Abstract
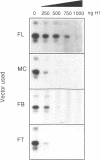
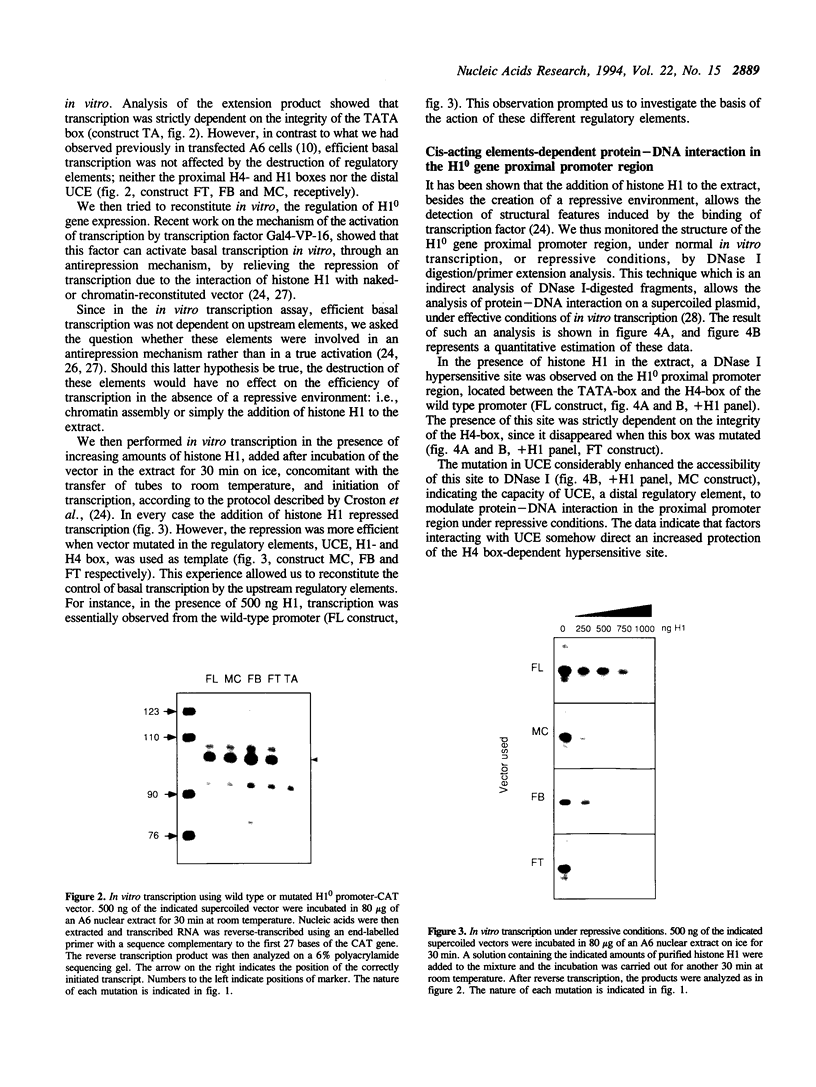
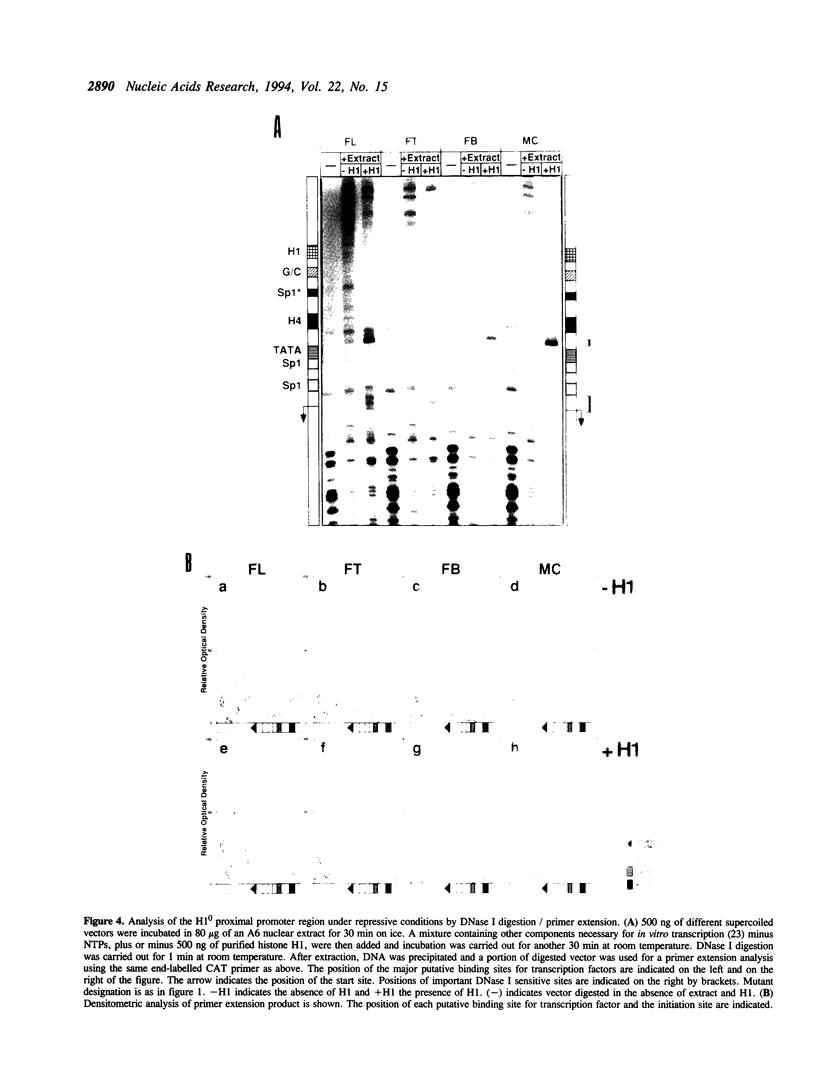
Histone H1(0) is encoded by a gene that is expressed only in cells committed to differentiation. We have previously cloned the Xenopus laevis H1(0) gene and studied elements involved in the regulation of its expression in transfected Xenopus laevis A6 cells, and in microinjected embryos. In this work, in order to understand the basis of the action of these elements, we used an A6 cell nuclear extract and showed that the H1(0) promoter is able to direct efficient in vitro transcription, which is highly dependent on a functional TATA box. However, in contrast to what we observed in vivo, in transfected A6 cells, the in vitro transcription was independent of major regulatory elements, defined in vivo. We then used this in vitro system to reconstitute H1(0) gene regulation. The creation of a repressive environment by the addition of purified histone H1 to the in vitro transcription system allowed us to obtain transcription dependent on the integrity of the regulatory elements. Investigating the basis of this regulation we found that protein-DNA interaction on the proximal promoter region was dependent on the integrity of proximal elements, and moreover the distal regulatory element, the UCE, was able to modulate this interaction. We conclude that the role of these regulatory elements is to maintain the basal TATA-dependent transcription of H1(0) under repressive condition: i.e., H1-mediated repression of transcription, or chromatin assembly in general.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter M., Côté J., Renaud J., Ruiz-Carrillo A. Regulation of histone and beta A-globin gene expression during differentiation of chicken erythroid cells. Mol Cell Biol. 1987 Oct;7(10):3663–3672. doi: 10.1128/mcb.7.10.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almouzni G., Wolffe A. P. Replication-coupled chromatin assembly is required for the repression of basal transcription in vivo. Genes Dev. 1993 Oct;7(10):2033–2047. doi: 10.1101/gad.7.10.2033. [DOI] [PubMed] [Google Scholar]
- Bouterfa H. L., Triebe S. M., Doenecke D. R. Differential regulation of the human H1 zero-histone-gene transcription in human tumor-cell lines. Eur J Biochem. 1993 Oct 1;217(1):353–360. doi: 10.1111/j.1432-1033.1993.tb18253.x. [DOI] [PubMed] [Google Scholar]
- Breuer B., Fischer J., Alonso A. Cloning and characterization of the mouse histone H1(0) promoter region. Gene. 1989 Sep 30;81(2):307–314. doi: 10.1016/0378-1119(89)90191-1. [DOI] [PubMed] [Google Scholar]
- Breuer B., Steuer B., Alonso A. Basal level transcription of the histone H1(0) gene is mediated by a 80 bp promoter fragment. Nucleic Acids Res. 1993 Feb 25;21(4):927–934. doi: 10.1093/nar/21.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard M. P., Rousseau D., Lawrence J. J., Khochbin S. Two mRNA species encoding the differentiation-associated histone H1(0) are produced by alternative polyadenylation in mouse. Eur J Biochem. 1994 Apr 1;221(1):421–425. doi: 10.1111/j.1432-1033.1994.tb18754.x. [DOI] [PubMed] [Google Scholar]
- Clarke H. J., Oblin C., Bustin M. Developmental regulation of chromatin composition during mouse embryogenesis: somatic histone H1 is first detectable at the 4-cell stage. Development. 1992 Jul;115(3):791–799. doi: 10.1242/dev.115.3.791. [DOI] [PubMed] [Google Scholar]
- Croston G. E., Laybourn P. J., Paranjape S. M., Kadonaga J. T. Mechanism of transcriptional antirepression by GAL4-VP16. Genes Dev. 1992 Dec;6(12A):2270–2281. doi: 10.1101/gad.6.12a.2270. [DOI] [PubMed] [Google Scholar]
- Dalton S., Wells J. R. A gene-specific promoter element is required for optimal expression of the histone H1 gene in S-phase. EMBO J. 1988 Jan;7(1):49–56. doi: 10.1002/j.1460-2075.1988.tb02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Almouzni G., Dasso M., Wolffe A. P. Chromatin transitions during early Xenopus embryogenesis: changes in histone H4 acetylation and in linker histone type. Dev Biol. 1993 Nov;160(1):214–227. doi: 10.1006/dbio.1993.1299. [DOI] [PubMed] [Google Scholar]
- Gjerset R., Gorka C., Hasthorpe S., Lawrence J. J., Eisen H. Developmental and hormonal regulation of protein H1 degrees in rodents. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2333–2337. doi: 10.1073/pnas.79.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J. D. Rapid "footprinting" on supercoiled DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3078–3081. doi: 10.1073/pnas.82.10.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley C., Perry M. Histone H2B gene transcription during Xenopus early development requires functional cooperation between proteins bound to the CCAAT and octamer motifs. Mol Cell Biol. 1992 Oct;12(10):4400–4411. doi: 10.1128/mcb.12.10.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hock R., Moorman A., Fischer D., Scheer U. Absence of somatic histone H1 in oocytes and preblastula embryos of Xenopus laevis. Dev Biol. 1993 Aug;158(2):510–522. doi: 10.1006/dbio.1993.1209. [DOI] [PubMed] [Google Scholar]
- Kamakaka R. T., Bulger M., Kadonaga J. T. Potentiation of RNA polymerase II transcription by Gal4-VP16 during but not after DNA replication and chromatin assembly. Genes Dev. 1993 Sep;7(9):1779–1795. doi: 10.1101/gad.7.9.1779. [DOI] [PubMed] [Google Scholar]
- Khochbin S., Wolffe A. P. Developmental regulation and butyrate-inducible transcription of the Xenopus histone H1(0) promoter. Gene. 1993 Jun 30;128(2):173–180. doi: 10.1016/0378-1119(93)90560-p. [DOI] [PubMed] [Google Scholar]
- Kress H., Tönjes R., Doenecke D. Butyrate induced accumulation of a 2.3 kb polyadenylated H1(0) histone mRNA in HeLa cells. Nucleic Acids Res. 1986 Sep 25;14(18):7189–7197. doi: 10.1093/nar/14.18.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase II. Science. 1991 Oct 11;254(5029):238–245. doi: 10.1126/science.254.5029.238. [DOI] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Threshold phenomena and long-distance activation of transcription by RNA polymerase II. Science. 1992 Sep 18;257(5077):1682–1685. doi: 10.1126/science.1388287. [DOI] [PubMed] [Google Scholar]
- Moorman A. F., de Boer P. A., Charles R., Lamers W. H. The histone H1(0)/H5 variant and terminal differentiation of cells during development of Xenopus laevis. Differentiation. 1987;35(2):100–107. doi: 10.1111/j.1432-0436.1987.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Ohsumi K., Katagiri C. Occurrence of H1 subtypes specific to pronuclei and cleavage-stage cell nuclei of anuran amphibians. Dev Biol. 1991 Sep;147(1):110–120. doi: 10.1016/s0012-1606(05)80011-9. [DOI] [PubMed] [Google Scholar]
- Rousseau D., Khochbin S., Gorka C., Lawrence J. J. Induction of H1(0)-gene expression in B16 murine melanoma cells. Eur J Biochem. 1992 Sep 15;208(3):775–779. doi: 10.1111/j.1432-1033.1992.tb17247.x. [DOI] [PubMed] [Google Scholar]
- Rousseau D., Khochbin S., Gorka C., Lawrence J. J. Regulation of histone H1(0) accumulation during induced differentiation of murine erythroleukemia cells. J Mol Biol. 1991 Jan 5;217(1):85–92. doi: 10.1016/0022-2836(91)90613-b. [DOI] [PubMed] [Google Scholar]
- Rousseau S., Renaud J., Ruiz-Carrillo A. Basal expression of the histone H5 gene is controlled by positive and negative cis-acting sequences. Nucleic Acids Res. 1989 Sep 25;17(18):7495–7511. doi: 10.1093/nar/17.18.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. C., Dworkin-Rastl E., Dworkin M. B. Expression of a histone H1-like protein is restricted to early Xenopus development. Genes Dev. 1988 Oct;2(10):1284–1295. doi: 10.1101/gad.2.10.1284. [DOI] [PubMed] [Google Scholar]
- Steuer B., Breuer B., Alonso A. Multiple cis-acting elements of the proximal promoter region are required for basal level transcription of the H1(0) histone gene. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1153–1160. doi: 10.1016/0006-291x(92)91352-q. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Wolffe A. P. In vitro transcription by RNA polymerase II in extracts of Xenopus oocytes, eggs, and somatic cells. Anal Biochem. 1992 Jun;203(2):340–347. doi: 10.1016/0003-2697(92)90322-x. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. Dominant and specific repression of Xenopus oocyte 5S RNA genes and satellite I DNA by histone H1. EMBO J. 1989 Feb;8(2):527–537. doi: 10.1002/j.1460-2075.1989.tb03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., van den Ent F. M., Lian J. B., Stein J. L., Stein G. S. Overlapping and CpG methylation-sensitive protein-DNA interactions at the histone H4 transcriptional cell cycle domain: distinctions between two human H4 gene promoters. Mol Cell Biol. 1992 Jul;12(7):3273–3287. doi: 10.1128/mcb.12.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]