Abstract
Background
Short-term changes in levels of fine ambient particulate matter (PM2.5) may increase the risk of acute ischemic stroke, but results from prior studies have been inconsistent. We examined this hypothesis using data from a multi-center prospective stroke registry.
Methods
We analyzed data from 9,202 patients hospitalized with acute ischemic stroke, having a documented date and time of stroke onset, and residing within 50 km of a PM2.5 monitor in 8 cities in Ontario, Canada. We evaluated the risk of ischemic stroke onset associated with PM2.5 in each city using a time-stratified case-crossover design, matching on day of week and time of day. We then combined these city-specific estimates using random-effects meta-analysis techniques. We examined whether the effects of PM2.5 differed across strata defined by patient characteristics and ischemic stroke etiology.
Results
Overall, PM2.5 was associated with a −0.7% change in ischemic stroke risk per 10-μg/m3 increase in PM2.5 (95% Confidence Interval = −6.3% to 5.1%). These overall negative results were robust to a number of sensitivity analyses. Among patients with diabetes mellitus, PM2.5 was associated with an 11% increase in ischemic stroke risk (1% to 22%). The association between PM2.5 and ischemic stroke risk varied according to stroke etiology, with the strongest associations observed for strokes due to large artery atherosclerosis and small vessel occlusion.
Conclusions
These results do not support the hypothesis that short-term increases in PM2.5 levels are associated with ischemic stroke risk overall. However, specific patient subgroups may be at increased risk of particulate-related ischemic strokes.
Higher levels of daily air pollution are associated with an increased risk of acute cardiovascular events, including myocardial infarction, ventricular arrhythmias, and decompensation in patients with congestive heart failure.1–3 These effects are likely mediated by a combination of changes in autonomic nervous system activity, systemic inflammation, changes in hematologic parameters, and vascular endothelial cell injury.1
Short-term elevations in particulate matter—an important component of ambient air pollution—may also increase the short-term risk of stroke.3–5 For example, Dominici et al.3 found that a 10-μg/m3 increase in ambient fine particulate matter (PM2.5) was associated with a 0.8% increased risk of hospital admission for cerebrovascular events among Medicare beneficiaries in 204 US counties (95% confidence interval [CI] = 0.3% to 1.3%), although many other studies have failed to find such a link.6–14 Some heterogeneity among prior studies may be attributable to the broad definition of cerebrovascular disease. Few studies have specifically examined the association between particulate matter and the risk of acute ischemic stroke. The results of these more focused studies have been inconsistent, with some studies finding positive associations 15–18 and others finding no evidence of an association.19–21
Ischemic stroke is etiologically diverse with distinct mechanistic subtypes, including atherosclerosis of the large extracranial and intracranial arteries that supply the brain (termed large-artery strokes), occlusion of the small penetrating arteries of the brain due to lipohyalinosis and fibrosis (termed small-vessel strokes or lacunar infarcts), and arterial occlusions due to emboli presumed to arise in the heart (termed cardioembolic strokes).22 Given that particulate matter may have different effects on the heart (e.g. ventricular arrhythmias2) and blood vessels (e.g. endothelial function, platelet activation and blood pressure23–25), the effects of particulate matter on the risk of ischemic stroke likely differ according to the pathophysiologic mechanism of ischemic stroke. However, only one prior study20 has evaluated whether the association between particulate matter and ischemic stroke varies according to ischemic stroke etiology. Based on the putative mechanisms of particulate matter health effects, we hypothesized that short-term increases in particulate matter would preferentially increase the risk of acute stroke due to intra-vascular thrombosis compared to cardioembolism. Findings that the effects of ambient particles differ by ischemic stroke subtype could provide important insights into the mechanisms of particulate-related acute vascular events.
An important limitation common to prior studies is that pollution exposure is assessed on the date of hospitalization rather than the date and time of stroke symptom onset. We have previously shown that this approach can lead to substantial exposure misclassification and thus bias health effects estimates towards the null hypothesis of no association.26 Most prior studies are also limited because patients with ischemic stroke are identified using discharge diagnosis codes that can have a low specificity and potentially further bias health-effect estimates toward the null. Moreover, discharge diagnosis codes in administrative datasets do not distinguish between etiologically distinct subtypes of ischemic stroke. Finally, identifying subgroups of the population who may be at greatest risk of particulate-related events is an important research priority, but few prior studies have had access to quality clinical and patient information. Evaluating whether patients with diabetes mellitus may be more susceptible is of particular interest given prior findings suggesting that these patients are more susceptible to the effects of particulate matter on the risk of all-cause mortality, cerebrovascular death, and cardiovascular hospitalizations.27–29
Accordingly, our goal was to evaluate the association between short-term changes in ambient fine particulate matter (PM2.5) levels and acute ischemic stroke onset in a cohort of patients hospitalized with clinically-confirmed acute ischemic stroke, documented date and time of stroke symptom onset, and detailed information on clinical and patient characteristics. We accomplished this using data from the Registry of the Canadian Stroke Network, a prospective multi-center registry of consecutive patients presenting with acute stroke to 11 regional stroke centers in the province of Ontario, Canada.30
Methods
Population
The Registry of the Canadian Stroke Network collects detailed clinical data on consecutive patients with acute stroke and transient ischemic attack seen in the emergency department or admitted to a regional stroke center in Ontario, Canada.30 Detailed clinical information, including stroke type and clinician-reported stroke etiology based on a classification scheme developed for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST),31 is captured through review of patient medical records. Chart abstraction is performed during and after the hospital admission by trained neurology research nurses using custom software. Chart re-abstraction studies have shown excellent agreement within the Registry database with κ scores of >0.8 for key variables (age, sex, stroke type, use of thrombolysis, comorbid conditions).30 A prior history of hypertension, atrial fibrillation, diabetes mellitus and smoking is based on documentation in the patient’s chart, and does not include a new diagnosis made during incident hospitalization. As part of the standardized case report form, time of stroke symptom onset or time last seen normal is abstracted from the medical records of all patients, and reported as “exact,” “estimated” or “unknown.” For the present study, we used data from Phase 3 of the Registry of the Canadian Stroke Network and included consecutive patients with acute ischemic stroke or transient ischemic attack seen at 11 Ontario regional stroke centers between 1 July 2003 and 31 March 2008. Patients with non-stroke etiology, intracerebral hemorrhage and subarachnoid hemorrhage were excluded. Computed tomography or magnetic resonance imaging of the brain was carried out on 98% of patients.
The Registry of the Canadian Stroke Network is “prescribed” under Ontario’s Personal Health Information Protection Act, and patient data are collected without patient consent for the purpose of facilitating the provision of stroke care in the province of Ontario. Approval for the Registry of the Canadian Stroke Network was obtained from the Research Ethics Board at each participating center. The present study protocol was reviewed and approved by the Registry of the Canadian Stroke Network Publications Committee and the Institutional Review Boards at the Beth Israel Deaconess Medical Center and Brown University.
Exposure Assessment
The Ontario Ministry of the Environment operates and maintains a network of more than 140 continuous air pollution monitors at 44 sites throughout the province. We obtained PM2.5 data from 19 monitoring stations in the vicinity of the 11 regional stroke centers participating in the Registry. PM2.5 levels are measured using a Tapered Element Oscillating Microbalance (TEOM 1400AB/SES) and reported on an hourly basis. Data were available from seven PM2.5 monitors within the greater Toronto metropolitan area (Toronto, Oakville, Mississauga, and Brampton), from six monitors within the Hamilton metropolitan area (Hamilton, Burlington, St. Catherine’s, and Brantford), and from one monitor in each of the other six cities with a regional stroke center (London, Ottawa, Kingston, North Bay, Thunder Bay, and Sudbury). For each metropolitan area, we averaged the values from all available monitors for each hour of the study period. No PM2.5 data were available for Kingston in 2005 or for Sudbury in 2003, leading to these periods in these cities being excluded from analysis. Otherwise, hourly PM2.5 values were available for 96.3% of all hours.
We obtained hourly meteorologic data from the US National Weather Service (National Climatic Data Center, Global Surface Hourly database), which maintains a database of global weather data. Hourly data on ambient temperature and dew point were available from 3 stations in the greater Toronto area, 3 stations in the Hamilton metropolitan area, and 1 station in each of the other cities. For each metropolitan area, we averaged the values available from all stations by hour. Meteorologic data was missing for fewer than 2% of hours. In each metropolitan area, we calculated hourly apparent temperature (AT), an index of thermal comfort,32 as AT = −2.653 + (0.994*Ta) + (0.0153*DPT2), where Ta denotes ambient temperature and DPT denotes dew point temperature.
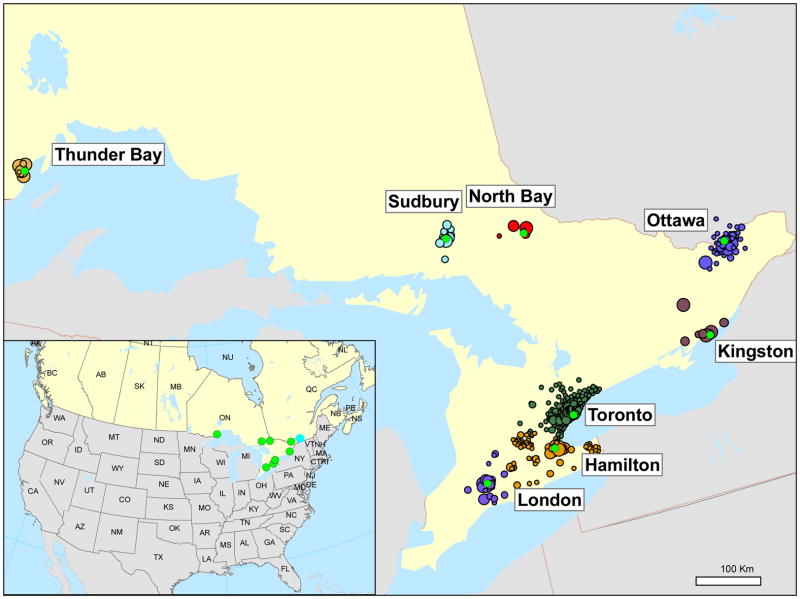
We assessed exposure to PM2.5 and meteorologic variables prior to stroke onset based on the first 3 characters of each subjects’ residential postal code (Figure 1). Specifically, each subject was matched to the metropolitan region nearest to the centroid of their residential postal code using ArcGIS (v 9.3, ESRI, Redlands, CA). The first 3 characters of postal codes tend to represent small geographic areas in the major metropolitan areas (eg: < 2 km2 in Toronto, Ottawa, and Hamilton) and larger geographic areas in smaller cities (eg: 3 to 14,000 km2 in North Bay). The average land area covered by the postal codes of patients included in this study was 124 km2. To reduce exposure misclassification, subjects residing more than 50 km from the nearest PM2.5 monitor were excluded from analysis. Moving averages of PM2.5, ambient temperature, and dew point temperature were calculated over periods of 24, 48, 72, 96, 120, 144, and 168 hrs. Moving averages were calculated when hourly data were available for at least 75% of the hours included in the moving average, and set to missing otherwise.
Figure 1.
Map of study area. Red circles represent the center of each metropolitan area. Colored circles represent the centroid of postal code of subjects’ residences with the diameter proportional to the number of participants living in each postal code. Circles of different colors are used to show the clustering of participants around each metropolitan area.
Statistical Analysis
We used a two-stage hierarchical model to evaluate the association between ambient PM2.5 and the risk of acute ischemic stroke, as previously described.17 In the first stage, we used the time-stratified case-crossover study design33 to separately estimate the effect of air pollution in each city. In this design, each subject’s exposure prior to a case-defining event (case period) is compared with his or her own exposure experience during one or more control periods when the subject did not become a case (control period). Control periods were chosen such that exposures during the case period were compared to exposures occurring on other days of the same month falling on the same day of the week and time of day as the case period. This design has been shown to be effective in controlling for seasonality, time-trends, and chronic and slowly-varying potential confounders.34
We performed conditional logistic regression, stratifying on each patient, to obtain estimates of odds ratios (ORs) and 95% confidence intervals. We controlled for temperature and dew point in all models. We modeled PM2.5, mean ambient temperature, and mean dew point temperature as linear continuous variables. In the second stage, we obtained a combined estimate from the city-specific effect estimates using standard random-effects meta-analysis methods.35 For comparability with prior studies, we report effect estimates as percent change in risk associated with a 10 μg/m3 increase in PM2.5.
In the primary analysis we evaluated the association between mean PM2.5 levels 0–47 hours prior to symptom onset and the risk of acute ischemic stroke among all subjects residing within 50 km from a PM2.5 monitor. Our decision to use the 0–47-hr moving average was made a priori based on published studies suggesting an increased risk of stroke within two days of increased PM2.5 levels.3,16–18 However, in sensitivity analyses we additionally considered moving averages of PM2.5 ranging from 24 hrs to 7 days, in 24-hour increments. We performed several additional sensitivity analyses to verify the robustness of our findings. First, to evaluate the potential effects of exposure misclassification, we repeated the main analysis alternately excluding patients with a residential postal code more than 40 or more than 20 km from the nearest ambient PM2.5 monitor, patients with “estimated” stroke onset times, patients presenting more than 48 hours after stroke symptom onset, patients presenting more than 12 hours after stroke symptom onset, and patients in whom symptoms were discovered on awakening. Second, we repeated the main analysis also including patients with transient ischemic attacks. Third, because the association between ambient temperature and the risk of stroke onset may be nonlinear, we entered temperature into models using linear and quadratic terms. Fourth, we repeated the main analysis adjusting for apparent temperature instead of ambient temperature and dew point. Finally, we evaluated the effects of PM2.5 separately in the warm (April-September) and cool seasons (October-March).
We hypothesized a priori that the effects of PM2.5 would differ by reported ischemic stroke etiology as classified by standard criteria.31 Those five classifications of ischemic stroke are: 1) large artery atherosclerosis, 2) small vessel occlusion, 3) cardioembolism, 4) stroke of other determined etiology, and 5) stroke of undetermined etiology. Strokes of other determined etiology and strokes of undetermined etiology were combined into a single stratum and referred to as “other strokes.” We estimated the association between PM2.5 levels and ischemic stroke onset separately for each of the 4 stroke classifications. In a post-hoc analysis, we compared the association between PM2.5 levels and ischemic stroke onset among participants with cardioembolic strokes versus all other strokes.
We hypothesized a priori that the association between PM2.5 and the risk of ischemic stroke onset would be more pronounced among participants with a medical history of diabetes mellitus. We evaluated whether the association between PM2.5 and ischemic stroke risk differed in patients with and without a medical history of diabetes mellitus, atrial fibrillation, hypertension, prior stroke or transient ischemic attack, and smoking by including interaction terms in the first-stage city-specific regression models.
Due to small numbers of cases in Sudbury, Thunder Bay, North Bay, and Kingston, we excluded these cities from subgroup analyses. We used the Wald test for homogeneity36 to evaluate whether associations differ across subgroups and denote the p-value from this test as ph. All reported p-values are based on two-sided tests at the α=0.05 level. Analyses were performed using SAS V9 (SAS Institute Inc., Cary, NC) and the R statistical package (R v 2.8.1 and rmeta v 2.15).37
Results
Between 1 July 2003 and 31 March 2008, 9,202 consecutive patients residing within 50 km of an ambient PM2.5 monitor were admitted to hospital or presented to an emergency department within the study catchment area with acute ischemic stroke (Figure 1). The mean age of patients was 72.5 years (SD = 13.8) and 49% were women. Other patient characteristics are presented in Table 1. More than 81% of eligible subjects resided in the metropolitan areas surrounding Toronto, Hamilton, Ottawa, or London.
Table 1.
Characteristics of 9202 patients hospitalized or presenting to the emergency department with acute ischemic stroke in the province of Ontario, Canada, overall and by ischemic stroke subtype.
| All (n = 9202) | Cardioembolic (n = 2232) | Large Artery (n = 1345) | Lacunar (n = 1585) | Other/Multiple/Undetermined (n = 4040) | |
|---|---|---|---|---|---|
| Women; no. (%) | 4492 (49) | 1237 (55) | 516 (38) | 739 (47) | 2000 (50) |
| Age (years); mean (SD) | 72.5 (13.8) | 75.6 (13.0) | 72.4 (11.5) | 71.4 (12.3) | 71.3 (15.0) |
| Medical History; no. (%) | |||||
| Diabetes | 2270 (25) | 461 (21) | 351 (26) | 467 (29) | 991 (25) |
| Hypertension | 6333 (69) | 1536 (69) | 991 (74) | 1166 (74) | 2640 (65) |
| Hyperlipidemia | 3374 (37) | 781 (35) | 573 (43) | 580 (37) | 1440 (36) |
| Atrial Fibrillation | 1726 (19) | 1146 (51) | 81 (6) | 77 (5) | 422 (10) |
| Prior Stroke | 2176 (24) | 531 (24) | 369 (27) | 327 (21) | 949 (23) |
| Prior Myocardial Infarction | 1392 (15) | 425 (19) | 224 (17) | 166 (10) | 577 (14) |
| Smoking; no. (%) | |||||
| Current | 1714 (19) | 262 (12) | 335 (25) | 347 (22) | 770 (19) |
| Past | 3578 (39) | 711 (32) | 668 (50) | 709 (45) | 1490 (37) |
PM2.5 levels varied substantially among cities and from day to day within cities (Table 2). Across the 8 metropolitan regions, the mean daily PM2.5 level was 6.9 μg/m3 (SD = 6.3), and exceeded the 24-hr Canada-wide standard of 30 μg/m3 on 163 days. Mean daily ambient temperature ranged from −32°C to 30°C, with an average of −2°C (SD = 8°C) from October to March and an average of 16°C (SD = 6°C) from April to September. PM2.5 levels averaged over 48 hours were moderately correlated with temperature (r = 0.44) and dew point (r = 0.45).
Table 2.
Summary of PM2.5 levels 1 July 2003 – 31 March 2008, by metropolitan region and overall.a
| City | Ischemic Strokes No. | Daily PM2.5 (μg/m3)
|
48 hour moving averages of PM2.5 (μg/m3)
|
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | IQR | Mean | SD | IQR | ||
| Toronto | 3829 | 8.0 | 6.9 | 6.5 | 8.0 | 6.1 | 5.9 |
| Hamilton | 1330 | 8.4 | 6.9 | 7.0 | 8.4 | 6.2 | 6.3 |
| Ottawa | 1258 | 6.6 | 6.0 | 5.9 | 6.5 | 5.1 | 5.2 |
| London | 1114 | 9.5 | 7.0 | 6.8 | 9.5 | 6.3 | 6.3 |
| Kingston | 603 | 7.5 | 6.4 | 6.4 | 7.5 | 5.6 | 5.7 |
| Thunder Bay | 480 | 4.7 | 3.9 | 4.0 | 4.7 | 3.4 | 3.5 |
| Sudbury | 423 | 4.7 | 5.0 | 4.2 | 4.7 | 4.5 | 3.8 |
| North Bay | 165 | 5.0 | 5.1 | 4.5 | 5.0 | 4.5 | 4.0 |
| Combined | 9202 | 6.9 | 6.3 | 6.1 | 6.8 | 5.6 | 5.6 |
The Canada Wide Standard for 24-hr PM2.5 is 30 μg/m3.
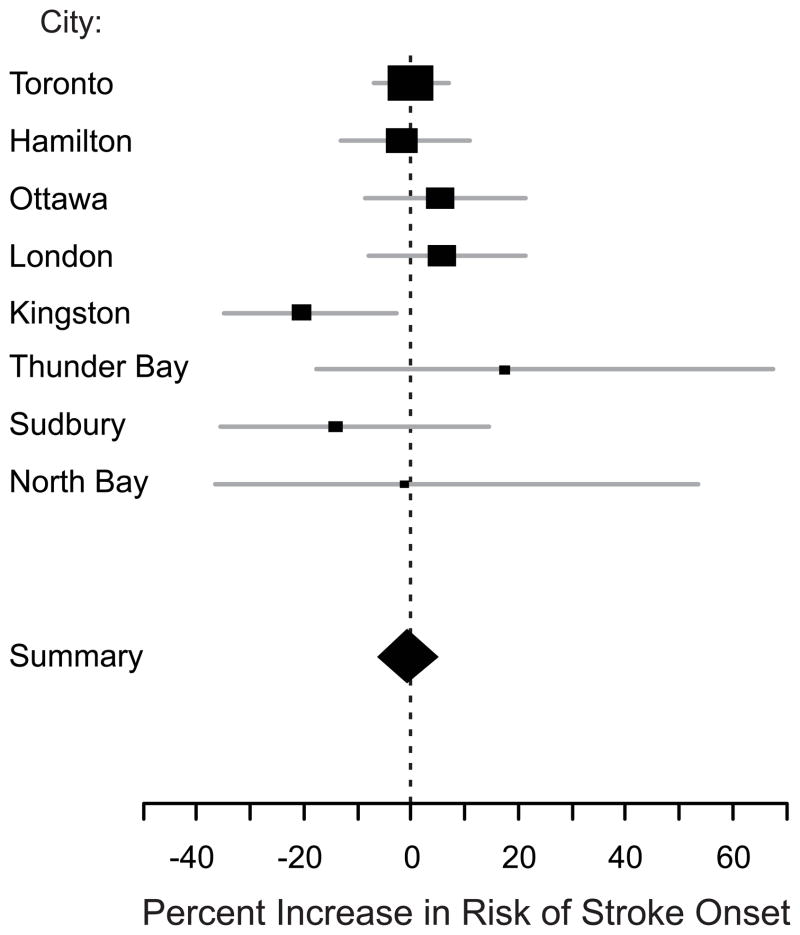
Overall, there was no association between the onset of ischemic stroke symptoms and PM2.5 levels 0–47 hr earlier (Figure 2). This finding was not materially altered by excluding patients living more than 40 km or more than 20 km from the nearest ambient PM2.5 monitor, those with “estimated” rather than “exact” stroke symptom onset times, those discovered with symptoms on awakening, or those presenting more than 48 or more than 12 hours after stroke symptom onset (Table 3). Results were also similar when we included subjects hospitalized with transient ischemic attack. In additional sensitivity analyses, we considered the effects of PM2.5 levels averaged over longer periods and found an inverse association between PM2.5 and ischemic stroke onset that peaked when PM2.5 levels 0–95 h prior to stroke onset were considered. Results were not materially different if only those subjects living in the 4 largest cities were considered.
Figure 2.
Percent increase in risk and 95% confidence interval of acute ischemic stroke associated with a 10-μg/m3 increase in mean PM2.5 levels 0–47 hr prior to stroke symptom onset (48-hr moving average). City-specific (squares, with height inversely proportional to variance of estimate; horizontal lines indicate 95% CI) and the random-effects summary estimate (diamond; horizontal limits indicate 95% CI) are shown.
Table 3.
Results of sensitivity analyses of the association between PM2.5 levels and the risk of stroke symptom onset.
| Percent Increase in Risk (95% CI)a |
||
|---|---|---|
| All Subjects | Subjects Residing in 4 Largest Citiesb | |
| Primary Analysis (48-hr moving average) | −0.7 (−6.3 to 5.1) | 1.1 (−4.0 to 6.5) |
| Sensitivity Analyses (48-hr moving average) | ||
| Subjects with “exact” stroke symptom times only | −0.3 (−9.8 to 10.1) | 1.2 (−5.4 to 8.2) |
| Subject residing ≤40 km of monitor only | −1.0 (−6.4 to 4.7) | 0.8 (−4.4 to 6.3) |
| Subject residing ≤20 km of monitor only | −1.8 (−7.4 to 4.0) | −0.5 (−5.9 to 5.1) |
| Subjects presenting ≤48 h after stroke symptom onset only | −0.9 (−6.7 to 5.3) | 1.0 (−4.2 to 6.5) |
| Subjects presenting ≤ 12 h after stroke symptom onset only | −1.0 (−8.0 to 6.5) | 0.5 (−5.4 to 6.7) |
| Subjects whose symptoms were not discovered on awakening only | 1.5 (−9.0 to 13.4) | 3.7 (−2.5 to 10.3) |
| Subjects with transient ischemic attacks also included | 0.0 (−5.9 to 6.1) | 1.6 (−3.5 to 6.9) |
| Adjusted for temperature modeled as a quadratic function | 0.4 (−5.1 to 6.1) | 0.9 (−5.0 to 7.0) |
| Adjusted for apparent temperature | 0.4 (−5.3 to 6.5) | 1.7 (−3.8 to 7.5) |
| Warm season only (April – September) | 0.2 (−6.4 to 7.3) | 0.4 (−6.7 to 8.1) |
| Cool season only (October – March) | −2.8 (−13.3 to 9.0) | 2.5 (−5.8 to 11.5) |
| Other PM2.5 Averaging Times | ||
| 24 h | −1.3 (−7.0 to 4.8) | −0.4 (−4.8 to 4.2) |
| 72 h | −5.1 (−11.3 to 1.5) | −2.8 (−8.3 to 3.1) |
| 96 h | −6.5 (−12.4 to −0.1) | −4.2 (−10.3 to 2.2) |
| 120 h | −4.0 (−10.1 to 2.6) | −2.0 (−8.6 to 5.2) |
| 144 h | −2.3 (−9.4 to 5.3) | 0.2 (−7.1 to 8.1) |
| 168 h | −0.2 (−8.0 to 8.3) | 2.3 (−5.7 to 11.0) |
per 10 μg/m3 increase in PM2.5.
Toronto, Hamilton, Ottawa, and London, Ontario.
Because ambient temperature has the potential to be an important confounder of the association between PM2.5 and stroke, we considered the effects of temperature in more detail. A 5°C increase in mean ambient temperature 0–47 hr preceding event onset was associated with a 12% decrease in risk of ischemic stroke onset during the cool season (95% CI = −2% to 24%) and a 7% decrease in risk during the warm season (−2% to 15%). We verified the assumption that the association between temperature and stroke onset was linear by modeling temperature as a quadratic function; the relationship was monotonic and approximately linear over a wide range of temperatures. The association between PM2.5 and ischemic stroke onset was not materially different in the warm and cool seasons, nor were the results for PM2.5 materially different when ambient temperature was modeled as a quadratic function (Table 3). Finally, adjusting for apparent temperature – an index of thermal comfort calculated from temperature and dew point – did not materially change the results.
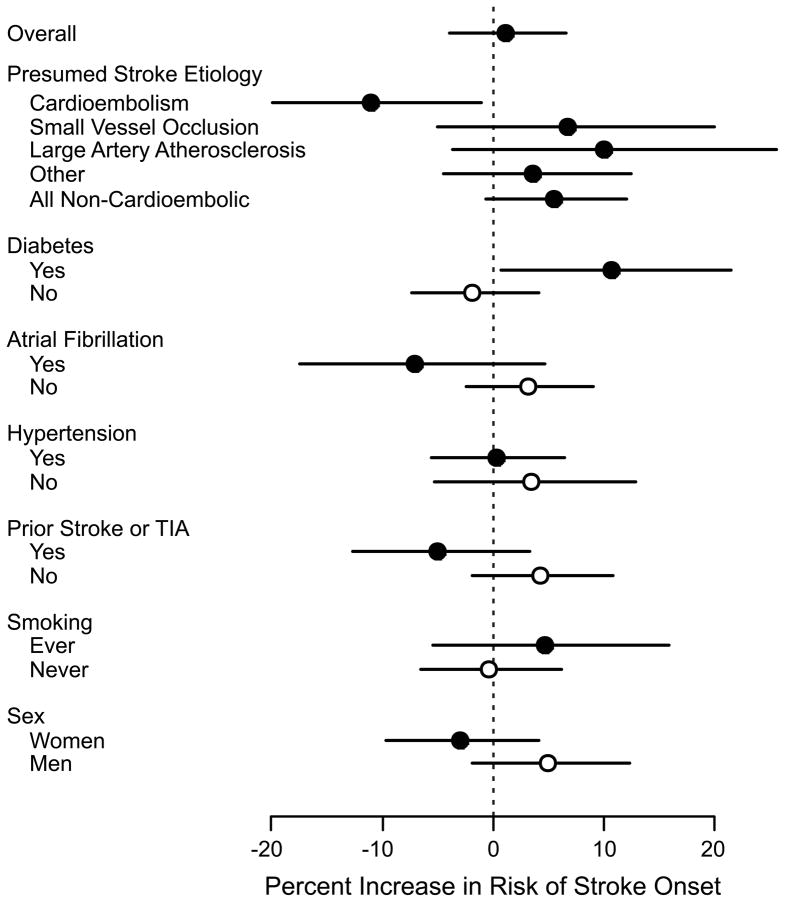
The effects of PM2.5 on stroke symptom onset differed across presumed ischemic stroke subtypes (Figure 3; eTable). Specifically, PM2.5 was positively associated with strokes due to small-vessel occlusion or large-artery atherosclerosis and inversely associated with strokes due to cardioembolism. In a post-hoc analysis, we found that among all patients with non-cardioembolic ischemic strokes (including strokes due to small-vessel occlusion, large-artery atherosclerosis, and ischemic strokes of other, multiple, or undetermined etiologies), a 10 μg/m3 increase in PM2.5 was associated with a 5.5% increased risk of stroke onset (95% CI = −0.6% to 12.0%), in sharp contrast to the inverse association observed for strokes due to cardioembolism.
Figure 3.
Percent increase in risk and 95% confidence interval of acute ischemic stroke associated with a 10-μg/m3 increase in the 48 hr moving average of PM2.5, overall and stratified by ischemic stroke subtype, by past medical history of diabetes, atrial fibrillation, hypertension, stroke or transient ischemic attack, and by smoking. Note that only subjects from the largest 4 metropolitan areas (Toronto, Hamilton, London, Ottawa) were included in these analyses.
PM2.5 was positively associated with the risk of ischemic stroke onset among patients with a medical history of diabetes mellitus (excess relative risk per 10-μg/m3 increase in PM2.5= 10.6% [95% CI = 0.8% to 21.5%]), while no association was observed among patients without such history (−1.8% [−7.4% to 4.1%]). Small positive associations were observed between PM2.5 levels and the risk of ischemic stroke onset among men and among subjects with a past medical history of atrial fibrillation or stroke/transient ischemic attack (Figure 3). There was no evidence that the effect of PM2.5 on stroke symptom onset was affected by a history of hypertension or smoking.
Discussion
In a large prospective multi-center stroke registry, we found no evidence of an association between short-term variability of PM2.5 levels and onset of ischemic stroke in the population overall. However, PM2.5 was associated with increased risk of ischemic stroke onset among patients with a history of diabetes mellitus and possibly those presenting with ischemic strokes not due to cardioembolism.
Prior studies suggest that a 10-μg/m3 increase in PM2.5 levels may be associated with a 1% to 5% increase in the risk of cerebrovascular events.3,5,18 Given the substantial reductions in misclassification of both exposure and outcome possible in the current study, we hypothesized that, if a similar association were present in Ontario, we would observe stronger associations between PM2.5 and ischemic stroke risk than previously observed. Although the results of the current study do not support this hypothesis, we are not able to exclude the possibility of a small 18 positive association between PM2.5 and ischemic stroke risk as suggested by some prior studies. Differences in population characteristics or particulate matter sources, composition, absolute levels or daily variability likely also contribute to the heterogeneity observed across studies. Of 3,18 note, PM2.5 levels in this study were similar to those observed in previous studies in the US, but substantially lower than those typically observed in studies in Asia.5
We are not aware of prior studies identifying patient characteristics that may alter the association between PM2.5 and ischemic stroke. However, our finding that a history of diabetes mellitus alters the effects of PM2.5 is consistent with evidence from prior studies suggesting that patients with diabetes may be more prone to particulate-related acute vascular events29 and changes in vascular function.23 A number of vascular mechanisms (including increased inflammation, vascular reactivity and endothelial dysfunction38) may account for this apparent enhanced vulnerability to the effects of PM2.5, although this remains an active area of investigation. If causal, our results do not necessarily imply that only patients with diabetes are at risk of particulate-related ischemic strokes; rather, they may be more susceptible to these effects in comparison to subjects without diabetes. We did not find strong evidence suggesting that sex or a history of other major stroke risk factors materially alters the association between PM2.5 and ischemic stroke onset. Additional studies are needed to confirm or refute these findings.
Differences between the pathogenesis of intra-arterial and intra-cardiac causes of ischemic stroke may explain the apparently diverse effects of PM2.5 by ischemic stroke etiology. Large-artery stroke usually results from the abrupt rupture of an unstable atherosclerotic plaque with thromboembolism, while small-vessel stroke is believed to result from progressive lipohyalinosis with in-situ thrombosis as an acute terminal event. In contrast, atrial fibrillation, the most common cause of cardioembolism, represents a more chronic model of thrombus formation similar to venous thromboembolism.39 Given the documented adverse effects of PM2.5 on endothelial function, platelet activation and hemodynamics,23–25 we hypothesized that short-term increases in particulate matter would preferentially increase the risk of acute stroke due to intra-vascular thrombosis compared with cardioembolism, as observed in this study. However, our results are in contrast to those from the Dijon Stroke Register in France showing that particulate matter was most strongly associated with strokes due to cardioembolism.20
There is some suggestion that certain ambient pollutants may increase the risk of atrial fibrillation.40–41 However, even if such an association exists for PM2.5, it is likely that the time interval between atrial fibrillation onset and stroke onset would be longer and more variable than the interval between intravascular thrombosis and ischemic stroke onset. Atrial fibrillation increases the risk of cardioembolism through atrial thrombus formation, mainly in the left atrial appendage. However, cardioembolism in patients with paroxysmal atrial fibrillation is most likely to occur following restoration of atrial mechanical function, which can be delayed relative to restoration of sinus rhythm by days or weeks.42–43 Indeed, these factors might paradoxically invert the association between PM2.5 and the onset of cardioembolic stroke as observed in the current study. Since a substantial proportion of all strokes are due to cardioembolism, these factors may be responsible for the more pronounced inverse association we observed at longer lags. These observations highlight the complex relationship between changes in PM2.5 and risk of stroke, and underscore the importance of additional research on the time course, direction and mechanisms of association between PM2.5 and specific stroke subtypes.
As noted above, it is plausible that variations in the distributions of patient and clinical characteristics may explain some of the heterogeneity observed in previous studies. For example, based on the results of the current study, prior studies from areas where cardioembolism is a more prevalent mechanism of ischemic stroke would find a smaller relative risk as compared with studies from areas where this mechanism is less common. There is some indirect evidence to support this assertion. Studies conducted in Asia, where cardioembolism is less common than in North America or Europe,44 have reported some of the strongest associations between particulate matter and ischemic stroke risk.16 Conversely, studies in areas where diabetes is more prevalent would be expected to find stronger associations between particulate matter exposure and ischemic stroke risk. Other factors, such as differences in sources or constituents of particulate matter, are also likely to contribute to heterogeneity in study findings. Nonetheless, our results underscore the importance in future studies of determining ischemic stroke subtypes, as well as patient and clinical characteristics.
Our finding that temperature was inversely associated with the risk of ischemic stroke onset in the cool months is consistent with the documented winter-time peak in stroke incidence and mortality45–47 and with prior studies directly examining the association between temperature and stroke.48 However, our finding of an inverse association between temperature and stroke onset in warmer months appears surprising, given the well-documented associations of excessive heat with cardiovascular and cerebrovascular mortality.49 However, some prior studies have reported inverse associations between temperature and cerebrovascular hospitalizations generally50 and ischemic stroke hospitalizations specifically.48 One possible explanation for this discrepancy is that cerebrovascular events occurring during periods of high temperatures may be more likely to be fatal, or more rapidly fatal, versus events occurring during periods of lower temperature.50 Studies with data on both fatal and non-fatal events would be necessary to evaluate this hypothesis.
Our study has several important limitations. First, the sample size is small compared with previous studies using administrative data, and, therefore, we may have failed to detect more modest health effects of the magnitude that we17 and others3 have previously found in the US Medicare population. Second, our exposure assessment strategy was based on residential postal code rather than actual addresses, potentially contributing to exposure misclassification that would be expected to bias our results toward the null. Because only Toronto and Hamilton had more than one PM2.5 monitor during this time period, opportunities to implement more-refined exposure assessments were limited. Third, a single stroke etiology was not identified in a large fraction of patients, consistent with previous studies of patients receiving routine clinical management.51 Stroke etiology may remain undetermined when patients die before the diagnostic workup is completed, when the cause is transient (eg: paroxysmal atrial fibrillation), or when a single cause is not identified with routine clinical tests. We expect that our classification of stroke etiology is highly specific, but not highly sensitive, leading to unbiased but less precise estimates of health effects within etiologic subgroups than might have been possible had more extensive clinical workup been undertaken. Fourth, our results may not be applicable to populations outside of North America where the composition, sources, and characteristics of particulate matter and distribution of ischemic stroke risk factors and subtypes may differ markedly.
On the other hand, this study offers several strengths in comparison with previous studies. Most patients enrolled in the Registry of the Canadian Stroke Network are seen by a stroke specialist, and almost all patients undergo neuroimaging. Accordingly, the diagnosis of ischemic stroke in the current study is expected to be much less prone to misclassification than in most prior studies in this area. Additionally, because patient and clinical characteristics were abstracted directly from patient records, the clinical characteristics evaluated in this study are also less likely to be misclassified than previous studies using administrative records. Indeed, it has not been possible to evaluate the effects of particulate matter by ischemic stroke subtype using administrative data. Finally, the time of stroke symptom onset was available for more than 80% of patients in the Registry, thereby substantially reducing exposure misclassification. We have previously shown that using the date of hospitalization rather than date and time of stroke symptom onset may bias health effects estimates towards the null by as much as 60%.26
It should be noted that time of symptom onset in the Registry is assessed as part of standard clinical care rather than for research purposes. Because thrombolysis is a viable treatment for acute stroke only among patients presenting within hours of stroke symptom onset, precise determination of stroke onset times beyond 12 hours is generally of less clinical interest. Accordingly, among Registry patients with documented stroke onset times, 89% had onset times within 12 hours of hospital presentation and 99% had onset times within 48 hours of hospital presentation. Thus, while the Registry provides a unique opportunity to evaluate the adverse effects of ambient air pollution with high quality clinical data, the results may not be generalizable to a more heterogeneous population of stroke patients.
In conclusion, data from a prospective, multi-center study suggest that short-term elevations of ambient fine particulate matter are associated with increased risk of ischemic stroke onset among patients with diabetes mellitus and those hospitalized with non-cardioembolic strokes. Additional research is required to confirm these findings in larger cohorts of patients with detailed information on stroke risk factors and presumed stroke etiology.
Supplementary Material
Acknowledgments
Financial Support:
The Registry of the Canadian Stroke Network is funded by grants from the Canadian Stroke Network and the Ontario Ministry of Health and Long-term Care, and is based at the Institute for Clinical Evaluative Sciences, which is also supported by an operating grant from the Ontario Ministry of Health and Long-Term Care. Dr. Moira Kapral is supported by the Canadian Stroke Network, the University Health Network Women’s Health Program and holds a New Investigator Award from the Canadian Institutes of Health Research. The project described was supported by grants ES015774, ES017125, and ES009825 from the National Institute of Environmental Health Sciences (NIEHS). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official vies of the sponsoring institutions.
Contributor Information
Martin J. O’Donnell, National University of Ireland, Galway, Ireland
Jiming Fang, Institute for Clinical Evaluative Sciences, Toronto, ON.
Murray A. Mittleman, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA
Moira K. Kapral, University of Toronto and Institute for Clinical Evaluative Sciences, Toronto, ON
Gregory A. Wellenius, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA and Center for Environmental Health and Technology, Brown University, Providence, RI
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 2.Rich DQ, Kim MH, Turner JR, Mittleman MA, Schwartz J, Catalano PJ, Dockery DW. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occup Environ Med. 2006;63(9):591–6. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wordley J, Walters S, Ayres JG. Short term variations in hospital admissions and mortality and particulate air pollution. Occup Environ Med. 1997;54(2):108–16. doi: 10.1136/oem.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan CC, Chuang KJ, Chien LC, Chen WJ, Chang WT. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur Heart J. 2006;27(10):1238–44. doi: 10.1093/eurheartj/ehi835. [DOI] [PubMed] [Google Scholar]
- 6.Wong TW, Lau TS, Yu TS, Neller A, Wong SL, Tam W, Pang SW. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup Environ Med. 1999;56(10):679–83. doi: 10.1136/oem.56.10.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, Simpson RW. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. 2006;114(7):1018–23. doi: 10.1289/ehp.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulphate. Occup Environ Med. 2001;58(8):504–10. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Simpson R, Corbett S. Associations between ambient air pollution and daily emergency department attendances for cardiovascular disease in the elderly (65+ years), Sydney, Australia. J Expo Sci Environ Epidemiol. 2006;16(3):225–37. doi: 10.1038/sj.jea.7500451. [DOI] [PubMed] [Google Scholar]
- 10.Halonen JI, Lanki T, Yli-Tuomi T, Tiittanen P, Kulmala M, Pekkanen J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology. 2009;20(1):143–53. doi: 10.1097/EDE.0b013e31818c7237. [DOI] [PubMed] [Google Scholar]
- 11.Burnett RT, Smith-Doiron M, Stieb D, Cakmak S, Brook JR. Effects of particulate and gaseous air pollution on cardiorespiratory hospitalizations. Arch Environ Health. 1999;54(2):130–9. doi: 10.1080/00039899909602248. [DOI] [PubMed] [Google Scholar]
- 12.Le Tertre A, Medina S, Samoli E, Forsberg B, Michelozzi P, Boumghar A, Vonk JM, Bellini A, Atkinson R, Ayres JG, Sunyer J, Schwartz J, Katsouyanni K. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health. 2002;56(10):773–9. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrieu S, Jusot JF, Blanchard M, Prouvost H, Declercq C, Fabre P, Pascal L, Tertre AL, Wagner V, Riviere S, Chardon B, Borrelli D, Cassadou S, Eilstein D, Lefranc A. Short term effects of air pollution on hospitalizations for cardiovascular diseases in eight French cities: the PSAS program. Sci Total Environ. 2007;387(1–3):105–12. doi: 10.1016/j.scitotenv.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Sunyer J, Ballester F, Tertre AL, Atkinson R, Ayres JG, Forastiere F, Forsberg B, Vonk JM, Bisanti L, Tenias JM, Medina S, Schwartz J, Katsouyanni K. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study) Eur Heart J. 2003;24(8):752–60. doi: 10.1016/s0195-668x(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 15.Linn WS, Szlachcic Y, Gong H, Jr, Kinney PL, Berhane KT. Air pollution and daily hospital admissions in metropolitan Los Angeles. Environ Health Perspect. 2000;108(5):427–434. doi: 10.1289/ehp.00108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai SS, Goggins WB, Chiu HF, Yang CY. Evidence for an Association Between Air Pollution and Daily Stroke Admissions in Kaohsiung, Taiwan. Stroke. 2003;34:2612–2616. doi: 10.1161/01.STR.0000095564.33543.64. [DOI] [PubMed] [Google Scholar]
- 17.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36(12):2549–53. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- 18.Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, Smith MA, Morgenstern LB. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64(1):53–9. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21(9):689–700. doi: 10.1007/s10654-006-9050-9. [DOI] [PubMed] [Google Scholar]
- 20.Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med. 2007;64(7):439–45. doi: 10.1136/oem.2006.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szyszkowicz M. Ambient air pollution and daily emergency department visits for ischemic stroke in Edmonton, Canada. Int J Occup Med Environ Health. 2008;21(4):295–300. doi: 10.2478/v10001-008-0029-5. [DOI] [PubMed] [Google Scholar]
- 22.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet. 2003;362(9391):1211–24. doi: 10.1016/S0140-6736(03)14544-8. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 24.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110(15):2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 25.Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116(7):898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lokken RP, Wellenius GA, Coull BA, Burger MR, Schlaug G, Suh HH, Mittleman MA. Air pollution and risk of stroke: underestimation of effect due to misclassification of time of event onset. Epidemiology. 2009;20(1):137–42. doi: 10.1097/ede.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15(2):143–9. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- 28.Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163(9):849–59. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- 29.Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13(5):588–92. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Kapral MK, Silver FL, Richards JA, Lindsay MP, Fang J, Shi S, Hill MD, Phillips SJ, Robertson A, Tu JV. Progress Report 2001–2005. Toronto: Institute for Clinical Evaluative Sciences; 2005. Registry of the Canadian Stroke Network. [Google Scholar]
- 31.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 32.Kalkstein L, Valimon K. An evaluation of summer discomfort in the United States using a relative climatological index. Bulletin Am Meterological Soc. 1986;67:842–848. [Google Scholar]
- 33.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12(2):186–92. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J, Zanobetti A, Bateson TF. Revised Analyses of Time-Series Studies of Air Pollution and Health. Special Report. Boston: Health Effects Institute; 2003. Morbidity and mortality among elderly residents in cities with daily PM measurements; pp. 25–58. [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Rothman KJ, Greenland S. Modern epidemiology. 2. Philadelphia: Lippincott- Raven; 1998. [Google Scholar]
- 37.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 38.O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton E, Schwartz J. Air pollution and inflammation in type 2 diabetes: A mechanism for susceptibility. Occup Environ Med. 2006 doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373(9658):155–66. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 40.Brauer M, Ebelt ST, Fisher TV, Brumm J, Petkau AJ, Vedal S. Exposure of chronic obstructive pulmonary disease patients to particles: respiratory and cardiovascular health effects. J Expo Anal Environ Epidemiol. 2001;11(6):490–500. doi: 10.1038/sj.jea.7500195. [DOI] [PubMed] [Google Scholar]
- 41.Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalano PJ, Speizer FE, Gold DR, Dockery DW. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114(1):120–3. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, Munson JT, Douglas PS. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23(7):1535–40. doi: 10.1016/0735-1097(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 43.Shite J, Yokota Y, Yokoyama M. Heterogeneity and time course of improvement in cardiac function after cardioversion of chronic atrial fibrillation: assessment of serial echocardiographic indices. Br Heart J. 1993;70(2):154–9. doi: 10.1136/hrt.70.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, Xie JX, Warlow C, Peto R. Indications for early aspirin use in acute ischemic stroke: A combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31(6):1240–9. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 45.Ricci S, Celani MG, Vitali R, La Rosa F, Righetti E, Duca E. Diurnal and seasonal variations in the occurrence of stroke: a community-based study. Neuroepidemiology. 1992;11(2):59–64. doi: 10.1159/000110913. [DOI] [PubMed] [Google Scholar]
- 46.Myint PK, Vowler SL, Woodhouse PR, Redmayne O, Fulcher RA. Winter excess in hospital admissions, in-patient mortality and length of acute hospital stay in stroke: a hospital database study over six seasonal years in Norfolk, UK. Neuroepidemiology. 2007;28(2):79–85. doi: 10.1159/000098550. [DOI] [PubMed] [Google Scholar]
- 47.Lanska DJ, Hoffmann RG. Seasonal variation in stroke mortality rates. Neurology. 1999;52(5):984–90. doi: 10.1212/wnl.52.5.984. [DOI] [PubMed] [Google Scholar]
- 48.Hong YC, Rha JH, Lee JT, Ha EH, Kwon HJ, Kim H. Ischemic stroke associated with decrease in temperature. Epidemiology. 2003;14(4):473–8. doi: 10.1097/01.ede.0000078420.82023.e3. [DOI] [PubMed] [Google Scholar]
- 49.Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michelozzi P, Accetta G, De Sario M, D’Ippoliti D, Marino C, Baccini M, Biggeri A, Anderson HR, Katsouyanni K, Ballester F, Bisanti L, Cadum E, Forsberg B, Forastiere F, Goodman PG, Hojs A, Kirchmayer U, Medina S, Paldy A, Schindler C, Sunyer J, Perucci CA. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med. 2009;179(5):383–9. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 51.Guercini F, Acciarresi M, Agnelli G, Paciaroni M. Cryptogenic stroke: time to determine aetiology. J Thromb Haemost. 2008;6(4):549–54. doi: 10.1111/j.1538-7836.2008.02903.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.