Abstract
OBJECTIVE
Current clinical guidelines recommend that orchidopexy be performed by the age of 1 in patients with congenital undescended testis. We sought to examine trends in surgical timing and to determine what factors are associated with age at surgery.
METHODS
The Pediatric Health Information System (PHIS) is a national database of >40 freestanding children’s hospitals. We searched the PHIS to identify boys with cryptorchidism who underwent orchidopexy between 1999 and 2008. Patient age at orchidopexy was evaluated, and we used multivariate models to determine factors associated with timing of surgery.
RESULTS
We identified 28 204 children who underwent orchidopexy at PHIS hospitals. Of these, 14 916 (53%) were white, and 17 070 (61%) had public insurance. Only 5031 patients (18%) underwent orchidopexy by the age of 1 year; only 12 165 (43%) underwent orchidopexy by the age of 2 years. These figures remained stable over time (P = .32). After adjusting for patient clustering, race (P < .001) and insurance status (P < .001) remained associated with patient age at orchidopexy; however, the treating hospital (P < .001) was the most important factor in predicting the timing of the procedure.
CONCLUSIONS
Only 43% had surgery by 2 years of age, which suggests that either significant numbers of boys with congenital cryptorchidism do not undergo surgery in a timely fashion or late-onset testicular ectopy is more common than generally recognized. Factors associated with the timing of orchidopexy include patient race, insurance status, and the hospital in which surgery is performed.
Keywords: cryptorchidism, pediatrics, testis, orchidopexy, clinical practice variation
Cryptorchidism, or undescended testis (UDT), occurs in 3% to 5% of male term infant births.1 The malpositioned testis is usually found along its normal developmental pathway from the abdominal cavity into the scrotum via the inguinal canal. The majority of UDTs will descend spontaneously, typically during the first 6 months of life.1,2 Beyond the age of 1, the percentage of boys with congenital UDT remains relatively stable at 0.8% to 1.1%.1
UDTs are associated with infertility, malignancy, and cosmetic concerns. UDT has been linked to abnormal testicular development, semen motility, and morphology3–5 and may lead to long-term infertility issues.6 In addition, there is a three- to eightfold increased relative risk of testicular cancer in boys with UDT.7 This risk is lower among boys who undergo early surgical treatment of the UDT with orchidopexy.8 Additional considerations include the psychological effects of childhood genital surgery and alterations in emotional development or body image related to the timing of intervention.9
Multiple medical organizations have made recommendations regarding the timing of intervention.2,9–11 On the basis of assessment of the benefits of early treatment, as well as the recognition that few cryptorchid testes descend spontaneously after the age of 6 months, the 1996 American Academy of Pediatrics guidelines formally recommend surgical repair by the age of 1 for boys with unilateral or bilateral UDT.
However, despite convincing evidence and consistent guidelines, a considerable number of orchidopexies continue to be performed beyond 1 year of age.11–14 The prevalence of such delays, and the factors that lead to the delays, are not well understood. Therefore, our aims for this study were to describe nationwide trends in compliance with guidelines for timing of orchidopexy for UDT, and to identify patient- and hospital-level factors associated with a child’s age at surgery.
METHODS
Data Source
We used the Pediatric Health Information System (PHIS), a national database of administrative and billing data from 41 freestanding children’s hospitals affiliated with the Child Health Corporation of America (Shawnee Mission, KS). The PHIS database comprises more than 125 discrete data points drawn from more than 1 000 000 pediatric patient encounters including data from inpatient admissions, ambulatory medical and/or surgical short-stay areas, and emergency department visits. PHIS data are screened for accuracy on a quarterly basis through the joint efforts of the Child Health Corporation of America, an independent data manager (Thomson Health care, Durham, NC), and each participating hospital. Data are accepted into the PHIS only when classified errors occur in <2% of a hospital’s quarterly data.
Patient Population
We identified all hospital or outpatient surgical visits that occurred between January 1999 and December 2008 for patients younger than 18 with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for cryptorchidism (752.5 and 752.51). We then selected patients who underwent surgical intervention for UDT by using ICD-9 procedure codes for orchidopexy (62.5). We excluded patients with the ICD-9 diagnosis code for testicular torsion (608.20 and 608.24) or retractile testes (752.52). To avoid dual counting of 2-stage orchidopexies or redo procedures, only the first encounter for any individual patient was included in the final data set. PHIS hospitals provide data on inpatient hospitalizations, with or without data on outpatients encounters (emergency department visits, outpatient surgery, etc); in this analysis we included data from a given hospital only during periods when that hospital provided both outpatient and inpatient data (n = 31). This resulted in the exclusion of a combined total of 171 procedures (0.6% of the total case volume).
Variable Selection
We selected predictor variables on the basis of biological plausibility and/or demonstrated associations in the literature: patient race/ethnicity, insurance type (private versus public), comorbidity level, procedure year, hospital factors (teaching status and mean annual orchidopexy volume), and US census region. The PHIS contains self-reported race defined as white, black, Asian, American Indian, or other. Ethnicity is defined as Hispanic or non-Hispanic. Self-reported Hispanic ethnicity was only found within the white race category. To simplify the analysis, we combined these variables into a single race/ethnicity variable with values of white/non-Hispanic, white/Hispanic, black, and other. Insurance status was defined as public or private payer. Patient comorbidity level was defined by the All Patient Refined Diagnosis Related Group (APR-DRG) 20 severity level (3M Health Information Systems, Salt Lake City, UT), and categorized as minor, moderate, major, or extreme. No patients in our sample were categorized as extreme. Surgeon type was defined by each hospital as urologist or pediatric surgeon. The PHIS surgical complication flag was used to identify adverse events. Hospital volume was stratified into quartiles on the basis of each hospital’s mean annual orchidopexy volume, with the low-volume quartile compared with higher volume quartiles. US census region was defined as Northeast, West, South, or Midwest on the basis of the location of the PHIS hospital.
Our primary outcome of interest, patient age at the time of orchidopexy, was dichotomized at various cutoff points: orchidopexy by 1, 2, or 3 years. Analysis results were similar for all 3 end points. Therefore, we chose surgery by age the 2 of as our primary outcome, in part to avoid an overly strict definition of the “most appropriate” age for surgery.
Statistical Methods
Bivariate tests of association were performed between the predictor variables and the binary outcome, orchidopexy before or after the age of 2, by using the Fisher’s exact test, χ2 test, or Mantel-Haenszel χ2 trend test as appropriate on the basis of data characteristics. Multivariate logistic regression models were constructed to further examine associations between the predictors of interest and patient age after adjusting for possible confounding. Model covariates were chosen on the basis of a priori hypotheses and/or a bivariate P value of <.2; the covariates entered into the final model were patient race/ethnicity, insurance type, APR-DRG comorbidity level, and hospital. Because region was defined only by the geographical location of the PHIS hospital, these covariates were collinear and could not be used in the multivariate model simultaneously. For this reason we chose to include only the more informative of the 2 (hospital identifier). The relative importance of each covariate in explaining the outcome variability was determined by comparing a series of nested models. Generalized estimating equations were used to control for surgeon-level patient clustering. Model diagnostics revealed no significant violations of regression assumptions. The predicted probability of surgery by the age of 2 at a particular hospital was determined by back-transforming data from the base logistic multivariate model. All analyses were performed by using SAS 9.2 (SAS Institute, Inc, Cary, NC). All test statistics were 2-sided, and P values of≤.05 were considered to be significant.
Institutional review board approval was obtained from Children’s Hospital Boston, and administrative approval was obtained from the PHIS before data extraction and analysis.
RESULTS
Patient Population
We identified 30 043 children who underwent orchidopexy between 1999 and 2008, and 28 204 remained after exclusions. Patient demographics are presented in Table 1. The majority of boys were white (14 916 [53%]), were publicly insured (17 070 [61%]), had minor comorbidity (26 045 [99%]), and were treated by a pediatric urologist (22 413 [84%]).
TABLE 1.
Demographic Characteristics of Boys Who Underwent Orchidopexy
| Characteristic | n (% of Total) |
|---|---|
| Age, y | |
| 0–1 | 5031 (18) |
| 1–2 | 7134 (25) |
| 3–6 | 7372 (26) |
| 7–10 | 5283 (19) |
| 10–18 | 3384 (12) |
| Race/ethnicity | |
| Black | 4032 (14) |
| White/Hispanic | 3908 (14) |
| White/non-Hispanic | 14 916 (53) |
| Other | 5348 (19) |
| Insurance type | |
| Public (or none) | 17 070 (61) |
| Private | 11 083 (39) |
| Region | |
| West | 4437 (16) |
| South | 9853 (35) |
| Midwest | 10 933 (39) |
| Northeast | 2981 (11) |
| Hospital teaching status | |
| Nonteaching | 1040 (3) |
| Teaching | 27 164 (96) |
| Comorbidity level | |
| Minor | 26 045 (99) |
| Moderate | 335 (1) |
| Major | 25 (0.1) |
| Surgeon type | |
| Urologist | 22 413 (84) |
| Pediatric surgeon | 4389 (16) |
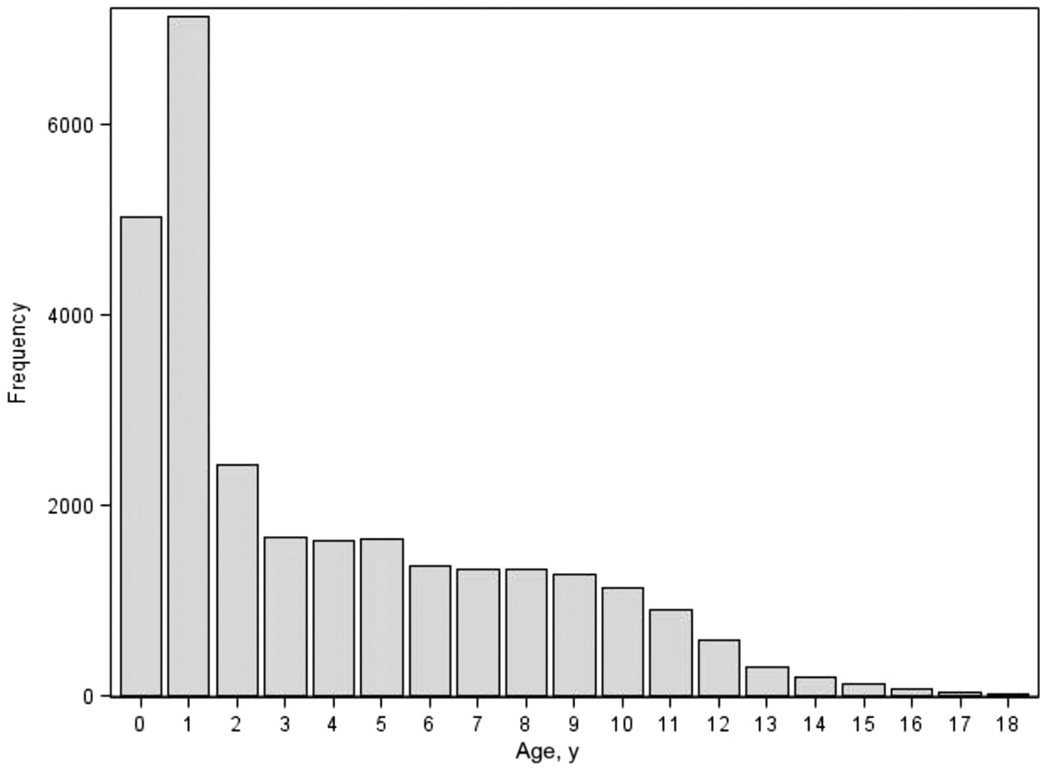
The number of orchidopexies performed by age is presented in Fig 1. The mean (±SD) age of the cohort at the time of surgery was 4.4 (±3.9) years. Overall, 5031 boys (18%) had surgery by the age of 1, 12 165 (43%) by the age of 2, and 14 598 (52%) by the age of 3. In 1999, 2003, and 2008, the proportion of procedures performed by the age of 2 was 40%, 44%, and 42%, respectively; there was no significant trend in age at the time of orchidopexy over the study period (P = .31, Mantel-Haenszel trend test). The overall complication rate was 0.6% and was not significantly different in children who underwent orchidopexy before or after the age of 2 (0.6% vs 0.5%; P = .17).
FIGURE 1.
Frequency of orchidopexy according to age.
Bivariate Analysis
Using bivariate analysis, white/non-Hispanic patients had a small but statistically significant increased likelihood of undergoing orchidopexy by 2 years old compared with blacks or white/Hispanics (43% vs 38% or 41%; P < .001) (Table 2). The proportion of surgeries before the age of 2 for hospital regions ranged from 41% to 44% (P = .03). Patient insurance status (P = .65), comorbidity level (P = .35), provider type (P = .71), hospital volume (P = .20 for lowest quartile versus other), and hospital teaching status (P = .48) were not significantly associated with age at orchidopexy.
TABLE 2.
Bivariate Analysis of Associations Between Patient- and Hospital-Level Factors and Performance of Orchidopexy by the Age of 2 in Boys With UDT
| Factor | OR (95% CI)a | Pb |
|---|---|---|
| Race/ethnicity | ||
| Black (referent) | — | <.001 |
| White/Hispanic | 1.10 (1.00–1.20) | |
| White/non-Hispanic | 1.23 (1.15–1.32) | |
| Other | 1.47 (1.35–1.60) | |
| Insurance type | ||
| Public (or none) (referent) | — | .65 |
| Private | 1.01 (0.96–1.06) | |
| Region | ||
| West (referent) | — | .03 |
| South | 1.09 (1.13–1.17) | |
| Midwest | 1.14 (1.03–1.25) | |
| Northeast | 1.10 (1.03–1.18) | |
| Hospital teaching status | ||
| Nonteaching (referent) | — | .48 |
| Teaching | 1.05 (0.93–1.19) | |
| Comorbidity level | ||
| Minor (referent) | — | .35 |
| Moderate | 1.17 (0.94–1.45) | |
| Major | 1.08 (0.49–2.38) | |
| Surgeon type | ||
| Urologist (referent) | — | .71 |
| Pediatric surgeon | 1.01 (0.95–1.08) | |
| Year of treatmentc | ||
| 1999 (referent) | — | .21 |
| 2004 | 1.20 (0.99–1.46) | |
| 2008 | 1.08 (0.89–1.31) | |
| Average annual hospital volume | ||
| Lowest quartile (referent) | — | .20 |
| Other quartiles | 1.03 (0.98–1.08) | |
| Hospital No. | ||
| 1 (referent) | — | <.001 |
| 15 | 1.84 (1.32–2.57) | |
| 31 | 2.75 (1.97–3.83) |
ORs were estimated from logistic regression.
P was calculated by using Fisher’s exact test for binary and χ2 test for categorical predictors.
No significant trend over time (P = .32 by Mantel-Haenszel χ2 trend test).
The hospital at which a patient sought treatment was highly associated with patient age at the time of surgery (P < .001). The proportion of orchidopexies performed before the age of 2 at a given hospital ranged from 29.5% to 53.5%. The treating hospital had the broadest range of unadjusted odds ratios (ORs) for any factor (Table 2).
Multivariate Analysis
After we adjusted for confounding effects as well as surgeon-level clustering of similar patients, both patient race (P < .001) and insurance payer (P < .001) were significantly associated with age at orchidopexy. Black patients were less likely to undergo orchidopexy by 2 years old than were either white/non-Hispanics or white/Hispanics (adjusted OR [aOR] of 1.33 and 1.18, respectively; P < .001). Similarly, privately insured boys had higher odds of undergoing orchidopexy by 2 years old compared with publicly insured boys (aOR: 1.12; P < .001). When individual hospitals were compared with the hospital with the lowest proportion of orchidopexies performed before the age of 2, aOR ranged from 1.37 to 2.79 (overall P < .001). Compared with all other covariates, the most important predictor of surgery by 2 years old was the hospital where surgery was performed (Table 3).
TABLE 3.
Multivariate Analysis of Associations Between Patient- and Hospital-Level Factors and Performance of Orchidopexy by the Age of 2 in Boys With UDT
| Factor | aOR (95% CI) | P |
|---|---|---|
| Race/ethnicity | ||
| Black (referent) | — | <.001 |
| White/Hispanic | 1.18 (1.06–1.31) | |
| White/non-Hispanic | 1.33 (1.23–1.45) | |
| Other | 1.68 (1.52–1.87) | |
| Insurance type | ||
| Public (or none) (referent) | — | <.001 |
| Private | 1.12 (1.06–1.19) | |
| Comorbidity level | ||
| Minor (referent) | — | .39 |
| Moderate | 1.20 (0.93–1.55) | |
| Major | 1.06 (0.49–2.29) | |
| Hospital No. | ||
| 1 (referent) | — | <.001 |
| 15 | 1.91 (1.49–2.46) | |
| 31 | 2.79 (2.18–3.60) |
ORs were estimated from logistic regression. Generalized estimating equations were used to adjust for surgeon-level clustering.
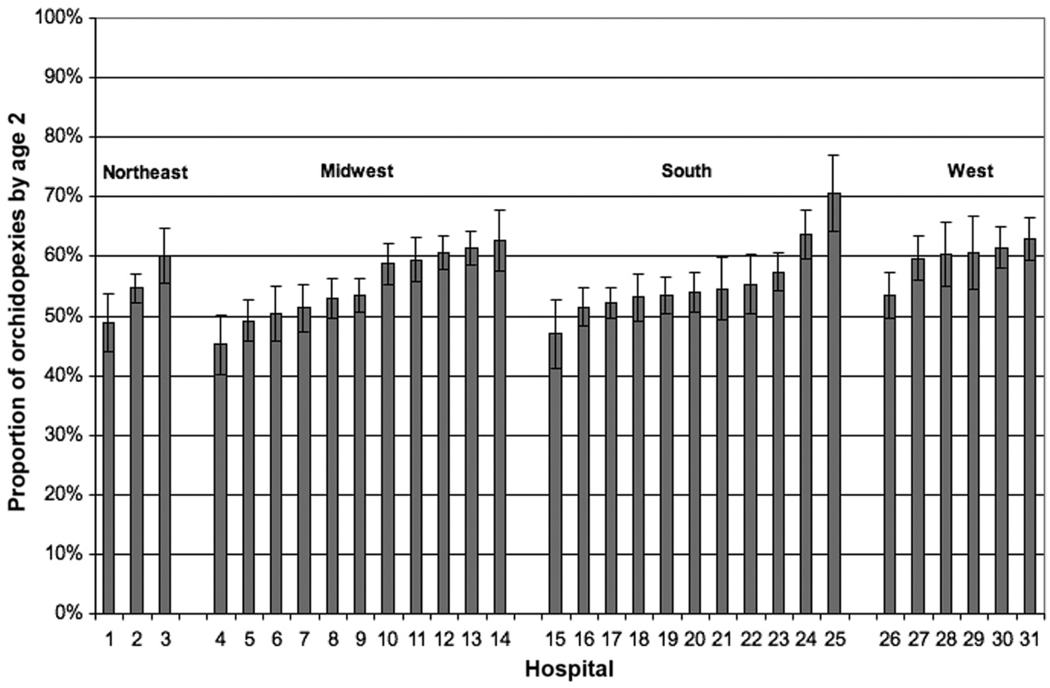
Using our multivariate model, we calculated the predicted probability of surgery by the age of 2 years at each study hospital for a boy who is white, has private insurance, and has an APR-DRG severity score of “minor.” Fig 2 displays the predicted probabilities for this typical patient to undergo surgery by the age of 2 years at each of the 31 PHIS hospitals. The predicted probability at a given hospital ranged from 45% (95% confidence interval [CI]: 40%–50%) to 71% (95% CI: 64%–76%).
FIGURE 2.
Multivariate model-adjusted probability of orchidopexy by the age of 2 for individual hospitals.
DISCUSSION
The primary aim of this study was to examine factors associated with the age at which orchidopexy is performed at freestanding children’s hospitals in North America. Contrary to our expectations, the majority of orchidopexies were still performed after the age of 2 in boys. In addition, this figure remained constant during the study period, despite the presence of guidelines and abundant evidence supporting surgical repair by the age of 1.
The finding that so many orchidopexies are performed outside of the expected time frame points to 1 of 2 basic explanations. In the first scenario, patients with congenital cryptorchidism (ie, patients with UDT at birth) were followed without surgery for longer than is recommended by current guidelines. Such delays could be the result of many factors distinct from medical decision-making, including family delays, insurance coverage issues, or problems with the timing of subspecialty referral. In the second scenario, patients who underwent late orchidopexy primarily comprised cases of acquired UDT (ie, patients whose testes were properly descended at birth but subsequently ascended to an abnormal location). Although long-recognized as a real entity, “ascending testis” is not typically thought of as a common condition, and the fact that more than half of the procedures we observed were performed in older boys suggests that the ascending testis is significantly more prevalent that has been generally recognized. We will address each of these scenarios in turn.
Delays in surgery to treat congenital cryptorchidism are concerning because the evidence for early repair is so strong. The most current guidelines for surgical management of congenital cryptorchidism are based on numerous studies that reveal the long-term consequences of untreated UDT and the advantages of early intervention. Cryptorchid testes tend to be smaller than their normally descended counterparts,15 and 1 trial found that catch-up growth of the cryptorchid testis occurred after orchidopexy was performed at 9 months, but not when surgery was delayed until the age of 3.16 Biopsy at the time of orchidopexy has shown that testicular histology tends to worsen with age.4,5,17 Germ-cell counts may be normal in the newborn period, but if left untreated beyond 2 years of age, intra-abdominal testes have a 30% to 40% chance of complete spermatogenic failure.18 Interstitial fibrosis and poor tubular characteristics are also more common in UDT repaired after 2 years.17 Conversely, both sperm counts and motility have been demonstrated to be higher in young men who had their orchidopexy before 1 year of age.5
If congenitally cryptorchid testes are followed nonoperatively for too long, is there anything that could be done to correct this? The available evidence suggests there is. One successful regional intervention revealed that considerable improvement in timing of orchidopexy can be achieved by heightening awareness of, and adherence to, available guidelines. In that study, before any intervention, 45% of referrals for UDT were initiated by parents, whereas the rest were initiated by local physicians. After the dissemination of educational materials and implementation of an improved system for communicating neonatal examination results, the median age at orchidopexy decreased by 2.5 years.12 Other investigators have found significant variations in the referral patterns of primary care providers, with up to 17% recommending orchidopexy after ages 3 to 10.19 These findings suggest that improvement is both needed and possible.
Regarding our second explanatory scenario (ie, that acquired UDTs are more common than previously assumed), there is evidence that suggests that the frequency of acquired UDT has been underestimated. One intriguing finding has been the apparent mismatch between the reported rates of congenital UDT and surgical orchidopexy. Despite the estimated 0.8% to 1.0% incidence of congenital UDT, some investigators in the United States have estimated that the rate of orchidopexy in the general population is 1.5% to 3% by the age of 17.11,14 In European studies, this figure has been estimated to be as high as 8.4%. These findings suggest that significant numbers of boys become “cryptorchid” later in life, despite having normal examinations at birth. Hack et al20 noted a bimodal distribution of age at which boys are referred for evaluation of UDT, with peaks at ages 2 and 10. They determined that 73% of boys who were referred late for UDT had previous documented normal examination(s).
Although the etiology of ascending testis is unclear, the associated testicular changes may be similar to those seen in children with congenital UDT.21 In published series of ascending testis, the mean age at orchidopexy ranges from 6 to 9 years.22 The natural spasticity of the cremasteric muscles, which peaks at 5 to 8 years of age, may account for a portion of the late orchidopexies; such testes might be better classified as retractile testes.23,24 Agarwal et al23 followed 204 boys with retractile testis and determined that their chance of secondary ascent was 32%. Perhaps most concerning is the finding by Rusnack et al21 that testicular biopsies from congenital and acquired UDT share similar reductions in germ cell counts. In our study, we specifically excluded boys with codes for retractile testis, although coding errors or misclassification may have allowed some of these boys to remain in our cohort.
In addition to the high proportion of late orchidopexies, our results also demonstrate unwarranted variation in the surgical management of UDT. Black and white/Hispanic boys were less likely to undergo orchidopexy by the age of 2 (Table 3), as were boys who were publicly insured. The source of these associations is unclear; however, racial disparities in the treatment and outcomes of urologic surgery have been noted previously.25–27 Although important to acknowledge, these relatively small changes in odds do little to explain the overall frequency of late orchidopexies; even the best-case race and payer groups did not come close to meeting the guidelines for orchidopexy timing. The specific mechanisms of these disparities cannot be adequately addressed with our current data and will require more specific investigation. We also observed considerable variability in the age at surgery among hospitals. Such variability may be because of differences in environmental or population characteristics among hospitals, or alternatively, because of modifiable, hospital-specific or health system-specific factors.
Our findings should be interpreted in light of their limitations. Data are limited to tertiary-care, freestanding children’s hospitals that are part of the PHIS system. As such, the patient population may not be generalizable to other academic or community hospitals. We are not aware of any specific important confounders not included in the model; however, given the observational nature of this investigation, there may be unmeasured, confounding influences on our results. Given the magnitudes of the effects seen in our model, the effect of any residual confounding would have to be considerable to render our findings null. No formal sensitivity analyses were performed to estimate the influence of unmeasured confounders. We can only speculate on the reasons for the large variation among hospitals. There may be differences in screening and referral mechanisms within local health care systems not captured by the PHIS database. Conversely, diversity in the proportion of acquired UDT among local patient populations, with or without systems variation, may be present.
Our diagnosis classifications rely on the ICD-9-CM coding system and represent secondary data extracted from the original medical chart. The potential for inaccurate data transfer or improper coding cannot be excluded and must be kept in mind when evaluating these data. This is particularly relevant when considering patients for whom there is missing data. The question of coding is specifically relevant with respect to the question of retractile testes. Although we excluded patients who had this diagnosis, it is possible that some patients with retractile testis were miscoded as having UDT. If widespread, this misclassification would tend to bias the average age at surgery upward because this condition is not typically treated surgically in young boys. However, we believe that the overall number of boys with retractile testis managed surgically at any age should be low, so effects from this bias should likewise be low.
CONCLUSIONS
Among patients who were treated at selected North American pediatric hospitals between 1999 and 2008, only 18% of boys with UDT underwent orchidopexy by the age of 1, and only 43% did so by the age of 2, despite long-standing guidelines that advocate early surgery. Patient race and insurance status were independently associated with likelihood of orchidopexy, after correcting for other patient- and hospital-level factors. The hospital where a patient is treated was the single most important factor associated with age at orchidopexy.
ACKNOWLEDGMENT
Dr Routh is supported by grant T32-HS000063 from the Agency for Healthcare Research and Quality.
ABBREVIATIONS
- UDT
undescended testis
- PHIS
Pediatric Health Information System
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- APR-DRG
All Patient Refined Diagnosis Related Group
- OR
odds ratio
- aOR
adjusted odds ratio
- CI
confidence interval
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Berkowitz GS, Lapinski RH, Dolgin SE, Gazella JG, Bodian CA, Holzman IR. Prevalence and natural history of cryptorchidism. Pediatrics. 1993;92(1):44–49. [PubMed] [Google Scholar]
- 2.Ritzén EM. Undescended testes: a consensus on management. Eur J Endocrinol. 2008;159 suppl 1:S87–S90. doi: 10.1530/EJE-08-0181. [DOI] [PubMed] [Google Scholar]
- 3.Trussell JC, Lee PA. The relationship of cryptorchidism to fertility. Curr Urol Rep. 2004;5(2):142–148. doi: 10.1007/s11934-004-0028-4. [DOI] [PubMed] [Google Scholar]
- 4.Tasian GE, Hittelman AB, Kim GE, DiSandro MJ, Baskin LS. Age at orchiopexy and testis palpability predict germ and Leydig cell loss: clinical predictors of adverse histological features of cryptorchidism. J Urol. 2009;182(2):704–709. doi: 10.1016/j.juro.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Canavese F, Mussa A, Manenti M, et al. Sperm count of young men surgically treated for cryptorchidism in the first and second year of life: fertility is better in children treated at a younger age. Eur J Pediatr Surg. 2009;19(6):388–391. doi: 10.1055/s-0029-1241171. [DOI] [PubMed] [Google Scholar]
- 6.Murphy F, Paran TS, Puri P. Orchidopexy and its impact on fertility. Pediatr Surg Int. 2007;23(7):625–632. doi: 10.1007/s00383-007-1900-3. [DOI] [PubMed] [Google Scholar]
- 7.Lee PA, Bellinger MF, Coughlin MT. Correlations among hormone levels, sperm parameters and paternity in formerly unilaterally cryptorchid men. J Urol. 1998;160(3 pt 2):1155–1157. doi: 10.1097/00005392-199809020-00052. discussion 1178. [DOI] [PubMed] [Google Scholar]
- 8.Wood HM, Elder JS. Cryptorchidism and testicular cancer: separating fact from fiction. J Urol. 2009;181(2):452–461. doi: 10.1016/j.juro.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics, Section on Urology. Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia. Pediatrics. 1996;97(4):590–594. [PubMed] [Google Scholar]
- 10.Gapany C, Frey P, Cachat F, et al. Management of cryptorchidism in children: guidelines. Swiss Med Wkly. 2008;138(33–34):492–498. doi: 10.4414/smw.2008.12192. [DOI] [PubMed] [Google Scholar]
- 11.Capello SA, Giorgi LJ, Jr, Kogan BA. Orchiopexy practice patterns in New York State from 1984 to 2002. J Urol. 2006;176(3):1180–1183. doi: 10.1016/j.juro.2006.04.052. [DOI] [PubMed] [Google Scholar]
- 12.Brown JJ, Wacogne I, Fleckney S, Jones L, Ni Bhrolchain C. Achieving early surgery for undescended testes: quality improvement through a multifaceted approach to guideline implementation. Child Care Health Dev. 2004;30(2):97–102. doi: 10.1111/j.1365-2214.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- 13.Lamah M, McCaughey ES, Finlay FO, Burge DM. The ascending testis: is late orchidopexy due to failure of screening or late ascent? Pediatr Surg Int. 2001;17(5–6):421–423. doi: 10.1007/s003830000535. [DOI] [PubMed] [Google Scholar]
- 14.Guven A, Kogan BA. Undescended testis in older boys: further evidence that ascending testes are common. J Pediatr Surg. 2008;43(9):1700–1704. doi: 10.1016/j.jpedsurg.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Cendron M, Huff DS, Keating MA, Snyder HM, III, Duckett JW. Anatomical, morphological and volumetric analysis: a review of 759 cases of testicular maldescent. J Urol. 1993;149(3):570–573. doi: 10.1016/s0022-5347(17)36151-7. [DOI] [PubMed] [Google Scholar]
- 16.Kollin C, Karpe B, Hesser U, Granholm T, Ritzen EM. Surgical treatment of unilaterally undescended testes: testicular growth after randomization to orchiopexy at age 9 months or 3 years. J Urol. 2007;178(4 pt 2):1589–1593. doi: 10.1016/j.juro.2007.03.173. discussion 1593. [DOI] [PubMed] [Google Scholar]
- 17.Park KH, Lee JH, Han JJ, Lee SD, Song SY. Histological evidences suggest recommending orchiopexy within the first year of life for children with unilateral inguinal cryptorchid testis. Int J Urol. 2007;14(7):616–621. doi: 10.1111/j.1442-2042.2007.01788.x. [DOI] [PubMed] [Google Scholar]
- 18.Hadziselimovic F, Herzog B, Buser M. Development of cryptorchid testes. Eur J Pediatr. 1987;146 suppl 2:S8–S12. doi: 10.1007/BF00452859. [DOI] [PubMed] [Google Scholar]
- 19.Steckler RE, Zaontz MR, Skoog SJ, Rushton HG., Jr Cryptorchidism, pediatricians, and family practitioners: patterns of practice and referral. J Pediatr. 1995;127(6):948–951. doi: 10.1016/s0022-3476(95)70034-x. [DOI] [PubMed] [Google Scholar]
- 20.Hack WW, Meijer RW, Van Der Voort-Doedens LM, Bos SD, De Kok ME. Previous testicular position in boys referred for an undescended testis: further explanation of the late orchidopexy enigma? BJU Int. 2003;92(3):293–296. doi: 10.1046/j.1464-410x.2003.04317.x. [DOI] [PubMed] [Google Scholar]
- 21.Rusnack SL, Wu HY, Huff DS, et al. The ascending testis and the testis undescended since birth share the same histopathology. J Urol. 2002;168(6):2590–2591. doi: 10.1016/S0022-5347(05)64223-1. [DOI] [PubMed] [Google Scholar]
- 22.Barthold JS, Gonzalez R. The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. J Urol. 2003;170(6 pt 1):2396–2401. doi: 10.1097/01.ju.0000095793.04232.d8. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal PK, Diaz M, Elder JS. Retractile testis: is it really a normal variant? J Urol. 2006;175(4):1496–1499. doi: 10.1016/S0022-5347(05)00674-9. [DOI] [PubMed] [Google Scholar]
- 24.Bingöl-Koloğlu M, Tanyel FC, Anlar B, Buyukpamukcu N. Cremasteric reflex and retraction of a testis. J Pediatr Surg. 2001;36(6):863–867. doi: 10.1053/jpsu.2001.23956. [DOI] [PubMed] [Google Scholar]
- 25.Hardy BE, Shah T, Cicciarelli J, Lemley KV, Hutchinson IV, Cho YW. Kidney transplantation in children and adolescents: an analysis of United Network for Organ Sharing Database. Transplant Proc. 2009;41(5):1533–1535. doi: 10.1016/j.transproceed.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CP. Evidence of variation by race in the timing of surgery for correction of pediatric ureteropelvic junction obstruction. J Urol. 2007;178(4 pt 1):1463–1468. doi: 10.1016/j.juro.2007.05.167. discussion 1468. [DOI] [PubMed] [Google Scholar]
- 27.Skoog SJ, Belman AB. Primary vesicoureteral reflux in the black child. Pediatrics. 1991;87(4):538–543. [PubMed] [Google Scholar]