Abstract
Imatinib is a tyrosine kinase inhibitor and is considered the first line of non stem cell transplantation treatment for patients diagnosed with CML. We evaluated the response rates and adverse reactions to Imatinib in our patients and tried to identify factors which affected the response to Imatinib. Eighty-four patients were diagnosed on the basis of clinical and haematological variables with confirmation by FISH, detecting Philadelphia chromosome or bcr-abl translocation and were then started on oral capsule Imatinib. A complete haematological response was seen in 78.04% patients, while complete cytogenetic response (CCR) was seen in 12.2% of patients and major cytogenetic response (MCR) was seen in 64.63% of patients. It was found that that a greater total leukocyte count (TLC) on presentation had a negative correlation with cytogenetic response. Cytopenias were seen in 36 patients (43.82%). 34.9% of patients having CCR/MCR required dose reduction while 73.6% of patients not achieving CCR/MCR required dose reduction. This was a significant difference, confirmed on statistical analysis (P < 0.05; P = 0.019), establishing the negative prognostic value of dose reduction due to cytopenias.
Keywords: CML, Imatinib, Cytopenias, Leukocytosis, Cytogenetic response
Introduction
Imatinib is a 2-phenylaminopyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes. It occupies the TK active site, leading to a decrease in activity. Because BCR-ABL tyrosine kinase activity is essential to the transforming function of BCR-ABL, the inhibition of the kinase should inhibit CML cells, which consist of a fusion protein of abl with bcr (breakpoint cluster region), termed bcr-abl. As this is now a continuously active tyrosine kinase, imatinib is used to decrease bcr-abl activity. The active sites of tyrosine kinases each have a binding site for ATP. The enzymatic activity catalyzed by a tyrosine kinase is the transfer of the terminal phosphate from ATP to tyrosine residues on its substrates, a process known as protein tyrosine phosphorylation. Imatinib works by binding to the ATP binding site of bcr-abl and inhibiting the enzyme activity of the protein competitively. Imatinib is quite selective for bcr-abl—it does also inhibit other targets mentioned above (c-kit and PDGF-R), but no other known tyrosine kinases. Imatinib also inhibits the abl protein of non-cancer cells but cells normally have additional redundant tyrosine kinases which allow them to continue to function even if abl tyrosine kinase is inhibited. Since being identified in the late 1990s by Novartis chemists, led by Dr Brian J. Druker, many of the key clinical trials have confirmed the efficacy of imatinib in CML [1–3]. Its development is the template for rational drug design. Imatinib received US FDA approval in May 2001 and is the treatment modality of choice in CML. The standard starting dose in therapy during the chronic phase is 400 mg once per day, with the minimum dose being 300 mg. Higher doses show hematological responses that are transient, so its role is undecided. Higher doses are also associated with earlier cytogenetic responses, but the long term benefit of this is unclear. The common side effects are superficial edema, nausea, muscle cramps, rash fatigue, hypopigmentation and depigmentation of skin and diarrhoea. Side effects occasionally noted, warranting further investigations are congestive cardiac failure, severe tumor lysis, retinal edema, cerebral edema and gynecomastia. Myelosuppression commonly occurs at treatment onset, requiring supportive therapy and withholding the drug till counts recover.
In the pre-Imatinib era, the indices used as prognostic indicators in patients with CML were age, spleen size, percentage of blasts, percentage of basophils and eosinophils, and platelet counts (Sokal score and Hasford scales). Currently there are no completely validated scoring scales for prognostic factors influencing the response to Imatinib specifically. The above mentioned variables, however correlate reasonably well with response to Imatinib. A study by the Hammersmith Imperial College [4], accepted in March 2003 indicated that a low neutrophil count & poor cytogenetic response at 3 months of imatinib therapy indicated poor overall outcome. A number of newer prognostic variables have come into the limelight and they require further validation [5]. Our study aims to evaluate the response and adverse reactions to Imatinib in patients with Chronic Myelogenous Leukemia and to evaluate for factors which affect the response to Imatinib.
Methods
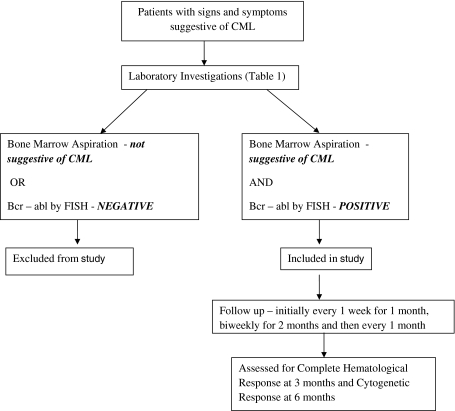
Patient Population & Study Design (Fig. 1)
Fig. 1.
Study design
Our study was an open level clinical trial in which patients of chronic myelogenous leukemia were included. Patients who attend the Medicine OPD, Hemat-Oncology OPD and those admitted in the Medical Wards of Gandhi Memorial & Associated Hospital, C.S.M.M.U were enrolled. Patients were eligible for inclusion in the study if they had been diagnosed with CML for the first time or if they were diagnosed earlier with CML and were on other medications, primarily Hydroxyurea and would be offered Imatinib for the first time. Patients having clinical and haematological features of CML, but bcr-abl negative were excluded. Diagnosis was made on the basis of clinical and haematological variables, with confirmation by cytogenetic method (FISH detecting Philadelphia chromosome or detection of bcr-abl translocation). Patients in all phases of CML (chronic phase, accelerated phase and blast crisis) were included in the study and a set of basic investigations was done prior to beginning treatment (Table 1). Exclusion and Inclusion criteria are summarized in Table 2. The duration of the study was one year, beginning May 2008, with a total enrolment of 84 patients. Eighty-two patients were diagnosed with chronic phase CML while one patient each of blast and accelerated phase were included in the study. However, both the latter mentioned patients were lost to follow-up. The study protocol was reviewed and accepted by the internal review committee of our Medicine Department.
Table 1.
Examination and Investigations prior to beginning treatment
| Complete general physical and systemic examination |
| Complete blood count |
| Liver function tests |
| Se-sodium, potassium, calcium |
| Blood urea nitrogen & Se-creatinine |
| Se-Protein & albumin |
| Se-LDH & Se-uric acid |
| Ultrasonography whole abdomen |
| Bone marrow aspiration |
| FISH for BCR-ABL |
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria |
| CML being diagnosed for the first time by Philadelphia Chromosome/BCR-ABL positivity |
| Patients, already diagnosed as CMl, but on other medications and being offered Imatinib for the first time |
| Exclusion criteria |
| Patients with clinic-haematological features of CML, but Philadelphia Chromosome negative |
Treatment, Follow-up Protocol and Outcome Measures
All patients were begun on Imatinib 400 mg once a day (Patients in the blast and accelerated phases were started on 800 mg per day). They were followed up on a weekly basis for the first one month, every 15 days for the next 2 months and monthly thereafter.
On each visit, the following were assessed:
Any fresh complaints, specifically for skin rashes, bleeding tendencies, fever, fatigue, edema, jaundice, nausea and vomiting
Perceptible decrease in spleen size and sense of well being, improved work performance
General physical examination and examination per abdomen
Complete blood counts
At the end of 3 months, the patients were assessed for presence or absence of complete hematological response (CHR) by the following parameters:
Platelet counts < 450,000/mm3
Total leukocyte counts < 10,000/mm3
Differential leukocyte counts—no immature cells and basophils < 5%
Disappearance of symptoms and signs of leukemia with absence of palpable splenomegaly
At the end of 6 months, FISH was done to evaluate for cytogenetic response and categorized as
Complete cytogenetic response (CCR) Ph+: 0%
Major cytogenetic response (MCR) Ph+: 1–35%
Minor cytogenetic response (MINOR CR) Ph+: 35–65%
No cytogenetic response (NCR) Ph+: >65%
Criteria for stopping treatment with Imatinib included
Bleeding or purpuric rashes
Platelet Counts < 30,000
Febrile neutropenia
TLC < 3000
Hemoglobin levels < 7 g% or progressive fall in values with associated features of fatigue and weakness
Statistical Analysis
All pre-treatment variables and variables gathered during monitoring of patients were compared to outcomes to identify factors affecting response by Kruskal–Wallis test of equality of populations. For primary endpoints, between group differences were calculated with the use of the chi square test of proportions (with a two sided alpha level of 5%). All predefined analyses were performed without adjustment for confounding factors. The calculation of the sample size was based on analysis of pooled data from previous studies.
Results (Table 3)
Table 3.
Important results obtained during study
| Patients with CHR (%) | 78.04 |
| Time to achieve CHR (weeks) | 10.9 |
| Patients with CCR (%) | 12.2 |
| Patients with MCR (%) | 64.63 |
| Patients with minor CR (%) | 18.29 |
| Patients with NCR (%) | 4.88 |
| Patients with cytopenias (any duration) requiring dose reduction (%) | 43.9 |
| Patients with cytopenias (>4 weeks) requiring dose reduction (%) | 20.73 |
| Mean TLC in patients with CCR (cells/mm3) | 27,034 |
| Mean TLC in patients with MCR (cells/mm3) | 39,828 |
| Mean TLC in patients with minor CR (cells/mm3) | 66,764 |
| Mean TLC in patients with NCR (cells/mm3) | 72,102 |
| Primary resistance to Imatinib (%) | 4.88 |
Between May 2008 and May 2009, a total of 84 patients were enrolled in the study and received Imatinib at a starting dose of 400 mg per day. Fifty-six males and 28 females (ratio 2:1) were enrolled in the study. This variable closely mirrored the incidence ratios found by Western estimations (1.5:1–2.0:1.1). The average age of patients included in the study was 36.61 years, with a majority of patients (67.86%) in the 21–40 years age group. As also estimated by earlier Indian studies [6,7], this was about one and a half decades earlier than those mentioned in Western studies (53 years). Of the 84 patients, 82 were diagnosed as having chronic phase CML, one patient had accelerated phase CMl and one patient had blast phase of CML. Both these patients were lost prior to the preliminary evaluation at 3 months for haematological response. The final outcomes of these patients is not known and has not been included in calculating results of the study. Demographic and baseline characteristics are shown in Table 4.
Table 4.
Demographic and baseline characteristics
| Age (years) | 36.61 (13–64) |
| Male sex (%) | 66.67 |
| Duration of illness prior to starting Imatinib (months) | 8.4 (0.5–96) |
| Haemoglobin (g%) | 9.85 (6–16) |
| Total leukocyte counts (cells/mm3) | 44,770 (12,550–136,000) |
| Patients with normal platelet counts (%) | 71.95 |
| Patients with thrombocytosis (%) | 20.73 |
| Patients with thrombocytopenia (%) | 7.32 |
| Patients with basophilia (%) | 90.3 |
| Patients with eosinophilia (%) | 93.9 |
| Patients with marked splenomegaly (%) | 58.54 |
| Patients with moderate splenomegaly (%) | 28.05 |
| Patients with spleen tip palpable (%) | 7.32 |
| Patients with absent splenomegaly (%) | 6.1 |
One of the major end points of our study was measuring cytogenetic response at the end of 6 months, assessing how it compared to earlier studies and looking for possible prognostic factors that might influence cytogenetic response. The degree of significant cytogenetic response {CCR (complete cytogenetic response) + MCR (major cytogenetic response)} was 76.83% (CCR: 12.2% and MCR: 64.63%) at the end of 6 months. This compares favourably with responses in Western studies (IRIS trial: 85%, Fransisco Cervantes et al.: 53%) [1,2] and is a much higher response rate when compared to previous Indian studies (A. Jacob et al.: 50%, Brijesh Arora et al.: 30%, Deshmukh et al.: 50%—all response rates for chronic phase CML) [6,7]. The figures in our study seem to correlate more with the response found in a study by M. Usman, N.N. Syed et al. in Pakistan (83.6%) [8]. The degree of significant cytogenetic response was almost equal in both males and females (Males: 75.9% vs Females: 78.5%). Due to the lack of patients in the accelerated/blast phases in our study, we could not assess this group by sub-group analysis.
On examining total leukocyte counts (TLC), we found a clear variation in cytogenetic response with increasing TLC. While the mean TLC over all cytogenetic response subgroups was 44770/cu mm with a range of 12,550–136,000, patients, the breakdown over the four cytogenetic response groups revealed a mean TLC of 27,034/cu mm, 39,828/cu mm, 66,764/cu mm and 72,102/cu mm for the complete cytogenetic response, major cytogenetic response, minor cytogenetic response and no response groups respectively. While this showed a negative correlation on univariate analysis, on further statistical analysis by chi square testing and equality of proportion testing by Kruskal–Wallis test, this negative correlation of rising TLC with degree of cytogenetic response was significant (P < 0.05; P = 0.001). No previous studies have documented such a relationship. It remains to be seen if this correlation between increased TLC and poor cytogenetic response can be further validated. Another point of discussion is whether the raised TLC is simply an indicator of prolonged disease and late presentation, as is commonly seen in Indian patients.
Cytopenias, of either of the three cell lines were seen in 36 out of 82 patients (43.82%), with the commonest being leukopenia (26/36:72.2%) followed by thrombocytopenia (12/36:33.33%) and anemia (4/36; 11.11%) respectively. (Side effects—Table 5) This was the only side effect which necessitated dose reduction, either temporarily or permanently. All patients who had cytopenias in our study had dose reduction in Imatinib from 400 mg to 200 mg/300 mg. Of the 36 patients who required dose reduction, a greater proportion were females (16/28:57% vs 20/54:37.04%) than males, although this was not significant enough on further analysis (P > 0.05). Nineteen of the 36 patients (52.77%) who required dose reduction were able to continue full dose of treatment (400 mg) after a delay of less than 4 weeks. On comparing the degree of cytogenetic response with the need for dose reduction, it was found that 22 of 63 (34.9%) patients having complete/major cytogenetic response required dose reduction while 14 out of 19 patients (73.6%) who did not achieve significant cytogenetic response required dose reduction. On further statistical analysis, this difference was found to be significant (P > 0.05; P = 0.019). This negative prognostic value of need for dose reduction due to cytopenias, resulting in a decreased cytogenetic response has been examined before and recommended as a negative prognostic indicator by Cervantes et al. (dose reduction > 4 weeks corresponded to decreased cytogenetic response) and by univariate analysis in other studies as well [2].
Table 5.
Distribution of side effects
| Side effects | Number of patients | Percentage of patients |
|---|---|---|
| Cytopenias | 36 | 43.9 |
| Hypopigmentation | 29 | 35.3 |
| Gastrointestinal | 22 | 26.8 |
| Pruritis | 7 | 8.5 |
| Edema | 8 | 9.7 |
| Headache | 10 | 12.1 |
Traditional risk factors, such as age, spleen size, percentage of basophils and eosinophils, and platelet counts, that have been taken into account by the Sokal scores and Hasford scales, had no correlation with response in our study. In our study, although a greater percentage of patients with minor cytogenetic response (11/15; 73.33%) and no cytogenetic response (3/4;75%) had marked splenomegaly compared to patients with complete cytogenetic response (4/10; 40%) and major cytogenetic response (30/53; 56.6%) in our study, there was no significance obtained on further statistical significance (P > 0.05; P = 0.34).
Discussion
In this open level clinical trial, patients with CML were benefitted by treatment with Imatinib. Besides the treatment benefit accrued by patients, the study showed up a number of differences in the way CML should be viewed as a disease in India, when compared to Western data.
The average age of diagnosis in our patients was 36.61 years, with a majority of patients (67.86%) in the 21–40 years age group. This is about one and a half decades earlier than those mentioned in Western studies. Another major difference between our study population and Western populations is the mode of diagnosis. While most western patients are picked up in an asymptomatic/early phase and are started immediately on ideal treatment, most of our patients were diagnosed well before starting on the ideal initial treatment, i.e. Imatinib. This included a subset of patients who were on treatment with predominantly Hydroxyurea. Thirty-seven percent patients of our patients were started on Imatinib after a delay of 6 months or greater after initial diagnosis. This was primarily due to lack of affordability and awareness (the most common reasons given by patients). The mean delay in starting treatment with Imatinib was 8.4 months with a range of 15 days–4 years. One of the theories postulated to explain the decreased response to Imatinib in Indian patients compared to Western patients was the delay in starting treatment in Indian patients. However, when we analysed these two variables (duration of illness prior to starting treatment with Imatinib and cytogenetic response), we found no such inverse relationship of statistical significance. Patients who experienced a complete cytogenetic response (CCR) (12.2%) had a mean delay of 15.25 months, compared to the mean of 8.4 months, which seems contrary to expectations. However, this was not of statistical significance. There was also no variation with respect to age and duration of illness prior to treatment with Imatinib.
A complete hematological response (CHR) (platelet counts < 450,000/cu mm, TLC < 10,000/cu mm, differential count—no immature granulocytes and less than 5% basophils, disappearance of signs and symptoms of leukemia with non palpable spleen) as assessed at 3 months was seen in 64 out of 82 patients (78.04%). This was much lower than that seen in previous Indian and international studies. While Deshmukh et al. recorded 95% and A. Gupta, K. Prasad et al. recorded a 93.75% complete hematological response respectively, the IRIS study [5] reported a 97% complete hematological response [5]. Of the 18 patients in our study who did not achieve CHR at 3 months, a further 50% (nine patients) did achieve CHR by 6 months. This response was obtained without further dose escalation. If these patients are included in the category of patients with haematological response, the percentage of patients in this category goes up to 85.3%. Some of the possible reasons for this lesser degree of hematological response could be a lack of initial compliance with medication and a longer duration of illness prior to starting treatment with Imatinib (as seen earlier, this did not correspond to cytogenetic response). However, there was no relationship of statistical significance between longer duration of illness prior to starting treatment with Imatinib to delayed hematological response as well. It was found that, although a majority of our patients achieved hematological response by 2 months (59.6%), a large number (40.4%) took more than this duration.
The mean time to achieve hematological response was 10.9 weeks. This is significantly more than that recorded by Deshmukh et al. (3 weeks) and Western data (3 weeks) [1, 5]. On comparing the time to achieve hematological response to cytogenetic response, it was found, paradoxically, that those patients who achieved either a complete or major cytogenetic response took a longer duration (11.6 & 11.82 respectively) to achieve hematological response compared to those with minor and no cytogenetic response, respectively. However, on further statistical investigation, there was no significant result found. We are unable to find a cause for the decreased hematological response as well as delayed responses in our patients. One way this problem could be surmounted is by assessment of cytogenetic response at 3 months as this would enable us to make a decision to increase dose or plan a different strategy in case of lack of at least a minor cytogenetic response (a lack of minor cytogenetic response at 3 months has been proposed as a criteria to measure response by some authorities).
One of the surprises we encountered while evaluating for risk factors was the strong negative correlation of total leukocyte counts (TLC) with cytogenetic response. We considered the possibility of this being a manifestation of late presentation and prolonged disease, as is commonly seen in Indian patients. Another confounding factor in our patients is the intermittent treatment with Hydroxyurea, which works as a cytoreductive agent and would cause a reduction in TLC. A number of our patients had a history of irregular intake of Hydroxyurea, the regularity of which could not be ascertained despite repeated questioning. While we had no means to account for the effect of Hydroxurea in our patients, there seemed to be no relationship between duration of illness prior to starting treatment with Imatinib and cytogenetic response. In the light of this, TLC may be considered as a probable prognostic factor in Indian patients.
The negative effects of prolonged duration of Imatinib being withheld or reduced was seen in our study. The only reason for discontinuing Imatinib in our patients was the development of significant cytopenias, which was seen in 36 of our 82 patients (43.82%) Most Western data quoter figures of myelosuppression in the range of 15–20%, predominantly on doses of 600–800 mg/day, while earlier Indian studies show approximately similar rates (Deshmukh et al.: 37%). All patients who had cytopenias in our study had dose reduction in Imatinib from 400 mg to 200 mg/300 mg. Of the 36 patients who required dose reduction, a greater proportion were females (16/28:57% vs 20/54:37.04%) than males, although this was not significant enough on further analysis (P > 0.05). It may have been argued that Indians, having a lower body mass index and being leaner, might respond to lower doses of Imatinib. But as seen, this is not the case and the recommended dose of Imatinib continues to be 400 mg/day for best response. Various theories have been propounded for the increased incidence of cytopenias seen in Indian patients—specific nutritional deficiencies, malnutrition, greater disease burden etc. These have to be validated further. As of now, a significant dose reduction during the course of Imatinib therapy seems to be a negative predictive prognostic indicator for response to Imatinib.
Our study is a 6 month short term study, thereby depriving us of the opportunity to follow up patients for a longer duration of time and assess patients for the long term effects of Imatinib. This is a significant issue, predominantly because there are no significant reports on how long Imatinib should be continued or as to whether it induces any degree of permanent remission, once the patient stops treatment. We were also unable to assess Cytogenetic Response at 3 months. Although estimation of cytogenetic response at 3 months is not completely validated as a requisite for assessing response, our study gave us the opportunity to have assessed this as a possible benchmark for future reference. It might have also helped explain the variance in hematological response. We are unable to explain the lower degree of hematological response as well as the longer duration taken by our patients to achieve hematological response. This is important because most earlier studies have convincingly demonstrated that hematological response is a good surrogate marker for assessing possible cytogenetic response. There are no solutions in the study regarding the relative benefits of stem cell transplantation versus Imatinib in younger patients. There were no patients of the accelerated and blast phases of CML in the study, thereby depriving us of comparisons between study groups.
Contributor Information
A. K. Tripathi, Email: tripathiak2005@hotmail.com
Ashutosh Kumar, Phone: +05-22-2343545, Email: ashutoshkumar53@rediffmail.com.
Anant Ramaswamy, Phone: +04-42-2242947, Email: anant_r13@rediffmail.com.
References
- 1.O Brien SG, Guilhot F, Larson RA et al (2003) Imatinib compared with interferon and low dose cytarabine for newly diagnosed chronic phase chronic myeloid leukemia. NEJM 348:994 [DOI] [PubMed]
- 2.Cervantes F, Hernandez-Boluda JC, Steegman JL et al (2003) Imatinib mesylate therapy of chronic phase chronic myeloid leukemia resistant or intolerant to interferon: results and prognostic factors for response and progression free survival in 150 patients. Haematologica 88:1117–1122 [PubMed]
- 3.Kantarjian H, Sawyers C, Hochhaus A et al (2002) Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. NEJM 346:645 [DOI] [PubMed]
- 4.Marin D, Marktel S, Bua M et al (2003) Prognostic factors for patients with chronic myeloid leukemia in chronic phase treated with imatinib mesylate after failure of interferon alfa. Leukemia 17:1448–1453 [DOI] [PubMed]
- 5.de Lemos JAR, de Oliveira CM, Scerni ACC et al (2005) Differential Molecular Responses of the transcripts B2A2 and B3A2 to imatinib mesylate in chronic myeloid leukemia. Genet Mol Res 4(4):803–811 [PubMed]
- 6.Arora B, Kumar L, Kumari M et al (2005) Therapy with imatinib mesylate for chronic myeloid leukemia. Ind J Med Pediatric Oncol 26(2):5–16
- 7.Deshmukh C, Saikia T, Bakshi A et al (2005) Imatinib mesylate in chronic myeloid leukemia: a prospective, single arm, non randomized study. JAPI 53:291–295 [PubMed]
- 8.Usman M, Syed NN, Kakepoto GN et al (2007) Chronic phase chronic myeloid leukemia: response of imatinab mesylate and significance of sokal score, age and duration in predicting the haematological and cytogenetic response. J Assoc Physician India 55:103–107 [PubMed]