC138 missing in this mutant is important for the axonemal localization of FAP120. As in the case of ida1, the cell body of bop5 mutants retains almost wild-type levels of FAP120 (Fig. S3).
Fig. 5.
The amounts of IC138 roughly correlate with those of FAP120 in alleles of bop5 mutants Immunoblot analyses on the axonemes of three bop5 alleles (bop5-3, bop5-4, bop5-6), mutants that have mutations in the IC138 gene, were performed using antibodies against IC138, IC97, p28 and FAP120. p28 is a subunit of single-headed inner arm dyneins and used as a control. Two alleles of bop5 (bop5-3, bop5-4) have extremely small amounts of FAP120 while one allele (bop5-6) has a reduced amount of FAP120. Mutant strains that have small amounts of IC138 such as bop5-6 and ida1 also have small amounts of FAP120 and IC97 in their flagella. These observations suggest that FAP120, as well as IC97, interacts with IC138.
Fig. 6.
bop5-1, a mutant expressing a truncated IC138 (Hendrickson et al., 2004), has no FAP120 in its flagella. Immunoblot analysis of axonemes from wild-type, ida7-1, bop5-1 and bop5-2 (6F5) using the anti-FAP120 antibody and the anti-IC140 antibody. Bop5-1 and bop5-2 axonemes contain IC140 but have only negligible amounts of FAP120. The Tropix detection system was used for this blot.
Presence of FAP120 in the Axonemes of mia1 and mia2
Since the above observations suggest that FAP120 is involved in the function of IC138, we examined whether the axonemes of the mia1 and mia2 mutants defective in the phosphorylation of IC138 (King and Dutcher, 1997) retain FAP120. Our results showed that these mutants have normal amounts of FAP120 in their flagella (Fig. S5).
DISCUSSION
We have shown that a novel ankyrin-repeat protein, FAP120 (Pazour et al., 2005), is associated with Chlamydomonas axonemes, but greatly reduced in flagella of mutants lacking inner arm dynein f (I1), ida1, ida2, ida3, and ida7. In addition, it is also missing or greatly diminished in the mutant bop5, a mutant deficient in the IC138 intermediate chain of dynein f. Importantly, in several alleles of these mutants, the amount of FAP120 in the axoneme is correlated with the amount of the residual IC138 and IC97 remaining in the axonemes. This observation strongly suggests that FAP120 and IC138 form a stoichiometric complex in vivo. FAP120 has four tandem repeats of ankyrin motif, one of the most common protein–protein interaction motifs (Li et al., 2006). FAP120 may well use this region for interaction with IC138. However, the interaction may be weak, since FAP120 does not co-elute with dynein f in ion-exchange chromatography/gel-filtration, and also since we have failed to detect their association in vitro using immuno-precipitation and chemical-crosslinking (Yamamoto et al., unpublished results).
IC138 has been shown to be the key regulator of microtubule sliding and is essential for the cell to display phototactic behavior. The regulation is effected through the phosphorylation state of IC138, which is controlled by as yet unidentified protein kinase(s) and phosphatase(s) (Habermacher and Sale, 1997; King and Dutcher, 1997). In vitro studies have suggested that a casein kinase 1-like enzyme is involved in its phosphorylation (Yang and Sale, 2000), and radial spokes and central pair microtubules function to promote dephosphorylation (Smith and Sale, 1992). In addition, genetic analysis identified two mutations, mia1 and mia2, that cause an abnormal increase in the IC138 phosphorylation level and loss of phototaxis (King and Dutcher, 1997). In all cases, dynein f with dephosphorylated IC138 is associated with higher (faster) motor activity. An interesting feature of IC138 is that it is not required for the assembly of dynein f complex; the alleles bop5-3 and bop5-4 lack IC138 almost completely, but retain DHCs and IC140 (Fig. S4). This is in good contrast with the IC140 intermediate chain, which is essential for the assembly of dynein f on the outer doublet (Perrone et al., 1998). Since our present study has shown that flagellar localization of FAP120 requires IC138, there appears to be a hierarchy of assembly in the dynein f complex: the core complex involving DHC1, DHC10 and IC140 is necessary for anchoring IC138, and IC138 is necessary for recruiting FAP120. The observation that the cell bodies of ida1 and bop5 have almost wild-type levels of FAP120 suggests that FAP120 requires IC138 for its transportation or attachment to the axoneme. These findings also raise the possibility that the motility regulation based on the phosphorylation state of IC138 involves a protein complex comprising IC138 and several other proteins including FAP120,IC97 and LC7b. The molecular entity of this putative complex of intermediate chains and FAP120, and the exact functions performed by FAP120 awaits further studies.
Supplementary Material
Acknowledgements
We thank Masafumi Hirono (University of Tokyo) for help with immunoblotting, and Haru-aki Yanagisawa (University of Tokyo) for helpful suggestion and discussion. This study has been supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology (R.K.), from the National Institutes of Health (M.E.P. (GM55667) and W.S.S. (GM51173)). R.Y. is a recipient of the JSPS Fellowship for Junior Scientists and M.W. is a recipient of an NIH National Research Service Award.
References
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskel. 1987;8:68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- DiBella LM, Sakato M, Patel-King RS, Pazour GJ, King SM. The LC7 light chains of Chlamydomonas flagellar dyneins interact with components required for both motor assembly and regulation. Mol Biol Cell. 2004;15:4633–4646. doi: 10.1091/mbc.E04-06-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBella LM, Smith EF, Patel-King RS, Wakabayashi K, King SM. A novel Tctex2-related light chain is required for stability of inner dynein arm I1 and motor function in the Chlamydomonas flagellum. J Biol Chem. 2004;279:21666–21676. doi: 10.1074/jbc.M313540200. [DOI] [PubMed] [Google Scholar]
- Dutcher SK, Gibbons W, Inwood WB. A genetic analysis of suppressors of the PF10 mutation in Chlamydomonas reinhardtii. Genetics. 1988;120:965–976. doi: 10.1093/genetics/120.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, Sale WS. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J Cell Biol. 1997;136:167–176. doi: 10.1083/jcb.136.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. The Chlamydomonas Sourcebook. San Diego, CA: Academic Press; 1989. p. 780. [Google Scholar]
- Harrison A, Olds-Clarke P, King SM. Identification of the t complex-encoded cytoplasmic dynein light chain tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J Cell Biol. 1998;140:1137–1147. doi: 10.1083/jcb.140.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson TW, Perrone CA, Griffin P, Wuichet K, Mueller J, Yang P, Porter ME, Sale WS. IC138 is a WD-repeat dynein intermediate chain required for light chain assembly and regulation of flagellar bending. Mol Biol Cell. 2004;15:5431–5442. doi: 10.1091/mbc.E04-08-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ikeda T, Morikawa K, Kamiya R. Axonemal localization of Chlamydomonas PACRG, a homologue of the human parkin-coregulated gene product. Cell Motil Cytoskel. 2007;64:814–821. doi: 10.1002/cm.20225. [DOI] [PubMed] [Google Scholar]
- Kagami O, Kamiya R. Translocation and rotation of microtubules causedby multiple species of Chlamydomonas inner-arm dynein. J Cell Sci. 1992;103:653–664. [Google Scholar]
- Kamiya R. Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int Rev Cytol. 2002;219:115–155. doi: 10.1016/s0074-7696(02)19012-7. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Kurimoto E, Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J Cell Biol. 1991;112:441–447. doi: 10.1083/jcb.112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chylamydomonas reinhardtii. J Cell Biol. 1988;107:2253–2258. doi: 10.1083/jcb.107.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathir P, LaVoie M, Brazelton WJ, Haas NA, Lefebvre PA, Silflow CD. Molecular map of the Chlamydomonas reinhardtii nuclear gnome. Eukaryot Cell. 2003;2:362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Minoura T, Hirono M, Kamiya R. Chlamydomonas inner-arm dynein mutant, ida5, has a mutation in an actin-encoding gene. J Cell Biol. 1997;137:649–656. doi: 10.1083/jcb.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Dutcher SK. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J Cell Biol. 1997;136:177–191. doi: 10.1083/jcb.136.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Kamiya R. Axonemal dyneins: assembly, structure and force generation. In: Witman George., editor. Chlamydomonas Source Book. Vol. III [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeDizet M, Piperno G. ida4-1, ida4-2, and ida4-3 are intron splicing mutations affecting the locus encoding p28, a light chain of Chlamydomonas axonemal inner dynein arms. Mol Biol Cell. 1995;6:713–723. doi: 10.1091/mbc.6.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mahajan A, Tsai MD. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- Luck D, Piperno G, Ramanis Z, Huang B. Flagellar mutants of Chlamydomonas: studies of radial spoke-defective strains by dikaryon and revertant analysis. Proc Natl Acad Sci U S A. 1977;74:3456–3460. doi: 10.1073/pnas.74.8.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Inoue S, Abiko S, Aoki H, Takeo K. Improved separation of alpha chains of collagen type I, type III, and type V by noninterrupted electrophoresis using thioglycolic acid as a negatively charged reducer. Electrophoresis. 1989;10:29–33. doi: 10.1002/elps.1150100108. [DOI] [PubMed] [Google Scholar]
- Myster SH, Knott JA, Wysocki KM, O’Toole E, Porter ME. Domains in the 1alpha dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas. J Cell Biol. 1999;146:801–818. doi: 10.1083/jcb.146.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita N, Isogai N, Hirono M, Kamiya R, Yoshimura K. Phototactic activity in Chlamydomonas ‘non-phototactic’ mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein. J Cell Sci. 2005;118:529–537. doi: 10.1242/jcs.01633. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CA, Myster SH, Bower R, O’Toole ET, Porter ME. Insights into the structural organization of the I1 inner arm dynein from a domain analysis of the 1beta dynein heavy chain. Mol Biol Cell 11. 2000:2297–2313. doi: 10.1091/mbc.11.7.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CA, Yang P, O’Toole E, Sale WS, Porter ME. The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol Biol Cell. 1998;9:3351–3365. doi: 10.1091/mbc.9.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, LeDizet M, Moscatelli A. Mutations in the ‘dynein regulatory complex’ alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol. 1994;125:1109–1117. doi: 10.1083/jcb.125.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol. 1992;118:1163–1176. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Immunofluorescence microscopy of cilia and flagella. Methods Cell Biol. 1995;47:163–169. doi: 10.1016/s0091-679x(08)60805-5. [DOI] [PubMed] [Google Scholar]
- Segal RA, Huang B, Ramanis Z, Luck DJ. Mutant strains of Chlamydomonas reinhardtii that move backwards only. J Cell Biol. 1984;98:2026–2034. doi: 10.1083/jcb.98.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science. 1992;257:1557–1559. doi: 10.1126/science.1387971. [DOI] [PubMed] [Google Scholar]
- Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell. 2002.;13:1015–1029. doi: 10.1091/mbc.01-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Hendrickson T, Sale WS. Eye on I1: I1 dynein as a modle for flagellar dynein assembly and regulation. Cell Motil Cytoskel. 2007;64:569–579. doi: 10.1002/cm.20211. [DOI] [PubMed] [Google Scholar]
- Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134:280–290. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]
- Wolf E, Kim PS, Berger B. MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 1997;6:1179–1189. doi: 10.1002/pro.5560060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Yanagisawa HA, Yagi T, Kamiya R. Novel 44-kilodalton subunit of axonemal Dynein conserved from Chlamydomonas to mammals. Eukaryot Cell. 2008;7:154–161. doi: 10.1128/EC.00341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa HA, Kamiya R. A tektin homologue is decreased in Chlamydomonas mutants lacking an axonemal inner-arm dynein. Mol Biol Cell. 2004;15:2105–2115. doi: 10.1091/mbc.E03-11-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Sale WS. The Mr 140,000 Intermediate Chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring. Mol. Biol. Cell. 1998;9:3335–3349. doi: 10.1091/mbc.9.12.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275:18905–18912. doi: 10.1074/jbc.M002134200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.