Abstract
Childhood obesity has become an epidemic on a worldwide scale. This article gives an overview of the progress made in childhood and adolescent obesity research in the last decade, with a particular emphasis on the transdisciplinary and complex nature of the problem. The following topics are addressed: 1) current definitions of childhood and adolescent overweight and obesity; 2) demography of childhood and adolescent obesity both in the US and globally; 3) current topics in the physiology of fat and obesity; 4) psychosocial correlates of childhood and adolescent overweight and obesity; 5) the three major obesity-related behaviors, i.e. dietary intake, physical activity and sleep; 6) genes components of childhood and adolescent obesity; 7) environment and childhood and adolescent obesity; and 8) progress in interventions to prevent and treat childhood obesity. The article concludes with recommendations for future research, including the need for large-scale, high dose and long-term interventions that take into account the complex nature of the problem.
Introduction
More than a decade ago, the World Health Organization (1997) declared obesity to be a global epidemic and proposed a set of strategies to prevent further rises in obesity rates. In 2001 the United States Surgeon General published a call to action to prevent and decrease overweight and obesity (U.S. Department of Health and Human Services, 2001). However, obesity prevalence has continued to rise exponentially in youth as well as adults. Childhood (or childhood and adolescent) obesity has been related to a host of adverse proximal and distal health outcomes, including high cholesterol and triglycerides, hypertension (Freedman, Dietz, Srinivasan, & Berenson, 1999), insulin resistance (Shaibi & Goran, 2008), type 2 diabetes (Pinhas-Hamiel & Zeitler, 1996), the metabolic syndrome, polycystic ovarian syndrome, nonalcoholic fatty liver disease (Cruz, et al., 2005) as well as breast, colorectal and some other cancers (Calle & Kaaks, 2004).
Childhood and adolescent obesity is a strong predictor of adult obesity. One study in a predominantly white US population found that in children 10 to 15 years-old, 80% of obese youth were obese by age 25 (Whitaker, Wright, Pepe, Seidel, & Dietz, 1997). In a recent review that included 25 longitudinal studies from around the world, each and every study showed that overweight and obese youth were at significantly increased risk of becoming overweight adults (Singh, Mulder, Twisk, van Mechelen, & Chinapaw, 2008). Evidence is convincing that childhood obesity is not a transient developmental phenomenon, but one that sustains adverse consequences over the entire lifespan.
The rise in childhood and adolescent obesity is not only deleterious to individual health, but comes at increasing cost to the public. Between 1998 and 2006 the medical burden of obesity increased from 6.5 percent to 9.1 percent of annual medical spending (Finkelstein, Trogdon, Cohen, & Dietz, 2009). The per capita medical spending for an obese person is roughly 42 percent higher than for a person of normal weight (Finkelstein, et al., 2009), and outranks the health costs of both smoking and drinking alcohol (Sturm, 2002). Understanding, preventing and treating childhood and adolescent obesity is therefore a top public health priority (Koplan, Liverman, & Kraak, 2005).
This article gives an overview of progress in childhood and adolescent obesity research in the last decade (1999–2009), and covers the following topics: 1) current definitions of childhood and adolescent overweight and obesity; 2) demography of childhood and adolescent obesity both in the US and globally; 3) current topics in the physiology of fat and obesity; 4) psychosocial correlates of childhood and adolescent overweight and obesity; 5) the three major obesity-related behaviors, i.e. dietary intake, physical activity and sleep; 6) genetic components of obesity, 7) environment and childhood and adolescent obesity, and concludes with: 8) a discussion of interventions to prevent and treat childhood obesity in the last decade, and the extremely difficult task of changing human behavior. Because childhood and adolescent obesity is a vast, complex and transdisciplinary field that involves complex interactions between many factors, this review is necessarily selective. It is meant to provide readers from disparate backgrounds with a common vocabulary and a series of small windows on otherwise vast repositories of research and inquiry.
Defining Childhood and adolescent Obesity
Obesity is generally defined as an excess of body fat. However, there is no clear delineation between how much fat is normal and how much fat is abnormal. Furthermore, body fat is difficult and expensive to measure directly in large samples. Therefore, obesity is often defined as excess weight after adjusting for height. Body Mass Index (BMI, calculated as weight in kg/ height in m2) is used in adults to ascertain obesity status, and a BMI ≥ 30 is considered obese. However, because children are growing, the age and gender of the child must be taken into account in order to evaluate a child’s obesity status. In 2000, the Centers for Disease Control and Prevention (CDC) published age and gender specific BMI percentile growth curves for youth from 2–20 years of age (Kuczmarski, et al., 2002). These Body Mass Index growth curves were based on nationally representative and ethnically diverse samples and took age as well as gender into account. The 2000 CDC growth curves replaced the National Center for Health Statistics growth charts that had been in use since 1977.
Terminology
To avoid stigmatization, the 2000 CDC growth curves were accompanied by the recommendation that youth between the 85th and 94th age and gender specific BMI percentile be referred to as ‘at risk for overweight’ and those at or above the 95th percentile be referred to as ‘overweight’ (Kuczmarski & Flegal, 2000). In 2005, an expert committee convened by the American Medical Association, Health Resources and Service Administration and the CDC advised that the term ‘at risk of overweight ’ (between the 85th and 94th age and gender specific BMI percentile) be replaced with ‘overweight’ and that the term ‘overweight’ (≥ the 95th age and gender specific BMI percentile or with a BMI > 30, whichever is lower) be replaced by ‘obese’, in order to more accurately reflect the serious health risks associated with excess body fat (Barlow & and the Expert Committee, 2007). This manuscript adheres to the expert committee’s terminology. These cutoffs are based on data from United States populations and are not necessarily appropriate for evaluation of childhood and adolescent obesity in international samples. To address this issue, the International Obesity Task Force (IOTF) (Cole, Bellizzi, Flegal, & Dietz, 2000) developed separate cutoffs for overweight and obesity based on data from international samples. However, the use of different cutoffs creates challenges in terms of comparing data internationally, particularly because the IOFF cutoffs correspond to higher BMIs than the US cutoffs.
Demography of Childhood and adolescent Obesity
Setting the stage
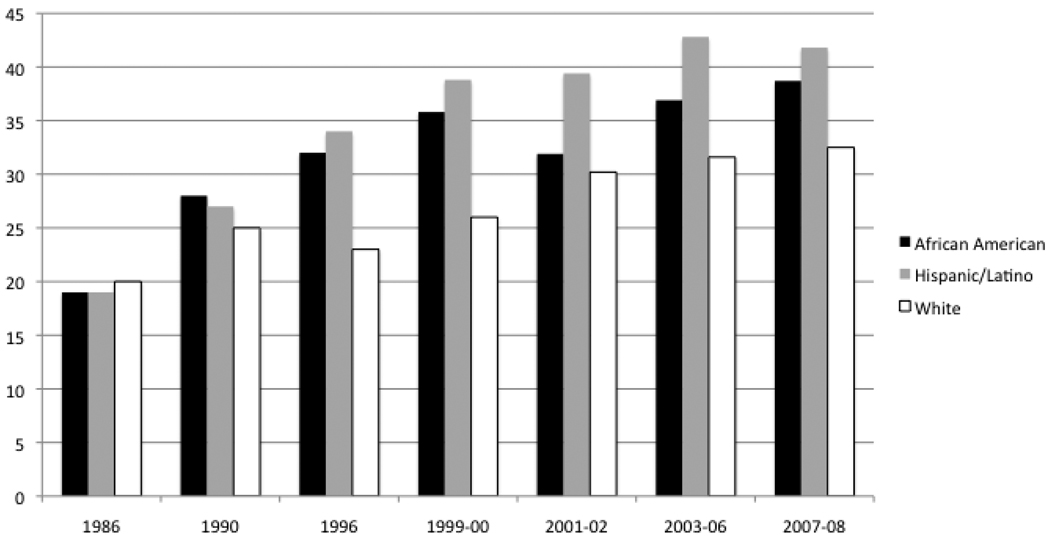
In the last three decades, obesity rates have tripled in youth aged 6–12 (Ogden, Flegal, Carroll, & Johnson, 2002). Between 1986–1998, data from the National Longitudinal Survey of Youth (NLSY) showed that obesity in children ages 4–12 years of age had increased by more than 120% among African Americans and Hispanics, and by more than 50% among whites. The relative weight of obese children had also increased by 144%. Thus, not only the prevalence, but also the severity of obesity had increased significantly between 1986–1998 (Strauss & Pollack, 2001).
The last decade: 1999–2009
The 1999–2000 NHANES data demonstrated continuing increases of overweight and obesity in youth between 1994 and 2000, showing an overall prevalence of obesity in 2–5 year-olds at 10% and in 6–19 year-olds at 15% (Ogden, et al., 2002). There were significant differences according to ethnicity. For instance, among 12–19 year-olds, the prevalence of obesity was 23.6% in non-Hispanic blacks, 23.4% in Mexican Americans, and 12.7% in non-Hispanic whites. NHANES data from 2003–2004 showed a significant increase in the prevalence of obesity in girls (from 13.8% in 1990–2000 to 16% in 2003–2004) and boys (from 14% to 18.2%) (Ogden, et al., 2006). The report on NHANES data from 2004–2006 did not show a continuing trend for increased obesity prevalence through 2006 (Ogden, Carroll, & Flegal, 2008). The report on the NHANES 2007–2008 data included a third category: High BMI (≥ the 97th percentile). This report showed that United States children continued to exhibit a high body mass index for age, with a significant increase only in boys with high BMI. BMI percentile differed significantly by ethnicity, with Hispanic boys more likely to have higher BMI at all 3 BMI cut points compared with non-Hispanic white boys, and non- Hispanic black girls more likely than to have high BMI at all 3 BMI cut points non-Hispanic white girls. According to this data, approximately one third of all US children are currently considered either overweight or obese (Ogden, Carroll, Curtin, Lamb, & Flegal, 2010) (see figure 1).
Figure 1.
Increasing prevalence of BMI ≥ 85th percentile in youth, 1986–2008
Data from the Longitudinal Survey of Youth 1986–1998 and NHANES 1998–2008
A note on measurement
In light of the importance of total body fat as well as the location of different fat depots, it is crucial to keep in mind that BMI and BMI percentiles are only proxy measures for adiposity (Barlow & and the Expert Committee, 2007). There are measures of body fat that also give some information on fat deposition, including dual X-ray absorptometry or DXA , air-displacement plethysmography or BodPod (Fields & Goran, 2000) and magnetic resonance imaging (Hu, Kim, Nayak, & Goran, 2009). These measures are extremely informative; however, they are expensive and can be somewhat invasive to obtain. Less precise, but also less invasive measures, include bioelectrical impedance (a proxy for total body fat) (Sung, Lau, Yu, Lam, & Nelson, 2001) and waist circumference (a proxy for visceral fat) (Fernández, Redden, Pietrobelli, & Allison, 2004).
Childhood and adolescent obesity: A global crises
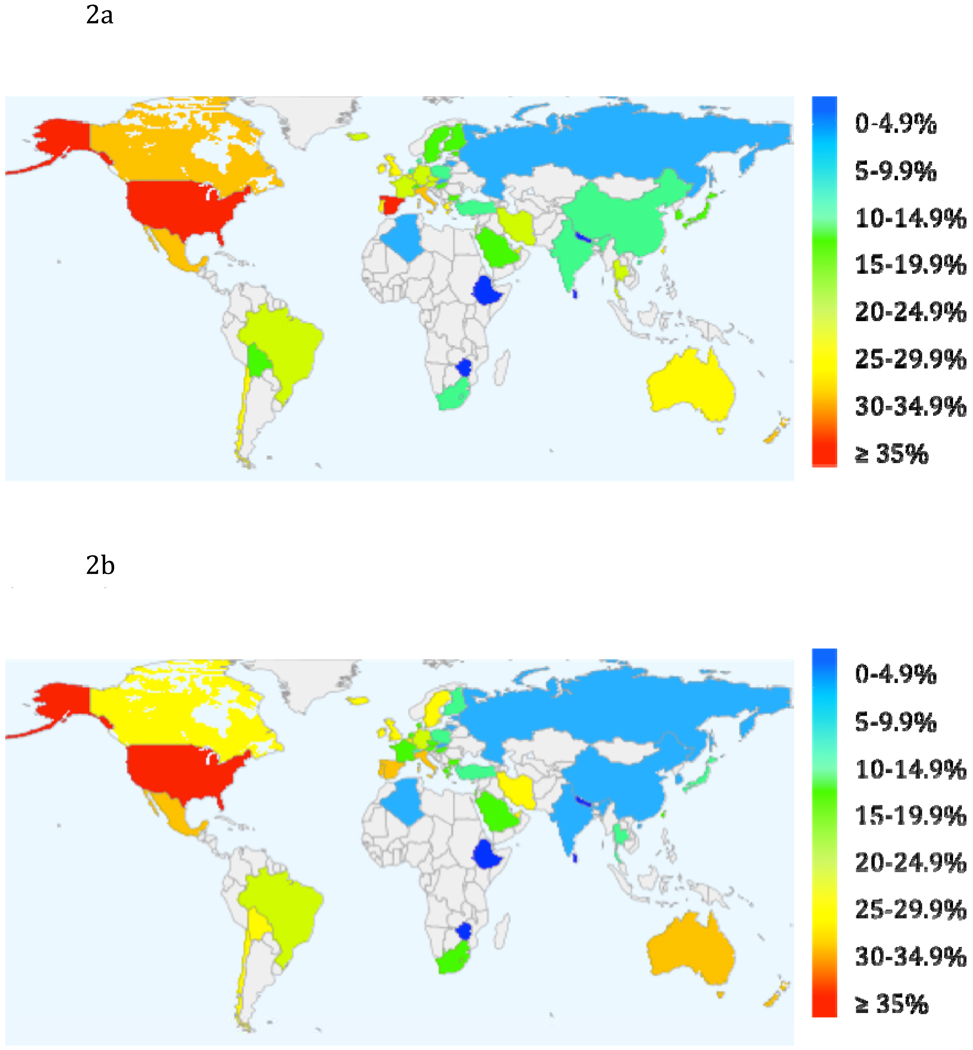
In 2004, the International Obesity Task Force (IOTF) released a report stating that 1 in 10 children were overweight, for a worldwide total of 155 million. Around 30–45 million of those were classified as obese - accounting for 2–3% of the world’s children aged 5–17 (Lobstein, Baur, & Uauy, 2004).In 2006, using the most recent data made available by the IOTF, Wang and Lobstein (2006) projected that 46.4% of youth in the Americas would be overweight or obese by 2010, 41.7% in Eastern Mediterranean countries, 38.2% in Europe, 22.9% in South East Asia and 27.2% in the Western Pacific nations. Data was not available to reliably project the prevalence of overweight or obese children in Africa. Figures 2a and 2b show current data on the global epidemic of childhood overweight and obesity (at or above the 85th percentile) for males and females by country. Data from earlier than 1995 could not be retrieved for countries in grey. Data ranged from 1995 (Ethiopia, Iran) to 2007 (Australia). Data came from the IOTF (2009) (made available July 21st, 2009), except for Russia (Wang, Monteiro, & Popkin, 2002), Israel and Lithuania (Lissau, et al., 2004). BMI percentile was calculated for most countries, including the United States, using the IOTF cut-offs (Cole, et al., 2000)
Figure 2.
Percentage of Male Children Meeting Criteria for Overweight by Country‡
Percentage of Female Children Meeting Criteria for Overweight by Country‡
‡ Data source: International Task Obesity Task Force (2009), except for Russia (Wang, Monteiro, & Popkin, 2002), Israel and Lithuania (Lissau, et al., 2004).
+BMI percentile calculated for most countries, including the US, using IOTF cut-offs (Cole, 2000).
Socioeconomic status and obesity
Obesity rates are influenced by socio-economic status (SES) and economic context (Goodman, 1999). This relationship is highly complex and a full review is beyond the scope of this manuscript. Many of the risks for childhood and adolescent obesity that are discussed in this article are negatively influenced by low socioeconomic status, making it difficult to sort out the effects of socioeconomic status alone. Thus, for instance, youth from lower socioeconomic status are less likely to have access to safe places to play (Gordon-Larsen, Adair, & Popkin, 2003) and available, affordable healthy food choices (Ver Ploeg, et al., 2009). Lower socioeconomic youth also more likely to be of minority status, which is in and of itself a risk factor for childhood and adolescent obesity (Ogden, et al., 2010).
The relationship between socioeconomic status and obesity is particularly salient when making global comparisons (Wang & Lobstein, 2006). For centuries, ‘plumpness’ was viewed as a sign of wealth, and in middle-income countries, members of wealthier households are still more likely to be overweight. However, in developed, higher income Western nations, there has been a marked shift in the relationship between SES and childhood and adolescent obesity, and overweight is most prevalent in the lower SES groups. A review of 34 studies in youth conducted between 1941–1989 found a positive, direct relationship in 26% of the studies, with higher SES related to higher BMI (Sobal & Stunkard, 1989). There was no association in 38% of the studies, and an inverse relationship in 36% of the studies. In a recent review of 45 studies between 1989–2008, higher SES was no longer associated with higher BMI in any of the studies. In 27% of the studies there was no relationship between SES and BMI, while 45% showed an inverse relationship, and 31% showed either no association or inverse associations, depending upon the population subgroup (Shrewsbury & Wardle, 2008). Thus, overweight is no longer associated with ‘plenty’ in developed countries. Rather, having the resources and access to eat well, exercise safely and receive adequate health care is now associated with lower adiposity in developed countries.
The prevalence of overweight and obesity continue to remain low in many lower income countries. However, this trend can reverse rapidly in the face of economic development that is inevitably accompanied by higher availability of motorized transportation, energy dense foods, small screen technologies and other obesogenic environmental influences (Popkin, 2009).
Physiology of obesity and its health consequences: Fat, gut and brain
One might ask: Why is fat bad (Bergman, et al., 2006)? The last decade has seen an upsurge of research aimed at understanding the mechanisms linking energy imbalance and obesity to disease risk.
Fat as an active endocrine organ
Excess calories are stored in fat cells, or adipocytes, leading to greater cell size (hypertrophy) and/or increased numbers of adipocytes (hyperplasia) (de Ferranti & Mozaffarian, 2008). Although the main purpose of adipose tissue was long thought to be for energy storage and thermal insulation, research now shows that adipose tissue secretes several major hormones and signaling factors that include adipokines, inflammatory mediators, and free fatty acids (FFAs) (Bastard, et al., 2006; Kriketos, et al., 2004; Krug & Ehrhart-Bornstein, 2005; Woods & Seeley, 2000). To regulate food consumption, the brain modulates appetite, and the core of appetite regulation lies in the gut-brain axis, which is sensitive to increases in adiposity (Cummings & Overduin, 2007). Several adipokines secreted by adipose tissue, such as leptin, contribute to the regulation of food intake (Korner & Leibel, 2003; Neary, Goldstone, & Bloom, 2004; Woods, 2004). These apidokines function in complex, evolved systems, together with gastrointestinal peptides and neuropeptides, to signal the brain to regulate appetite and energy intake (Markward, Markward, & Peterson, 2009). Higher levels of several of these circulating adipokines are related to adverse health outcomes, including type 2 diabetes and atherosclerosis (de Ferranti & Mozaffarian, 2008).
It is now recognized that obesity also leads to a physiological state of chronic inflammation, which is attributed to elevated plasma levels of inflammatory markers (cytokines) such as tumor necrosis factor ∞ (TNF∞) and interleukin 6 (IL-6), many of which are secreted by adipocytes themselves (Bastard, et al., 2006). Although acute inflammation is a signal of the body’s healing processes, chronic inflammation is linked to several diseases including type 2 diabetes and the metabolic syndrome (Juge-Aubry, Henrichot, & Meier, 2005; Krug & Ehrhart-Bornstein, 2005). Finally, elevated FFA concentrations reduce muscle glucose uptake (Randle, 1998; Randle, Garland, Hales, & Newsholme, 1963), increase liver glucose production (Lam, et al., 2003; Yoshii, et al., 2006) and stimulate insulin secretion (Bergman, et al., 2006; Boden, 2005), contributing to a cascade of effects that can increase insulin resistance (Randle, 1998).
What is insulin resistance?
Insulin resistance is a failure of insulin to act normally on these targeted tissues, which hampers glucose uptake and conversion (Frayn, 2007). It is associated with a cluster of risk factors for cardiovascular disease and type 2 diabetes. Increased adiposity, particularly visceral adiposity (fat accumulated around abdomen), is directly related to insulin resistance in both adults (Fujioka, Matsuzawa, Tokunaga, & Tarui, 1987; Kissebah, et al., 1982) and youth (Goran, 2001). Insulin resistance, in turn, has been directly related to a host of diseases, including cardiovascular disease, type 2 diabetes, and cancer (DeFronzo & Ferrannini, 1991; Jee, Kim, & Lee, 2005). In healthy youth, after consumption of a meal, carbohydrates (both starches and complex sugars) in the food are directly broken down into glucose molecules, which pass into the bloodstream. At a certain concentration of glucose, the pancreas is stimulated to release insulin into the bloodstream. Insulin must then cross the capillary endothelium and attach to insulin receptors on muscle cells, adipose tissue and other organs, allowing the uptake of the glucose and conversion of glucose to energy. Increased adiposity can increase FFA levels, which force liver, muscle and other tissues to shift towards increased storage and oxidation of fats for their energy production, leading to a reduced capacity of these tissues to absorb, store and metabolize glucose (Randle, et al., 1963).
Insulin resistance might thus develop as a metabolic adaptation to increased circulating levels of free fatty acids (FFAs) (Calle & Kaaks, 2004), which, as we have seen, are constantly released from adipose tissue (Bergman & Ader, 2000). If the pancreas fails to produce enough insulin to compensate for this insulin resistance, or if the insulin receptors fail to function properly, glucose uptake and conversion may be disrupted. Insulin resistance is compensated by hyperinsulinemia, i.e. increased pancreatic insulin secretion by the beta cells and/or decreased clearance of insulin by the liver (Bergman, 2005; Bergman, Prager, Volund, & Olefsky, 1987). When beta cells fail to compensate, type 2 diabetes can ensue. Insulin resistance in youth can be ameliorated through exercise, including aerobic (Bell, et al., 2007) and resistance training (Shaibi, et al., 2006), and through caloric restriction (Monzillo, et al., 2003). Interventions that reduce added sugar consumption (Davis, Ventura, et al., 2007) or emphasize low glycemic index foods (Ebbeling, Leidig, Sinclair, Hangen, & Ludwig, 2003) have shown some promise but require further testing.
Pubertal insulin resistance
Both cross-sectional and longitudinal studies show that youth become transiently insulin resistant during pubertal development. Transitions from Tanner pubertal development stage 1 (pre-pubertal) to Tanner pubertal development stage 3 (females: enlarged breast and aroela, males: penis growth in length, less in width, some pubic hair in both sexes)(Marshall & Tanner, 1969, 1970) is associated with an approximate 30% decrease in insulin sensitivity (Goran, Ball, & Cruz, 2003). This profound decline occurs regardless of gender, ethnicity and obesity status (Amiel, Sherwin, Simonson, Lauritano, & Tamborlane, 1986; G. Ball, Huang, Gower, & Goran, 2006; Ball, et al., 2005; Goran & Gower, 2001; Hoffman, Vicini, Sivitz, & Cobelli, 2000; Moran, et al., 1999; Travers, Jeffers, Bloch, Hill, & Eckel, 1995). However, there is an independent effect of minority status on pubertal insulin resistance. Minority youth, including African Americans, Hispanics, Pima Indians, and Asians, are more insulin resistant than their white counterparts. In most studies, this is true regardless of adiposity, diet and physical activity (Arslanian & Suprasongsin, 1996; Arslanian, Suprasongsin, & Janosky, 1997; Arslanian, Saad, Lewy, Danadian, & Janosky, 2002; Goran, Cruz, Bergman, & Watanabe, 2002; Goran, Herd, & Gower, 2000; B. A. Gower, Nagy, & Goran, 1999; Gower, Nagy, Trowbridge, Dezenberg, & Goran, 1997; Klein, et al., 2004; Misra, et al., 2004; Svec, et al., 1992; Young-Hyman, Schlundt, Herman, De Luca, & Counts, 2001). Interestingly, a recent study in 44 white and 46 African American participants showed that the influence of genetic factors as reflected by African genetic admixture (AfADM) on insulin sensitivity was superseded by adiposity (Casazza, et al., 2009). This demonstrates that there is a strong and independent influence of adiposity on pubertal insulin resistance, (Cruz, et al., 2005; Roemmich, et al., 2002; Steinberger, Moran, Hong, Jacobs, & Sinaiko, 2001). In summary, puberty, minority status and adiposity exert independent influences on the severity of pubertal insulin resistance.
Psychosocial correlates and consequences of childhood and adolescent obesity
Setting the stage
It has been known for several decades that overweight and obese youth are subject to stigmatization, particularly in Western cultures (Kimm, et al., 1997; Tiggemann & Anesbury, 2000). A longitudinal study in a nationally representative sample showed that women who were obese as adolescents consequently grew up to have lower education, income, and likelihood of marriage compared with their thinner counterparts (Gortmaker, Must, Perrin, Sobol, & Dietz, 1993). Obese youths experience discrimination throughout their lives, including experiencing lower admission rates into college (Canning & Mayer, 1966). Overweight has been associated with increased experiences of anxiety, depression, suicidal thoughts, body dissatisfaction and hopelessness (Lobstein, et al., 2004), particularly in Western youth (Ge, Elder, Regnerus, & Cox, 2001). A landmark study in 1967 in 90 youth aged 6–11 showed that children attributed negative personality characteristics such as cheating, laziness, sloppiness, lying, naughtiness, meanness, and being ugly, dirty and stupid to overweight body types (Staffieri, 1967). These results were replicated in 2001 (Latner & Stunkard, 2001) showing that, although pediatric obesity has become increasingly common, the negative social reaction to obesity has not abated with its increased prevalence. In fact, emerging research techniques that allow for complex analyses of social networks have been able to examine friendship networks, and have shown that overweight and obese children have fewer friends than their normal weight counterparts (Strauss & Pollack, 2003; Valente, Fujimoto, Chou, & Spruijt-Metz, 2009).
The last decade – 1999–2009
One important development in transdisciplinary pediatric obesity research in the last decade involves the reciprocal relationships between stress and obesity. Twenge has shown that today’s youth suffer from increased anxiety relative to those of the past (Twenge, 2000). In particular, inner-city, minority youth are frequently exposed to specific environmental factors that are also linked to chronic stress, including crime, disturbed family and social connections, racial discrimination, and lower SES (Baum, Garofalo, & Yali, 1999; Williams, 1999). A lower SES is clearly related to increased obesity in both adults and adolescents (Everson, Maty, Lynch, & Kaplan, 2002; E. Goodman, Slap, & Huang, 2003; Treiber, Harshfield, Davis, Kapuku, & Moore, 1999). Stress often results in a preference for, and the ingestion of increased calories, fat, and calorically dense snack-type foods (McCann, Warnick, & Knopp, 1990; Nguyen-Michel, Unger, & Spruijt-Metz, 2007; Oliver, Wardle, & Gibson, 2000; Wardle, Steptoe, Oliver, & Lipsey, 2000). These associations provide some explanation for Roemmich et al’s (2007) findings that a change in perceived stress was predictive of BMI percentile, such that stress-induced excess energy intake, particularly of energy-dense “comfort” foods can lead to increased BMI. Further, research with minority adolescents has shown an association between perceived stress and emotional eating (eating in response to negative affect, not hunger), regardless of weight status (Nguyen-Rodriguez, Chou, Unger, & Spruijt-Metz, 2008)
Dallman and colleagues have proposed a physiological explanation for the association between stress and the ingestion of energy-dense foods as well as its link to obesity (Dallman, et al., 2003). Stress activates the hypothalamic pituitary axis (HPA) thereby releasing glucocorticoids, including cortisol. Chronically high concentrations of these glucocorticoids have been shown to increase the expression of corticotropin-releasing factor mRNA in the central nucleus of the amygdala, which plays an important role in processing emotion. This enables recruitment of a ‘chronic stress response network’, which induces a chronic elevation of glucocorticoids, which in turn increase the salience of pleasurable or compulsive activities such as ingestion of sucrose, fat, or other ‘comfort foods.’ At the same time, glucocorticoids foster the development of central adiposity. This occurs via neuroendocrine mechanisms, indicating that chronic stress may result in increased visceral adiposity, insulin resistance, and metabolic syndrome (the presence of at least three of the following abnormalities: abdominal obesity, hypertriglyceridemia, low HDL cholesterol, hypertension and/or impaired glucose tolerance (Cruz, et al., 2004; Bjorntorp & Rosmond, 2000a, 2000b; Charmandari, Kino, Souvatzoglou, & Chrousos, 2003; Chrousos, 2000; Pasquali & Vicennati, 2000). This area of research has sparked the development of innovative intervention modalities that aim to reduce childhood and adolescent obesity through helping youth to reduce stress (Weigensberg, et al., 2009).
Behavioral determinants of obesity: Advances in research on diet in youth
Over the last several decades, an increase in variety, availability, price and aggressive marketing have encouraged a shift in children’s food choices from healthy foods such as fruit and vegetables to energy-dense, refined foods including fast-foods and sugar-laden beverages (Chopra, Galbraith, & Darnton-Hill, 2002). The energy balance equation (Woods & Seeley, 2005) holds that obesity is the consequence of chronic positive energy imbalance. In otherwise healthy youth, this occurs when more energy is consumed than expended. One fast-food meal (including a double cheeseburger, french fries, soft drink, and dessert) contains a total of about 2200 kilocalories. To put this into perspective, let’s assume that a runner burns 85 kilocalories per mile, this caloric load would take a full marathon to burn off (Ebbeling, Pawlak, & Ludwig, 2002).
It is not merely the fact that children and adolescents are exceeding recommended caloric intake (Wright, Wang, Kennedy-Stephenson, & Ervin, 2003). Nutrient makeup of food also seems to matter. For decades, (over-) consumption of dietary fat was believed to be the main cause of obesity (Rolls & Shide, 2009). Indeed, one gram of carbohydrate or protein yields 4 kilocalories, while one gram of fat yields 9 kilocalories. However, new evidence suggests that it is not (over-) consumption of dietary fat per se, but maternal obesity during pregnancy, that pre-programs children to be susceptible to dietary fat (White, Purpera, & Morrison, 2009). Furthermore, the association between dietary fat and adiposity in children has not been consistently shown, and the prevalence of obesity has increased while the proportion of total calories consumed from fat in the diet of US children has actually decreased (Ebbeling, et al., 2002). Many of the packaged foods currently marketed as ‘low fat’ or ‘all natural’ are extremely high in their total calorie content (Maziak, Ward, & Stockton, 2008). Furthermore, lower fat intake may be related to higher sugar intake (Gibson, 1996; Gregory, Foster, Tyler, & Wiseman, 1990; McColl, 1988). Thus, it is not merely the high caloric content of children’s diets, but also the type of nutrients consumed that are having a direct influence on childhood and adolescent obesity. In particular, the shift to high sugar, low fiber diets might have a strong effect on obesity and related diseases.
Soda intake is a topic of particular interest at the moment for the media and research communities. There have been several studies showing that soda consumption is related to adiposity in youth (Berkey, Rockett, Field, Gillman, & Colditz, 2004; Ebbeling, et al., 2006), although one recent review found no significant relationship (Forshee, Anderson, & Storey, 2008). This might be because sugar-laden drinks alone do not account for the high levels of added sugars in children’s diets today. Our group has shown cross-sectionally that total added dietary sugars, and not dietary fat or glycemic index, are directly related to adiposity in Latino youth (Davis, Alexander, et al., 2007). In a 16-week nutrition and strength training intervention in 54 overweight Latino youth, those who reduced sugar intake by an amount approximately equivalent to the sugar content of one soda per day improved insulin secretion and glucose uptake (Ventura, et al., 2009). Furthermore, those participants who increased their dietary fiber intake by the equivalent of approximately ½ cup of beans decreased their BMI by 2% and visceral fat by 10% over the 16-week period (Ventura, et al., 2009). Finally, regular breakfast consumption is inversely related to visceral fat (Alexander, et al., 2009) and BMI (Timlin, Pereira, Story, & Neumark-Sztainer, 2008) in youth. Taken together, this literature suggests that encouraging energy restriction can best be accompanied by recommendations that also include the reduction of added dietary sugars, increase in total fiber, and regular consumption of breakfast.
Behavioral determinants of obesity: Advances in research on physical activity in youth
Physical activity is important during youth not only because it can prevent and reduce obesity (Bergman, Ader, Huecking, & Van Citters, 2002; Goran, Reynolds, & Lindquist, 1999), but it also reduces the risk for cardiovascular disease (Ruiz & Ortega, 2009) and type 2 diabetes (Diabetes Prevention Program Research, 2002). Physical activity can reduce breast cancer risk by as much as 40%, particularly with sufficient exercise during childbearing years (McTiernan, et al., 2003; Patel & Bernstein, in press; Thune, Brenn, Lund, & Gaard, 1997; Verloop, Rookus, van der Kooy, & van Leeuwen, 2000). Physical activity can also improve mental health and well-being in youth (Connor, 2004).
The research on physical activity in youth in the last decade has been driven by vast improvements in measurement methodologies (Spruijt-Metz, et al., 2009; Troiano, 2005). The broader use of objective measures of physical activity, particularly the use of accelerometers (small electronic devices that measure acceleration caused by bodily movement, usually worn on a belt at the waist) in nationally representative samples of youth, has provided much deeper insight into the epidemiology of physical activity in youth. And, taking into account the 2008 recommendations for 60 minutes of moderate to vigorous physical activity (MVPA) per day for youth under 18 (Physical Activity Guidelines Advisory Committee, 2008), the findings are grim. Only 48.9 % of boys and 34.7 % of girls aged 6–11 meet the physical activity guidelines (Troiano, et al., 2008). Longitudinal data shows that physical activity declines by 37.6 minutes per year between the ages of 9–15 (Nader, Bradley, Houts, McRitchie, & O'Brien, 2008, 2009), with only 11.9 % of boys and 3.4% of girls meeting the guidelines by ages 12–15 (Troiano, et al., 2008). The complex bio-behavioral and socio-cultural etiology of this pubertal decline in physical activity is not yet well understood. Once this decline in physical activity occurs, it does not reverse. Figure 3 shows cross-sectional evidence for the pubertal decline in physical activity in a nationally representative sample of over 6000 participants.
Figure 3.
Decline in time spent (in mean minutes per day) in Moderate to Vigorous physical activity, United States, 2003–2004‡
‡ Data source: Troiano et al, 2008
* Females significantly less active than males at all ages
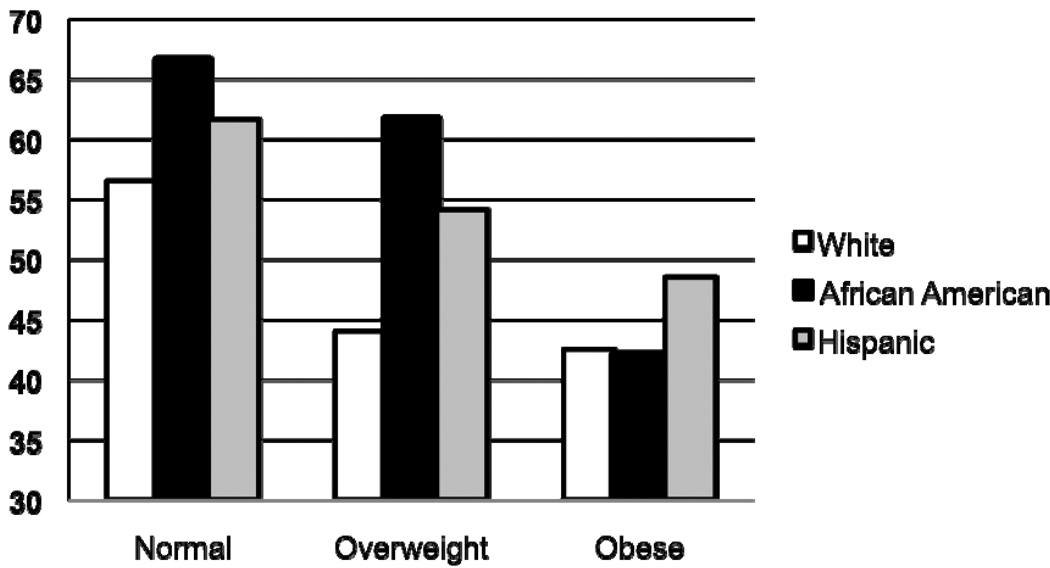
In a data from 3,698 youth participating in the 2003-4 and 2005-6 National Health and Nutritional Examination Survey, our group recently showed that obese youth recorded less MVPA than overweight youth, and both obese and overweight youth recorded less activity than normal weight youth, with some differences according to ethnicity (see Figure 4) (Belcher, et al., in press). White youth recorded the fewest mean minutes of MVPA and African American youth recorded the most minutes of MVPA. The difference between boys and girls was pronounced. Females recorded about 27 fewer minutes per day of MVPA than males, which can be equated with approximately 600 kg per day (Ekelund, et al., 2006).
Figure 4.
Significant differences in time spent (mean minutes per day) in Moderate to Vigorous Physical Activity by obesity status and ethnicity, United States 2003–2006‡
‡ Data source, Belcher, et al, in press
*BMI percentile negatively associated with MVPA in all race/ethnic groups for both genders (p<.05 for all)
Research on Sedentary Behavior
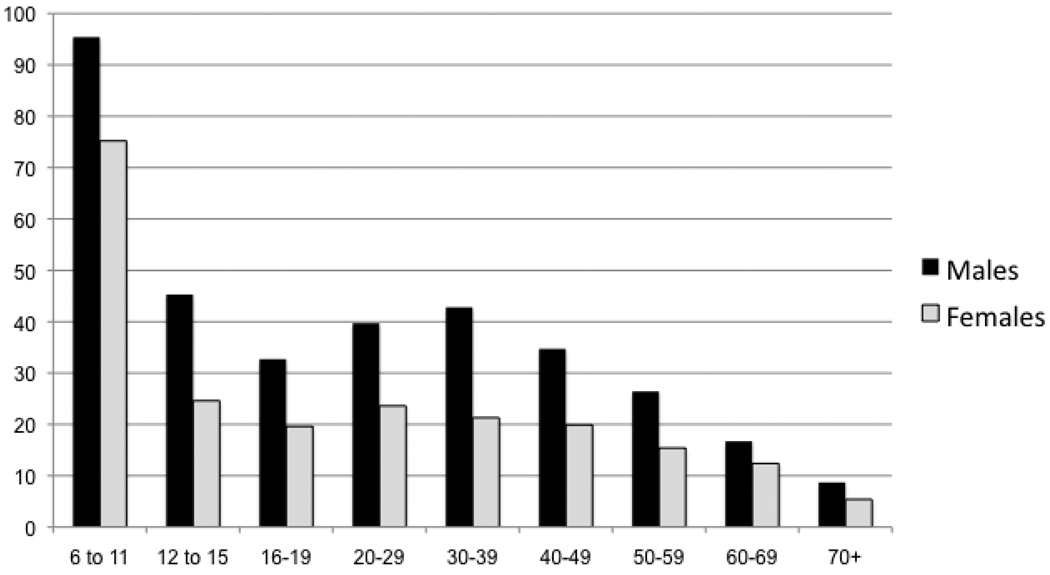
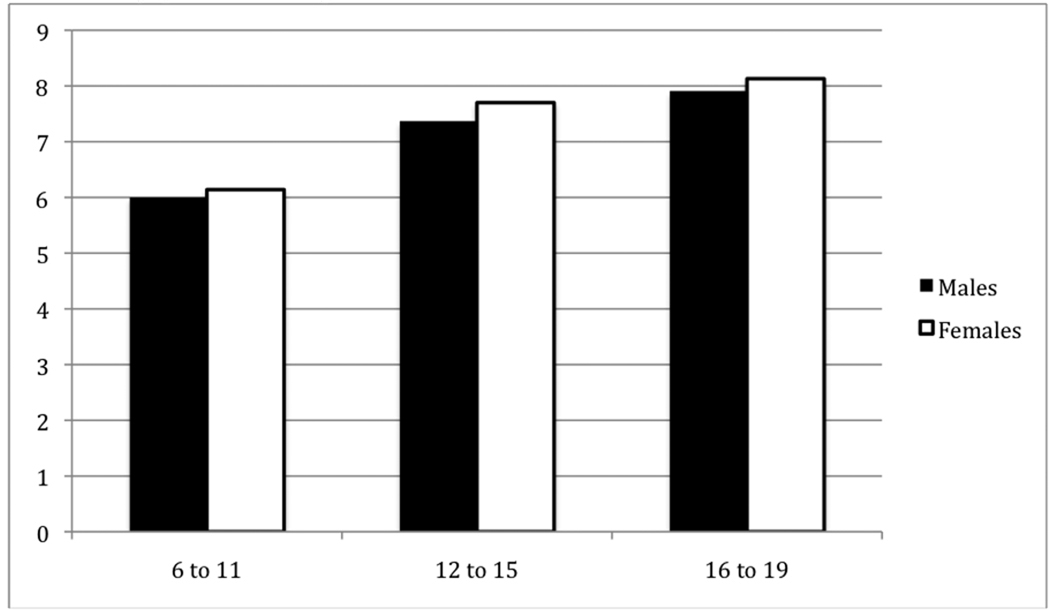
Another major advance in physical activity research is the serious attention that is being given to sedentary behaviors, i.e. as television viewing, computer use, video gaming and other small screen recreation and other low intensity/low energy-expenditure activities (Hardy, Bass, & Booth, 2007; Must & Tybor, 2005). This is important because the amount of time spent in sedentary activity has been independently associated with increased obesity, (Andersen, Crespo, Bartlett, Cheskin, & M. Pratt, 1998; Jago, Baranowski, Baranowski, Thompson, & Greaves, 2005) risk for cardiovascular disease (Raitakari, et al., 1994), and metabolic syndrome (Hsu, et al., 2009) in youth. Minority youth watch more TV and spend more time in sedentary pastimes than their white counterparts (Andersen, Crespo, Bartlett, Cheskin, & Pratt, 1998; Eisenmann, Bartee, & Wang, 2002). In a representative sample of US youth, time spent in sedentary behaviors measured objectively by accelerometry, increased with age, with girls being significantly more sedentary than boys (Matthews, et al., 2008) (Figure 5).
Figure 5.
Increases in amount of time (mean hours/day), spent in sedentary behavior by gender and age, United States, 2003–2004‡
‡ Data source: Matthews et al, 2008
* Females significantly more sedentary at all ages (6–11, p = 0.002, 12–15, p = 0.003, 16–19, p = 0.028.
** p for trend of increasing sedentary behavior, p < 0.001
Taken together, recent research on physical activity and sedentary behavior in youth suggests that innovative interventions to increase physical activity are needed, and that targeting a decrease in sedentary behavior may be equally as important.
Behavioral determinants of obesity: Advances in research on sleep in youth
Another advance in the literature is growing recognition of the third pillar of metabolic health: Sleep. The risk for obesity is nearly three times higher for children who sleep less than eight hours per night (Sekine, et al., 2002), and a short sleep cycle leads to same year increases in BMI in adolescent females (Berkey, Rockett, & Colditz, 2008). At the same time, sleep deprivation has also increased in youth, with > 33% of teens getting less than the recommended 9 hours of sleep with objective evidence of increasing daytime sleepiness (Carskadon, Wolfson, Acebo, Tzischinsky, & Seifer, 1998). Sleep deprivation can lead to increased hunger for high energy-dense foods (Spiegel, Leproult, et al., 2004; Spiegel, Leproult, Tasali, Penev, & Van Cauter, 2003). Shortened sleep patterns have also led to an increase in ghrelin and a decrease in leptin (Spiegel, Tasali, Penev, & Van Cauter, 2004; Spilsbury, et al., 2004; Taheri, Lin, Austin, Young, & Mignot, 2004). While ghrelin is a hormone that stimulates appetite, leptin conversely signals satiety. This highlights the implications for increased obesity - short sleep can lead to increased energy intake, which can lead to overweight/obesity if not counteracted by an equal increase in energy expenditure. Insufficient sleep has also been related to decreased levels of physical activity in adolescents (Gupta, 2001). Chaput and colleagues (2008) found that both short sleep and long sleep present a risk for future weight gain, even after adjusting for dietary intake and physical activity. Finally, experimental sleep deprivation of healthy, young adults causes elevations of evening cortisol levels, reduced glucose tolerance, and an altered profile of growth hormone secretion (Leproult, Copinschi, Buxton, & Van Cauter, 1997; Spiegel, 2008; Spiegel, Leproult, & Van Cauter, 1999; Van Cauter, 1992; Van Cauter, et al., 2007). It is thus clear that obesity prevention and treatment efforts that aim to increase physical activity, decrease sedentary behavior and improve dietary habits should be accompanied by efforts to improve sleep.
Several socio-environmental changes in the last decade have been hypothesized to adversely affect sleep onset and duration in youth, including nighttime technology use (Calamaro, Mason, & Ratcliffe, 2009) and increased stress and anxiety (Bruusgaard, Smedbraten, & Natvig, 2000; Twenge, 2000). Thus, interventions to improve sleep in youth might consider environmental modifications, such as the removal of technology from the bedroom, as well as methods to help children reduce stress.
Genetic influences on childhood and adolescent obesity
The complex system that regulates body fat, described earlier, comprising multiple pathways and systems involved in energy homeostasis, is susceptible to genetic regulation at each junction (Butte, Bacino, Cole, & Comuzzie, 2006). Except for very rare instances where obesity can be related to one genetic mutation (Bochukova, et al., 2009), obesity is an oligogenic disorder attributed to multiple genes whose expression is modulated by gene-gene and gene-environment interactions (Butte, et al., 2006). The prevalence of obesity is increasing far to rapidly to attribute everything to evolutionary changes in the gene pool (Grilo & Pogue-Geile, 1991; Hewitt, 1997), and the inventory of genes that influence obesity in children may only partially overlap with those that influence obesity in adults (Hebebrand & Thiesen, 2005). Different genes may be selectively activated or repressed as part of childhood development or as plastic responses to changing environmental conditions. Furthermore, there exists a profound mismatch between our genetic background and the current environment. Therefore, it might be more accurate to say that adiposity is determined by the interaction between the genetic makeup of an individual and the environment in which that person is living, along with their behavior and physiology (Smith & Ravussin, 2005). According to the ‘thrifty genotype’ hypothesis, people prone to overconsumption, efficient energy storage and energy conservation were more likely to survive and thus successfully propagate ‘thrifty’ genes (Neel, 1962). However, these ‘thrifty genes’ are maladaptive to our current obesigenic environment, which encourages the consumption of energy dense, highly palatable, nutrient poor foods in large portions, and discourages expenditure of energy through reductions in physical education time, motorized transportation, and increased time spent in sedentary behaviors, including television and small screen time (Hill & Peters, 1998; Hill, Wyatt, Reed, & Peters, 2003). Thus, some people are more susceptible to this obesigenic environment than others. Therefore, the epidemic in childhood and adolescent obesity that we have seen over the past two decades is likely due to susceptibility genes exerting their influence in an environment that is highly permissive, rather than to changes in the human gene pool (Butte, et al., 2006).
Built and social-environmental influences on childhood and adolescent obesity
There has been an upsurge of research on the influence of the built environment on obesity and obesity-related behavior in recent years (Sallis & Glanz, 2006). The ‘built environment’ has come to refer to the many forms of surroundings that influence human activity. Physical activity levels of youth may depend on environmental features that encourage or discourage physical activity, such as access to recreational facilities, walkability of the environment and low neighborhood crime rates (Davison & Lawson, 2006; Ferreira, et al., 2007). The school environment, including availability of healthy foods and professionally led physical activity classes, also plays an important role in childhood and adolescent obesity (Story, Nanney, & Schwartz, 2009). Physical activity levels are inversely related to a low socio-economic status (Gordon-Larsen, McMurray, & Popkin, 2000; Sallis, Zakarian, Hovell, & Hofstetter, 1996), and this might be due in part to an inequality in community tax base and fiscal health, amenity levels in neighborhood environments, and access to these key public facilities and services such as recreation facilities (Joassart-Marcelli, Musso, & Wolch, 2005). A recent review of built-environmental influences on children’s physical activity showed that levels in youth are positively associated with publicly provided recreational infrastructure (access to recreational facilities and schools) and transport infrastructure (presence of sidewalks and controlled intersections, access to destinations and public transportation) (Davison & Lawson, 2006). Living within ½-1 mile of a public park increases adolescent girls’ physical activity significantly (Cohen, et al., 2006), while traffic density within 150 meters around the home was related to higher BMI in both girls and boys (Jerrett, et al., 2009).
Fewer studies have examined the effects of physical activity-related built environmental variables on actual weight status in youth, with inconsistent results (Dunton, Kaplan, Wolch, Jerrett, & Reynolds, 2009). In their comprehensive review, Dunton et al (2009) found that school play space, road safety, proximity to supermarkets and lower population density have been found to be related to lower obesity rates in young children. In adolescents, the number of recreational facilities seemed to be the only factor that has been clearly related to obesity. Furthermore, there appear to be gender differences in physical environment effects on obesity status. One study in preschool children showed relationships between intersection density, walkability and lower BMI in young girls only (Spence, Cutumisu, Edwards, & Evans, 2008). In a nationally representative sample of more than 20,000 adolescents in 19% of all US census-block groups, Gordon Larsen et al (Gordon-Larsen, Nelson, Page, & Popkin, 2006) found that lower-SES and high-minority block groups had reduced access to key facilities, which in turn was associated with decreased physical activity and increased overweight.
Environmental factors also influence dietary intake. Access to healthy food resources positively influences healthy diets and is related to lower obesity rates (Morland, Diez Roux, & Wing, 2006; Morland, Wing, & Diez Roux, 2002). Although the relationship between exposure to neighborhood fast food outlets and poor diet is fairly consistent, some studies find no relationship between density or proximity of fast food restaurants and adiposity in youth (Burdette & Whitaker, 2004; Crawford, et al., 2008). Lower prices for fruit and vegetables have also been directly related to lower BMI in youth, while fast food prices and proximity of outlets were not (Sturm & Datar, 2005). Poor nutritional choices available in school environments, including vending machines and a la carte fast foods have been related to poor diet (Kubik, Lytle, Hannan, Perry, & Story, 2003) and higher BMI (Fox, Dodd, Wilson, & Gleason, 2009). The pervasive influence of the built environment on physical activity and diet in youth has recently been recognized in the White House Task Force on Childhood Obesity, which includes a sweeping set of recommendations for fundamental changes to these environments (White House Task Force on Childhood Obesity, 2010).
Elements of the built environment, such as noise pollution and use of social networking sites at bedtime have adverse influences on sleep in youth. For example, having a television, computer or other technologies available in the bedroom has been linked to short sleep in youth. In a study of youth (mean age of 9 years-old) in 8 cities in China using self-reported measures, television or computer presence in the bedroom was associated with later bedtimes, later awakening times, and a shorter duration of sleep during weekdays and weekends (Li, et al., 2007). Another study using self-report in 1656 Belgian youth from 13–17 years of age found that using mobile phones for calling or texting after lights out was significantly related to feeling tired during the day. (Van den Bulck, 2007). In a study of 11 children that used objectively measured sleep by polysomnography, children were exposed to voluntary excessive television and computer game use. Computer game playing resulted in prolonged sleep-onset latency, significantly reduced amounts of slow-wave sleep as well as significant declines in verbal memory performance (Dworak, Schierl, Bruns, & Struder, 2007).
The social environment: The influence of family
The influence of family on BMI is strongly supported in heritability research (see section above). Beyond genetics and heritability, the family also has profound influences on obesity-related behaviors. A recent review of 58 papers found fairly consistent associations between parental dietary intake and children’s fat, fruit and vegetable consumption (van der Horst, et al., 2007). The same review found a consistent influence of parent and sibling energy and fat intake with adolescent’s energy and fat intakes. Lower parental education was related to lower adolescent fruit and vegetable intake. Higher frequency of family meals eaten together was related to healthier dietary intake in youth, as was home availability of healthy foods (Story, Kaphingst, Robinson-O'Brien, & Glanz, 2008). Parental child-feeding practices have been studied in depth by Birch and colleagues, providing evidence that restrictive practices, such as parental restriction of palatable foods, can lead to eating in the absence of hunger and predicts adiposity both cross-sectionally and longitudinally (Anzman & Birch, 2009; Birch, Fisher, & Davison, 2003). Where physical activity is concerned, parental support of physical activity (James F. Sallis, Prochaska, & Taylor, 2000), higher parental physical activity levels (Davison, Cutting, & Birch, 2003) and higher parental education levels are all related to higher levels of physical activity in children and adolescents (Gordon-Larsen, McMurray, & Popkin, 2000). Parents also influence sleep in children, and this influence extends past setting and monitoring bedtimes. As shown above, computers, cell phones and television in the bedrooms can have negative effects on sleep duration and onset in youth. One study found that longer sleep duration was related to parenting practices that encouraged social maturity (Spilsbury, et al., 2005). In sum, for each of the three main obesity-related behaviors addressed here, families play important roles as models, rule-setters, and guides.
Ravussin et al (1994), in their landmark article on the effects of traditional environments on obesity in Pima Indians, observed that, in studies within populations where environments are similar, the variance in obesity that is explained by the environment is likely to be underestimated. This is because the environmental effects that we are able to estimate in any given study are the result of the degree of variability of environments observed in that specific study population. Since we usually cannot perform controlled experiments on environmental variables, and important influences on obesity such as pollution, crime and fast food, are often ubiquitous in any given sample, our observations cannot be used to infer the possible effects of actually altering that environment (Musani, Erickson, & Allison, 2008). These modern ‘obesogenic’ environmental factors, including (but not limited to) urban sprawl, traffic density, availability of energy dense foods, ubiquitous technology, and the decreasing frequency of family meals, interact with genetic predisposition to contribute to the epidemic of childhood and adolescent obesity. However, a genetic predisposition for obesity may not necessarily rule out behavioral or environmental control (Rampersaud, et al., 2008).
Changing Human Behavior: Interventions to prevent and treat childhood obesity
The obvious conclusion from this review of a decade of childhood and adolescent obesity research is that we need to turn the tide, with a focus on reducing childhood and adolescent obesity through treatment and prevention. However, changing behavior, particularly behaviors that are necessary for daily living, has proven particularly challenging. One cannot ‘quit’ eating or ‘quit’ sedentary behavior. Therefore, behavioral modifications must be made in the face of continuing temptation. Increasing physical activity has proven difficult in the current environment that favors motorized transportation, screen use, hours spent sedentary in school with decreased physical education time, and parents who do not have time to encourage home meals or child’s physical activity. Even when behavior change is successful, the magnitude of change is frequently not large enough to affect BMI or adiposity. Table 1 provides outcomes from three recent reviews of interventions to treat and three recent reviews to prevent childhood and adolescent obesity, chosen for their diversity and comprehensiveness. Several excellent reviews were not included here, such as (Brown & Summerbell, 2009; van Sluijs, McMinn, & Griffin, 2007; Flodmark, Marcus, & Britton, 2006; Connelly, Duaso, & Butler, 2007; Kamath, et al., 2008). It is clear that, depending upon the analyses and inclusion criteria, different reviews may provide different outlooks on the endeavor (Doak, Heitmann, Summerbell, & Lissner, 2009).
Table 1.
Six comprehensive reviews of obesity treatment and prevention trials.
| Treatment | |||
|---|---|---|---|
| Source | Inclusion Criteria | Number of Studies | N (%) studies that significantly reduced BMI or adiposity |
| (McGovern, et al., 2008) |
|
14 Pharmaceutical 17 Physical activity 3 Reduce Sedentary 26 Lifestyle |
5 (36%) 2 (12%) 3 (0%) 7 (27%) Overall: 23% |
| (Atlantis, Barnes, & Singh, 2006) |
|
14 | 2 (14%) |
| (Oude Luttikhuis, et al., 2009) |
|
64 8 lifestyle and 4 drug trials used for pooled analyses |
(Of the 12 studies in pooled analyses) 5 of 8 (63%) (at 6 months) 3 of 4 (74%) |
| Prevention | |||
| (Dobbins, De Corby, Robeson, Husson, & Tirilis, 2009) |
|
26 Only 14 studies report BMI |
(Of 14 studies reporting BMI) 4 (29%) |
| (Stice, Shaw, & Marti, 2006) |
|
61 | 13 (21%) |
| (Summerbell, et al., 2005) |
|
22 19 school-based 1 community-based 2 family-based |
4 (18%) |
Interventions to treat childhood and adolescent obesity
McGovern et al (2008) reviewed 60+ interventions to treat childhood and adolescent obesity. Only 23% of these resulted in significant decrease in adiposity. Most of these interventions were short-term and follow-up data was, for the most part, not available. Atlantis et al (2006) examined the effect of 14 studies in youth that included an exercise intervention. Only 14% of these interventions significantly decreased adiposity. However, interventions with higher doses of exercise, between 120–150 minutes per week of moderate to vigorous physical activity, were likely to be successful, which is still lower than the 60 minutes a day prescribed by the 2008 guidelines for physical activity (Physical Activity Guidelines Advisory Committee, 2008). The most recent Cochrane review of interventions to treat childhood and adolescent obesity reviewed 64 studies (Oude Luttikhuis, et al., 2009). Only 12 of these studies lent themselves to pooled analyses. In general, most of the studies that were included in the pooled analyses achieved positive outcomes. Family-targeted behavioral lifestyle interventions seemed most effective, and combined diet, physical activity and behavioral interventions were suggested as promising areas for future efforts. However, according to another recent review it is unclear whether it is more effective to target single or multiple behaviors, and there is evidence for effectiveness of both simultaneous and sequential behavior change interventions (Brown & Summerbell, 2009). Unfortunately, most of the studies included in the Oude Luttikhuis, et al. review were underpowered. Recommendations for the future include larger-scale interventions with process and fidelity measures and costeffectiveness analyses. The authors emphasized the need for including psychosocial mediators of behavior change and building interventions based on psychosocial theory. Psychosocial theories of obesity-related behavior in youth have been fairly well described (see for instance McClain, et. al (2009) for correlates of eating behavior, Sallis, Prochaska, & Taylor (2000) for correlates of physical activity, and Lubans, Foster, & Biddle (2008) for mediators of physical activity change). Although it has been acknowledged for more than a decade that theory-based interventions are more likely to significantly impact obesity-related behaviors in youth (Baranowski, Cullen, & Baranowski, 1999; Baranowski, Lin, Wetter, Resnicow, & Hearn, 1997; Spruijt-Metz, 1999), few theories have been fully tested in childhood obesity interventions, and many interventions are still not truly based in theory.
Interventions to prevent childhood and adolescent obesity
In the Cochrane review of school-based interventions to prevent obesity, only 14 studies provided information on BMI (Dobbins, De Corby, Robeson, Husson, & Tirilis, 2009). Of these, only 4 (18%) significantly impacted BMI, and all 4 of these studies succeeded only in limiting the rise in BMI percentile in the intervention compared to the control group. There were no significant reductions in BMI percentile. However, there have been several landmark school-based interventions that have successfully improved obesity-related behaviors and reduced obesity (Gortmaker, et al., 1999), for instance through changing. Therefore, improving school food and activity environments remains a priority (White House Task Force on Childhood Obesity, 2010). Stice (2006) reviewed 61 discrete interventions to prevent obesity and found that only 21% prevented BMI gain. In general, this review found that these interventions failed to prevent increases in BMI. For most programs that did produce significant prevention of weight gain, the effect sizes were clinically meaningful but often confined to pre–post effects, with no long-term staying power. Finally, the most recent Cochrane review on prevention (Summerbell, et al., 2005) suggests that prevention efforts have not been able to significantly impact the weight status of children. The authors emphasize the need for multifactorial theoretical approaches that consider the impact of system, environment and organizational issues, and address individual as well as group behavior change. They also encourage inclusion of stakeholders (families, schools, policy-makers) in the decision-making process about intervention strategies to be implemented.
Conclusions: Challenges for the next decade
Today’s children may be the first in more than a century to live less healthy, and possibly even shorter lives than their parents (Olshansky, et al., 2005). The impact on health and life expectancy will likely be the most severe in minority populations, where the increase in childhood obesity has been rapid and precipitous. Reversing the epidemic of childhood obesity and associated health consequences – through the development of effective interventions to prevent and reduce childhood and adolescent obesity – is the most important challenge for the next decade. To do this, progress must be made in each of the areas discussed here. A few of the many questions left to answer are outlined below. Answers to each of these questions need to be formulated with two key stipulations: 1) children are by definition in development – answers to any of these questions may differ according to cognitive, emotional and physiological developmental status, and 2) there are profound ethnic disparities in childhood and adolescent obesity as well as each of the topics covered here – answers to these questions may be specific to race/ethnic group, and be particular to SES.
Physiology and neurobiology of childhood and adolescent obesity
What are the physiological and neurological mechanisms that might regulate obesity-related behaviors in youth? How do development and puberty influence behavior and how are these influences mediated physiologically? How can we integrate knowledge being gained in studies on how the brain modulates energy balance into interventions? How might metabolic and biological influences precede behavior and how can this knowledge be integrated into prevention efforts?
Psychosocial influences on childhood and adolescent obesity
Is human motivation for obesity-related behaviors affected by physiological developmental phenomena, such as pubertal insulin resistance? How can current knowledge from relevant fields of research best be incorporated into transdisciplinary theories of behavior that truly predict behavior change, and how can we ensure that these theories are properly utilized in intervention development and evaluation?
Obesity-related behaviors in context: Diet, physical activity and sleep
Research has shown that objectively measured environments can differ significantly from how those environments are perceived, and both actual and perceived environments influence obesity-related behaviors (Ball, Timperio, & Crawford, 2006). So, how can influence stakeholders and policy makers to make the necessary changes (such as reduction of serving sizes, healthy school menus, access to clean and safe public parks, walkable neighborhoods, etc.), as well as change perceptions of environments as they become safer or more amenable to healthy behaviors?. We know that diet can influence physical activity (Spruijt-Metz, et al., 2009), and that physical activity can influence sleep (Atkinson & Davenne, 2007) How do obesity-related behaviors fold back on one another and interact to influence obesity, and at which junctures in this complex cascade of cause and effect can we best intervene?
Genes & Environment
How might genetic variation interact with behavioral factors to influence the regulation of body weight and adiposity? There is currently great variability in how individuals respond to obesity prevention and treatment interventions, and evidence suggests that these responses might be partially regulated by genes. Do (specific) genes regulate responses to particular interventions? If so, what are the mechanisms and how might this knowledge be best harnessed to develop effective interventions?
Interventions: The future
Taking the above into account, possibly the best place to tackle the epidemic of childhood and adolescent obesity is in real time. Using new technologies such as cell phones and wireless body area networks to capture behavior, emotions and thoughts, physiological reactions and location in real-time will give us new understanding of how children live, think and feel in their environments, and how we can best help them to live healthy lives (Stone, Shiffman, Atienza, & Nebeling, 2007). New technological platforms will enable provision of adaptive and personalized interventions (Collins, Murphy, & Bierman, 2004) that are flexible (Thatte, et al., 2009), adaptive to the personal behaviors and needs (Patrick, Griswold, Raab, & Intille, 2008) of the child, and give just-in-time feedback on real-life behaviors.
In conclusion, the multiple factors that contribute to childhood and adolescent obesity seem to be heterarchical (McCulloch, 1945), in that there is no well defined highest or lowest level, no one determinant and no unidirectional model. Rather, they interact, overlap, and re-array into divergent, yet co-existent patterns of relation. There are thus many different places of entry or junctures to intervene. To stem the tide of childhood and adolescent obesity, the next decade will need to see development of highly transdisciplinary interventions that recognize the complex, heterarchical structure of the disease.
Acknowledgements
I would like to thank Dr. David Berrigan (NCI) and Dr. Joyce Richey (USC) for their helpful comments on earlier drafts of parts of this manuscript, Dr. Adar Emken (USC) and Javier Diaz (USC) for programming the map of global childhood obesity.
This work was supported by the University of Southern California’s Center for Transdisciplinary Research on Energetics and Cancer (National Institutes of Cancer U54 CA 116848) and the University of Southern California’s Comprehensive Minority Health Research Center of Excellence (National Centers for Minority Health and Health Disparities P60 MD002254).
References
- Alexander KE, Ventura EE, Spruijt-Metz D, Weigensberg MJ, Goran MI, Davis JN. Association of Breakfast Skipping With Visceral Fat and Insulin Indices in Overweight Latino Youth. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty:A contributing factor to poor glycemic control in adolescents with diabetes. The New England Journal of Medicine. 1986;315(4):215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: Results from the third national health and nutrition examination survey. JAMA: Journal of the American Medical Association. 1998;279(12):938–942. doi: 10.1001/jama.279.12.938. [DOI] [PubMed] [Google Scholar]
- Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(12):938–942. doi: 10.1001/jama.279.12.938. [see comment] [DOI] [PubMed] [Google Scholar]
- Anzman S, Birch L. Low Inhibitory Control and Restrictive Feeding Practices Predict Weight Outcomes. The Journal of Pediatrics. 2009 doi: 10.1016/j.jpeds.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslanian S, Suprasongsin C. Difference in the in vivo secretion and sensitivity of healthy black versus white adolescents. J Pediatrics. 1996;129:440–443. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. JCEM. 1997;82(6):1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiology & Behavior. 2007;90(2–3):229–235. doi: 10.1016/j.physbeh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlantis E, Barnes EH, Singh MA. Efficacy of exercise for treating overweight in children and adolescents: a systematic review. Int J Obes (Lond) 2006;30(7):1027–1040. doi: 10.1038/sj.ijo.0803286. [DOI] [PubMed] [Google Scholar]
- Ball G, Huang T-T, Gower B, Goran M. Longitudinal changes in insulin sensitivity, insulin secretion and beta-cell function during puberty in Caucasian and African American Youth. J Pediatrics. 2006;148:16–22. doi: 10.1016/j.jpeds.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Ball GD, Weigensberg MJ, Cruz ML, Shaibi GQ, Kobaissi HA, Goran MI. Insulin resistance and beta-cell function across Tanner stages in overweight Hispanic children with a family history of type 2 diabetes. Int J Obes. 2005;29(12):1471–1477. doi: 10.1038/sj.ijo.0803044. [DOI] [PubMed] [Google Scholar]
- Ball K, Timperio AF, Crawford DA. Understanding environmental influences on nutrition and physical activity behaviors: where should we look and what should we count? Int J Behav Nutr Phys Act. 2006;3:33. doi: 10.1186/1479-5868-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski T, Cullen KW, Baranowski J. Psychosocial correlates of dietary intake: advancing dietary intervention. Annu Rev Nutr. 1999;19:17–40. doi: 10.1146/annurev.nutr.19.1.17. [DOI] [PubMed] [Google Scholar]
- Baranowski T, Lin LS, Wetter DW, Resnicow K, Hearn MD. Theory as mediating variables: Why aren't community interventions working as desired? Annals of Epidemiology. 1997;7(7) [Google Scholar]
- and the Expert Committee. Barlow SE. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120 Supplement_4:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European Cytokine Network. 2006;17(1):4–12. [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann.N.Y.Acad.Sci. 1999;896:131. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Belcher B, Berrigan D, Dodd K, Emken A, Chou C-P, Spruijt-Metz D. Physical Activity in US Youth: Impact of Race/Ethnicity, Age, Gender, & Weight Status. doi: 10.1249/MSS.0b013e3181e1fba9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LM, Watts K, Siafarikas A, Thompson A, Ratnam N, Bulsara M, et al. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab. 2007;92(11):4230–4235. doi: 10.1210/jc.2007-0779. [DOI] [PubMed] [Google Scholar]
- Bergman R. Minimal model: perspective from 2005. Horm Res. 2005;64 Suppl 3:8–15. doi: 10.1159/000089312. [DOI] [PubMed] [Google Scholar]
- Bergman R, Kim S, Catalano K, Hsu I, Chiu J, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14(2s):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- Bergman R, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. Journal of Clinical Investigation. 1987;79(3):790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11(9):351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51 Suppl 1:S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HRH, Colditz GA. Weight Gain in Older Adolescent Females: The Internet, Sleep, Coffee, and Alcohol. The Journal of Pediatrics. 2008 doi: 10.1016/j.jpeds.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Rockett HRH, Field AE, Gillman MW, Colditz GA. Sugar-Added Beverages and Adolescent Weight Change. Obes Res. 2004;12(5):778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls' eating in the absence of hunger. American Journal of Clinical Nutrition. 2003;78(2):215–220. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int.J.Obes.Relat Metab Disord. 2000a;24 Suppl 2:S80. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000b;16(10):924. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2009 doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G. Free fatty acids and insulin secretion in humans. Curr Diab Rep. 2005;5(3):167–170. doi: 10.1007/s11892-005-0004-5. [DOI] [PubMed] [Google Scholar]
- Brown T, Summerbell C. Systematic review of school-based interventions that focus on changing dietary intake and physical activity levels to prevent childhood obesity: an update to the obesity guidance produced by the National Institute for Health and Clinical Excellence. Obes Rev. 2009;10(1):110–141. doi: 10.1111/j.1467-789X.2008.00515.x. [DOI] [PubMed] [Google Scholar]
- Bruusgaard D, Smedbraten BK, Natvig B. Bodily pain, sleep problems and mental distress in schoolchildren. Acta Paediatrica. 2000;89(5):597–600. doi: 10.1080/080352500750027925. [DOI] [PubMed] [Google Scholar]
- Burdette HL, Whitaker RC. Neighborhood playgrounds, fast food restaurants, and crime: relationships to overweight in low-income preschool children. Preventive Medicine. 2004;38(1):57–63. doi: 10.1016/j.ypmed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Butte NF, Bacino CA, Cole SA, Comuzzie AG. Genetics of childhood obesity. In: Goran MI, Southern M, editors. Handbook of Pediatric Obesity: Etiology, Pathophysiology and Prevention. Boca Raton, FL: Taylor & Francis Books/CRC Press; 2006. pp. 79–96. [Google Scholar]
- Calamaro CJ, Mason TB, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123(6):e1005–e1010. doi: 10.1542/peds.2008-3641. [DOI] [PubMed] [Google Scholar]
- Calle E, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Canning H, Mayer J. Obesity's possible effect on college acceptance. New England Journal of Medicine. 1966;275(1):172–171. [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Casazza K, Phadke RP, Fernandez JR, Watanabe RM, Goran MI, Gower BA. Obesity attenuates the contribution of African admixture to the insulin secretory profile in peripubertal children: a longitudinal analysis. Obesity (Silver Spring) 2009;17(7):1318–1325. doi: 10.1038/oby.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31(4):517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm.Res. 2003;59(4):161. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Chopra M, Galbraith S, Darnton-Hill I. A global response to a global problem: the epidemic of overnutrition. Bulletin of the World Health Organization. 2002;80(12):952–958. [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int.J.Obes.Relat Metab Disord. 2000;24 Suppl 2:S50. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Ashwood JS, Scott MM, Overton A, Evenson KR, Staten LK, et al. Public Parks and Physical Activity Among Adolescent Girls. Pediatrics. 2006;118(5):e1381. doi: 10.1542/peds.2006-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JB, Duaso MJ, Butler G. A systematic review of controlled trials of interventions to prevent childhood obesity and overweight: a realistic synthesis of the evidence. Public Health. 2007;121(7):510–517. doi: 10.1016/j.puhe.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Connor JM. Physical activity and well-being. 2004 [Google Scholar]
- Crawford D, Timperio A, Salmon J, Baur L, Giles-Corti B, Roberts R, et al. Neighbourhood fast food outlets and obesity in children and adults: the CLAN Study. International Journal of Pediatric Obesity. 2008;3(4):249–256. doi: 10.1080/17477160802113225. [DOI] [PubMed] [Google Scholar]
- Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GDC, Goran MI. Pediatric Obesity and Insulin Resistance: Chronic Disease Risk and Implications for Treatment and Prevention Beyond Body Weight Modification. Annual Review of Nutrition. 2005;25(1):435–468. doi: 10.1146/annurev.nutr.25.050304.092625. [DOI] [PubMed] [Google Scholar]
- Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. Journal of Clinical Endocrinology & Metabolism. 2004;89(1):108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. Journal of Clinical Investigation. 2007;117(1):13. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: A new view of "comfort food". Proceedings of the National Academy of Sciences. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JN, Alexander KE, Ventura EE, Kelly LA, Lane CJ, Byrd-Williams CE, et al. Associations of dietary sugar and glycemic index with adiposity and insulin dynamics in overweight Latino youth. Am J Clin Nutr. 2007;86(5):1331–1338. doi: 10.1093/ajcn/86.5.1331. [DOI] [PubMed] [Google Scholar]
- Davis JN, Ventura EE, Alexander KA, Salguero LE, Weigensberg MJ, Crespo N, et al. Development and testing of a culturally tailored nutrition education program for reducing sugar and increasing fiber intake in overweight Latina adolescents. Int J Ped Obes. 2007;2:22–30. [Google Scholar]
- Davison K, Lawson C. Do attributes in the physical environment influence children's physical activity? A review of the literature. Int J Behav Nutr Phys Act. 2006;3:19. doi: 10.1186/1479-5868-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison KK, Cutting TM, Birch LL. Parents' activity-related parenting practices predict girls' physical activity. Medicine & Science in Sports & Exercise. 2003;35(9):1589–1595. doi: 10.1249/01.MSS.0000084524.19408.0C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison KK, Lawson CT. Do attributes in the physical environment influence children's physical activity? A review of the literature. The International Journal of Behavioral Nutrition and Physical Activity. 2006;3:19. doi: 10.1186/1479-5868-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clinical Chemistry. 2008;54(6):945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research, G. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doak C, Heitmann BL, Summerbell C, Lissner L. Prevention of childhood obesity - what type of evidence should we consider relevant? Obes Rev. 2009;10(3):350–356. doi: 10.1111/j.1467-789X.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- Dobbins M, De Corby K, Robeson P, Husson H, Tirilis D. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6–18. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD007651. CD007651. [DOI] [PubMed] [Google Scholar]
- Dunton GF, Kaplan J, Wolch J, Jerrett M, Reynolds KD. Physical environmental correlates of childhood obesity: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2009 doi: 10.1111/j.1467-789X.2009.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworak M, Schierl T, Bruns T, Struder H. Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics. 2007;120(5):978. doi: 10.1542/peds.2007-0476. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117(3):673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A Reduced-Glycemic Load Diet in the Treatment of Adolescent Obesity. Arch Pediatr Adolesc Med. 2003;157(8):773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC, Bartee RT, Wang MQ. Physical activity, TV viewing, and weight in U.S. youth: 1999 Youth Risk Behavior Survey. Obesity Research. 2002;10(5):379–385. doi: 10.1038/oby.2002.52. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Sarnblad S, Brage S, Ryberg J, Wareham NJ, Aman J. Does physical activity equally predict gain in fat mass among obese and nonobese young adults? Int J Obes. 2006;31(1):65–71. doi: 10.1038/sj.ijo.0803361. [DOI] [PubMed] [Google Scholar]
- Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J.Psychosom.Res. 2002;53(4):891. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Fernández J, Redden D, Pietrobelli A, Allison D. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. The Journal of pediatrics. 2004;145(4):439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Ferreira I, Van der Horst K, Wendel-Vos W, Kremers S, Van Lenthe F, Brug J. Environmental correlates of physical activity in youth a review and update. Obesity Reviews. 2007;8(2):129–154. doi: 10.1111/j.1467-789X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Fields DA, Goran MI. Body composition techniques and the four-compartment model in children. Journal of Applied Physiology. 2000;89(2):613–620. doi: 10.1152/jappl.2000.89.2.613. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs. 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Flodmark CE, Marcus C, Britton M. Interventions to prevent obesity in children and adolescents: a systematic literature review. Int J Obes (Lond) 2006;30(4):579–589. doi: 10.1038/sj.ijo.0803290. [DOI] [PubMed] [Google Scholar]
- Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. American Journal of Clinical Nutrition. 2008;87(6):1662–1671. doi: 10.1093/ajcn/87.6.1662. [DOI] [PubMed] [Google Scholar]
- Fox MK, Dodd AH, Wilson A, Gleason PM. Association between school food environment and practices and body mass index of US public school children. Journal of the American Dietetic Association. 2009;109(2 Suppl):S108–S117. doi: 10.1016/j.jada.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Frayn K. Visceral fat and insulin resistanceócausative or correlative? British Journal of Nutrition. 2007;83(S1):71–77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103(6 Pt 1):1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism: Clinical and Experimental. 1987;36(1):54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Ge X, Elder CH, Jr, Regnerus M, Cox C. Pubertal transitions, perceptions of being overweight, and adolescents' psychological maladjustment: Gender and ethnic differences. Social Psychology Quarterly. 2001;64(4):363–375. [Google Scholar]
- Gibson S. Are high-fat, high-sugar foods and diets conducive to obesity? International Journal of Food Sciences and Nutrition. 1996;47(5):405–415. doi: 10.3109/09637489609006954. [DOI] [PubMed] [Google Scholar]
- Goodman E. The role of socioeconomic status gradients in explaining differences in US adolescents' health. American Journal of Public Health. 1999;89(10):1522–1528. doi: 10.2105/ajph.89.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. Am.J.Public Health. 2003;93(11):1844. doi: 10.2105/ajph.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI. Metabolic precursors and effects of obesity in children: a decade of progress, 1990–1999. American Journal of Clinical Nutrition. 2001;73(2):158–171. doi: 10.1093/ajcn/73.2.158. [DOI] [PubMed] [Google Scholar]
- Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. Journal of Clinical Endocrinology & Metabolism. 2003;88(4):1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- Goran MI, Cruz ML, Bergman RN, Watanabe RM. Insulin resistance and the associated compensatory response in Caucasian, African American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- Goran MI, Gower BA. Longitudinal study of pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- Goran MI, Herd SL, Gower BA. Influence of total body fat versus visceral and abdominal fat on insulin action and insulin secretion in African American and Caucasian children. Diabetes. 2000 [Google Scholar]
- Goran MI, Reynolds KD, Lindquist CH. Role of physical activity in the prevention of obesity in children. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1999;23 Suppl 3:S18–S33. doi: 10.1038/sj.ijo.0800880. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Adair LS, Popkin BM. The relationship of ethnicity, socioeconomic factors, and overweight in US adolescents. Obesity Research. 2003;11(1):121–129. doi: 10.1038/oby.2003.20. [erratum appears in Obes Res. 2003 Apr;11(4):597] [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, McMurray R, Popkin B. Determinants of Adolescent Physical Activity and Inactivity Patterns. Pediatrics. 2000;105(6):1–8. doi: 10.1542/peds.105.6.e83. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, McMurray RG, Popkin BM. Determinants of adolescent physical activity and inactivity patterns. Pediatrics. 2000;105(6):E83. doi: 10.1542/peds.105.6.e83. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the Built Environment Underlies Key Health Disparities in Physical Activity and Obesity. Pediatrics. 2006;117(2):417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. New England Journal of Medicine. 1993;329(14):1008–1012. doi: 10.1056/NEJM199309303291406. [see comment] [DOI] [PubMed] [Google Scholar]
- Gortmaker SL, Peterson K, Wiecha J, Sobal AM, Dixit S, Fox MK, et al. Reducing Obesity via a School-Based Interdisciplinary Intervention Among Youth: Planet Health. Archives of Pediatric& Adolescent Medicine. 1999;153(4):409–418. doi: 10.1001/archpedi.153.4.409. [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI. Fat distribution and insulin response in pre-pubertal African American and Caucasian children. American Journal of Clinical Nutrition. 1997;67:821–827. doi: 10.1093/ajcn/67.5.821. [DOI] [PubMed] [Google Scholar]
- Gregory J, Foster K, Tyler H, Wiseman M. The Dietary and Nutritional Survey of British Adults. London: HMSO. 1990;12 [Google Scholar]
- Grilo CM, Pogue-Geile MF. The nature of environmental influences on weight and obesity: a behavior genetic analysis. Psychological Bulletin. 1991;110(3):520–537. doi: 10.1037/0033-2909.110.3.520. [DOI] [PubMed] [Google Scholar]
- Gupta NK. Is obesity associated with poor sleep quality in adolescents? University of Texas Health Science Center at Houston, School of Public Health; 2001. [Google Scholar]
- Hardy LL, Bass SL, Booth ML. Changes in Sedentary Behavior among Adolescent Girls: A 2.5-Year Prospective Cohort Study. Journal of Adolescent Health. 2007;40(2):158–165. doi: 10.1016/j.jadohealth.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Thiesen F. Genetic aspects of obesity. In: Antel J, Finer N, Heal D, Krause G, editors. Obesity and metabolic disorders. Amsterdam: IOS Press; 2005. [Google Scholar]
- Hewitt JK. The genetics of obesity: what have genetic studies told us about the environment. Behavior Genetics. 1997;27(4):353–358. doi: 10.1023/a:1025687930765. [DOI] [PubMed] [Google Scholar]
- Hill J, Peters J. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Hoffman RP, Vicini P, Sivitz WI, Cobelli C. Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartment minimal model. Pediat.Res. 2000;48(3):384–388. doi: 10.1203/00006450-200009000-00022. [DOI] [PubMed] [Google Scholar]
- Hsu Y-W, Ventura EE, Davis J, Belcher BR, Byrd-Williams C, Goran MI, et al. Relationships between Physical Activity and Metabolic Syndrome in Minority Youth. Obesity. 2009;17(S2):149. [Google Scholar]
- Hu H, Kim H, Nayak K, Goran M. Comparison of Fat-Water MRI and Single-voxel MRS in the Assessment of Hepatic and Pancreatic Fat Fractions in Humans. Obesity. 2009 doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Obesity Task Force. [Retrieved December 19, 2009];2009 July 21; 2009, from http://www.iotf.org/database/documents/GlobalChildhoodOverweightJuly09_000.pdf.
- Jago R, Baranowski T, Baranowski J, Thompson D, Greaves K. PEDIATRIC HIGHLIGHT BMI from 3–6 y of age is predicted by TV viewing and physical activity, not diet. International Journal of Obesity. 2005;29:557–564. doi: 10.1038/sj.ijo.0802969. [DOI] [PubMed] [Google Scholar]
- Jee SH, Kim HJ, Lee J. Obesity, insulin resistance and cancer risk. Yonsei Medical Journal. 2005;46(4):449–455. doi: 10.3349/ymj.2005.46.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, McConnell R, Chang CC, Wolch J, Reynolds K, Lurmann F, et al. Automobile traffic around the home and attained body mass index: A longitudinal cohort study of children aged 10–18 years. Preventive Medicine. 2009 doi: 10.1016/j.ypmed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joassart-Marcelli PM, Musso JA, Wolch JR. Fiscal Consequences of Concentrated Poverty in a Metropolitan Region. Annals of the Association of American Geographers. 2005;95(2):336–356. [Google Scholar]
- Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19(4):547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Kamath CC, Vickers KS, Ehrlich A, McGovern L, Johnson J, Singhal V, et al. Clinical review: behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. Journal of Clinical Endocrinology and Metabolism. 2008;93(12):4606–4615. doi: 10.1210/jc.2006-2411. [DOI] [PubMed] [Google Scholar]
- Kimm SY, Barton BA, Berhane K, Ross JW, Payne GH, Schreiber GB. Self-esteem and adiposity in black and white girls: the NHLBI Growth and Health Study. Annals of Epidemiology. 1997;7(8):550–560. doi: 10.1016/s1047-2797(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. Journal of Clinical Endocrinology and Metabolism. 1982;54(2):254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- Klein DJ, Aronson Friedman L, Harlan WR, Barton BA, Schreiber GB, Cohen RM, et al. Obesity and the development of insulin resistance and impaired fasting glucose in black and white adolescent girls: a longitudinal study. Diabetes Care. 2004;27(2):378–383. doi: 10.2337/diacare.27.2.378. [DOI] [PubMed] [Google Scholar]
- Koplan JP, Liverman CT, Kraak VI. Preventing childhood obesity: health in the balance: executive summary. Journal of the American Dietetic Association. 2005;105(1):131–138. doi: 10.1016/j.jada.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Korner J, Leibel RL. To eat or not to eat - how the gut talks to the brain. New England Journal of Medicine. 2003;349(10):926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- Kriketos AD, Greenfield JR, Peake PW, Furler SM, Denyer GS, Charlesworth JA, et al. Inflammation, insulin resistance, and adiposity: a study of first-degree relatives of type 2 diabetic subjects. Diabetes Care. 2004;27(8):2033–2040. doi: 10.2337/diacare.27.8.2033. [DOI] [PubMed] [Google Scholar]
- Krug AW, Ehrhart-Bornstein M. Newly discovered endocrine functions of white adipose tissue: possible relevance in obesity-related diseases. Cellular and Molecular Life Sciences. 2005;62(12):1359–1362. doi: 10.1007/s00018-005-4555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik MY, Lytle LA, Hannan PJ, Perry CL, Story M. The association of the school food environment with dietary behaviors of young adolescents. American Journal of Public Health. 2003;93(7):1168–1173. doi: 10.2105/ajph.93.7.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R, Ogden C, Guo S, Grummer-Strawn L, Flegal K, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11, Data from the national health survey. 2002;(246):1. [PubMed] [Google Scholar]
- Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. American Journal of Clinical Nutrition. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003;284(5):E863–E873. doi: 10.1152/ajpendo.00033.2003. [DOI] [PubMed] [Google Scholar]
- Latner J, Stunkard A. Schoolchildren, stigma, and obesity. Obesity Research. 2001;9:169–169. [Google Scholar]
- Lee G, Tsai C, Griswold W, Raab F, Patrick K. PmEB: a mobile phone application for monitoring caloric balance. 2006 [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep(New York, NY) 1997;20(10):865–870. [PubMed] [Google Scholar]
- Li S, Jin X, Wu S, Jiang F, Yan C, Shen X. The impact of media use on sleep patterns and sleep disorders among school-aged children in China. Sleep. 2007;30(3):361–367. doi: 10.1093/sleep/30.3.361. [DOI] [PubMed] [Google Scholar]
- Lissau I, Overpeck MD, Ruan WJ, Due P, Holstein BE, Hediger ML. Body Mass Index and Overweight in Adolescents in 13 European Countries, Israel, and the United States. Archives of Pediatrics and Adolescent Medicine. 2004;158(1):27–33. doi: 10.1001/archpedi.158.1.27. [DOI] [PubMed] [Google Scholar]
- Livingstone MBE, Robson PJ. Measurement of dietary intake in children. Proceedings of the Nutrition Society. 2000;59:279–293. doi: 10.1017/s0029665100000318. [DOI] [PubMed] [Google Scholar]
- Lobstein T, Baur L, Uauy R. Obesity in children and young people: A crisis in public health. London, England: IASO International Obesity Task Force; 2004. [DOI] [PubMed] [Google Scholar]
- Lubans DR, Foster C, Biddle SJ. A review of mediators of behavior in interventions to promote physical activity among children and adolescents. Preventive Medicine. 2008;47(5):463–470. doi: 10.1016/j.ypmed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Markward NJ, Markward MJ, Peterson CA. Biological and genetic influences. In: Heinberg LJ, Thompson JK, editors. Obesity in youth: Causes, consequences, and cures. Washington, DC: American Psychological Association; 2009. [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of Time Spent in Sedentary Behaviors in the United States, 2003–2004. American Journal of Epidemiology. 2008 doi: 10.1093/aje/kwm390. kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Stockton MB. Childhood obesity: are we missing the big picture? Obes Rev. 2008;9(1):35–42. doi: 10.1111/j.1467-789X.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom.Med. 1990;52(1):97. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- McClain AD, Chappuis CK, Nguyen-Rodriguez ST, Yaroch AL, Spruijt-Metz D. Psychosocial correlates of eating behavior in children and adolescents: a review. Int J Behav Nutr Phys Act. 2009;6(1):54. doi: 10.1186/1479-5868-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl KA. The sugar-fat seesaw. Nutrition Bulletin. 1988;13(2):114–118. [Google Scholar]
- McCulloch W. A heterarchy of values determined by the topology of nervous nets. Bulletin of Mathematical Biology. 1945;7(2):89–93. doi: 10.1007/BF02478429. [DOI] [PubMed] [Google Scholar]
- McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. Journal of Clinical Endocrinology and Metabolism. 2008;93(12):4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290(10):1331–1336. doi: 10.1001/jama.290.10.1331. [see comment] [DOI] [PubMed] [Google Scholar]
- Misra A, Vikram NK, Arya S, Pandey RM, Dhingra V, Chatterjee A, et al. High prevalence of insulin resistance in postpubertal Asian Indian children is associated with adverse truncal body fat patterning, abdominal adiposity and excess body fat. Int J Obes Relat Metab Disord. 2004;28(10):1217–1226. doi: 10.1038/sj.ijo.0802704. [DOI] [PubMed] [Google Scholar]
- Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obesity Research. 2003;11(9):1048–1054. doi: 10.1038/oby.2003.144. [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs DRJ, Steinberger J, Hong C-P, Prineas R, Luepker RV, et al. Insulin resistance during puberty: Result form clamp studies in 357 childred. Diabetes. 1999;48(10):2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- Morland K, Diez Roux A, Wing S. Supermarkets, Other Food Stores, and Obesity The Atherosclerosis Risk in Communities Study. American Journal of Preventive Medicine. 2006;30(4):333–339. doi: 10.1016/j.amepre.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Morland K, Wing S, Diez Roux A. The contextual effect of the local food environment on residents' diets: the atherosclerosis risk in communities study. Am J Public Health. 2002;92(11):1761–1767. doi: 10.2105/ajph.92.11.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musani SK, Erickson S, Allison DB. Obesity--still highly heritable after all these years. American Journal of Clinical Nutrition. 2008;87(2):275–276. doi: 10.1093/ajcn/87.2.275. [DOI] [PubMed] [Google Scholar]
- Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes Relat Metab Disord. 2005;29(S2):S84–S96. doi: 10.1038/sj.ijo.0803064. [DOI] [PubMed] [Google Scholar]
- Nader P, Bradley R, Houts R, McRitchie S, O'Brien M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA. 2008;300(3):295. doi: 10.1001/jama.300.3.295. [DOI] [PubMed] [Google Scholar]
- Nader P, Bradley R, Houts R, McRitchie S, O'Brien M. Incorrect Data in: Moderate-to-Vigorous Physical Activity From Ages 9 to 15 Years. JAMA. 2009;301(20):2095–2098. doi: 10.1001/jama.2009.701. [DOI] [PubMed] [Google Scholar]
- Neary NM, Goldstone AP, Bloom SR. Appetite regulation: from the gut to the hypothalamus. Clinical Endocrinology. 2004;60(2):153–160. doi: 10.1046/j.1365-2265.2003.01839.x. [DOI] [PubMed] [Google Scholar]
- Neel JV. Diabetes mellitus: a "thrifty" genotype rendered detrimental by "progress"? American Journal of Human Genetics. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Michel ST, Unger JB, Spruijt-Metz D. Dietary correlates of emotional eating in adolescence. Appetite. 2007 doi: 10.1016/j.appet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Rodriguez ST, Chou CP, Unger JB, Spruijt-Metz D. BMI as a moderator of perceived stress and emotional eating in adolescents. Eating Behaviors. 2008;9(2):238–246. doi: 10.1016/j.eatbeh.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of High Body Mass Index in US Children and Adolescents, 2007–2008. JAMA. 2010:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of Overweight and Obesity in the United States, 1999–2004. Am Med Assoc. 2006;Vol. 295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends of in overweight among US children and adolescents, 1999–2000. Journal of the American Medical Association. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom.Med. 2000;62(6):853. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. New England Journal of Medicine. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [see comment] [DOI] [PubMed] [Google Scholar]
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O'Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD001872.pub2. CD001872. [DOI] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int.J Obes.Relat Metab Disord. 2000;24 Suppl 2:S47. [PubMed] [Google Scholar]
- Patel A, Bernstein L. Physical activity and cancer incidence: Breast cancer. In: McTiernan A, editor. Physical Activity, Energy Balance, and Cancer: Etiology and Prognosis. New York, NY: Marcel Dekker, Inc; (in press) [Google Scholar]
- Patrick K, Griswold W, Raab F, Intille S. Health and the mobile phone. American Journal of Preventive Medicine. 2008;35(2):177–181. doi: 10.1016/j.amepre.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington DC: Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- Pinhas-Hamiel O, Zeitler P. Insulin resistance, obesity, and related disorders among black adolescents. Journal of Pediatrics. 1996;129(3):319–320. doi: 10.1016/s0022-3476(96)70060-4. [editorial; comment] [DOI] [PubMed] [Google Scholar]
- Popkin BM. Recent dynamics suggest selected countries catching up to US obesity. American Journal of Clinical Nutrition. 2009 doi: 10.3945/ajcn.2009.28473C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitakari OT, Porkka KV, Taimela S, Telama R, Rasanen L, Viikari JS. Effects of persistent physical activity and inactivity on coronary risk factors in children and young adults. The Cardiovascular Risk in Young Finns Study. Am J Epidemiol. 1994;140(3):195–205. doi: 10.1093/oxfordjournals.aje.a117239. [DOI] [PubMed] [Google Scholar]
- Rampersaud E, Mitchell B, Pollin T, Fu M, Shen H, O'Connell J, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Archives of Internal Medicine. 2008;168(16):1791. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes/Metabolism Reviews. 1998;14(4):263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Valencia ME, Esparza J, Bennett PH, Schulz LO. Effects of a traditional lifestyle on obesity in Pima Indians. Diabetes Care. 1994;17(9):1067–1074. doi: 10.2337/diacare.17.9.1067. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2002;26(5):701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- Rolls B, Shide D. The influence of dietary fat on food intake and body weight. Nutrition Reviews. 2009;50(10):283–290. doi: 10.1111/j.1753-4887.1992.tb02466.x. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Ortega F. Physical activity and cardiovascular disease risk factors in children and adolescents. Current Cardiovascular Risk Reports. 2009;3(4):281–287. [Google Scholar]
- Sallis JF, Glanz K. The role of built environments in physical activity, eating, and obesity in childhood. The Future of Children. 2006;16(1):89–108. doi: 10.1353/foc.2006.0009. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Medicine & Science in Sports & Exercise May. 2000;32(5):963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Zakarian JM, Hovell MF, Hofstetter CR. Ethnic, socioeconomic, and sex differences in physical activity among adolescents. Journal of Clinical Epidemiology. 1996;49(2):125–134. doi: 10.1016/0895-4356(95)00514-5. [see comments] [DOI] [PubMed] [Google Scholar]
- Sekine M, Yamagami T, Handa K, Saito T, Nanri S, Kawaminami K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health and Development. 2002;28(2):163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Salem GJ, Crespo NC, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Medicine and Science in Sports and Exercise. 2006;38(7):1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- Shaibi GQ, Goran MI. Examining Metabolic Syndrome Definitions in Overweight Hispanic Youth: A focus on insulin resistance. Journal of Pediatrics. 2008;152(2):171–176. doi: 10.1016/j.jpeds.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: A systematic review of cross-sectional studies 1990ñ2005. Obesity. 2008;16(2):275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- Smith SR, Ravussin E. Genetic and physiological factors in obesity. Journal of the Louisiana State Medical Society. 2005;157(Spec No 1):S12–S18. [PubMed] [Google Scholar]
- Sobal J, Stunkard A. Socioeconomic status and obesity: a review of the literature. Psychological Bulletin. 1989;105(2):260–275. doi: 10.1037/0033-2909.105.2.260. [DOI] [PubMed] [Google Scholar]
- Spence J, Cutumisu N, Edwards J, Evans J. Influence of neighbourhood design and access to facilities on overweight among preschool children. International Journal of Pediatric Obesity. 2008;3(2):109–116. doi: 10.1080/17477160701875007. [DOI] [PubMed] [Google Scholar]
- Spiegel K. Sleep loss as a risk factor for obesity and diabetes. International Journal of Pediatric Obesity. 2008;3:27–28. doi: 10.1080/17477160802404681. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology & Metabolism. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Tasali E, Penev P, Van Cauter E. Sleep curtailment results in decreased leptin levels and increased hunger and appetite. Sleep. 2003;26:A174. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [see comment][summary for patients in Ann Intern Med. 2004 Dec 7;141(11):I52; PMID: 15583222] [DOI] [PubMed] [Google Scholar]
- Spilsbury J, Storfer-Isser A, Drotar D, Rosen C, Kirchner H, Redline S. Effects of the home environment on school-aged children's sleep. Sleep. 2005;28(11):1419. doi: 10.1093/sleep/28.11.1419. [DOI] [PubMed] [Google Scholar]
- Spilsbury JC, Storfer-Isser A, Drotar D, Rosen CL, Kirchner LH, Benham H, et al. Sleep behavior in an urban US sample of school-aged children. Archives of Pediatrics & Adolescent Medicine. 2004;158(10):988–994. doi: 10.1001/archpedi.158.10.988. [DOI] [PubMed] [Google Scholar]
- Spruijit-Metz D, Davis JN, Nguyen-Rodriguez ST. Behavior, Energy Balance and Cancer, Overview. In: Berger N, editor. Energy Balance and Cancer. New York: Springer; (in press) [Google Scholar]
- Spruijt-Metz D. Adolescence, affect and health. London: Psychology Press; 1999. [Google Scholar]
- Spruijt-Metz D, Belcher B, Anderson D, Lane CJ, Chou CP, Salter-Venzon D, et al. A high-sugar/low-fiber meal compared with a low-sugar/high-fiber meal leads to higher leptin and physical activity levels in overweight Latina females. Journal of the American Dietetic Association. 2009;109(6):1058–1063. doi: 10.1016/j.jada.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt-Metz D, Berrigan D, Kelly L, McConnell R, Dueker D, Lindsey G, et al. Measures of physical activity and exercise. In: Allison DB, Baskin ML, editors. Handbook of Assessment Methods for Eating Behaviors and Weight-Related Problems: Measures, Theory, and Research. 2009. pp. 187–254. [Google Scholar]
- Staffieri J. A study of social stereotype of body image in children. Journal of Personality and Social Psychology. 1967;7(1):101–104. doi: 10.1037/h0021227. [DOI] [PubMed] [Google Scholar]
- Steinberger J, Moran A, Hong CP, Jacobs DR, Jr, Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. Journal of Pediatrics. 2001;138(4):469–473. doi: 10.1067/mpd.2001.112658. [comment] [DOI] [PubMed] [Google Scholar]
- Stice E, Shaw H, Marti CN. A Meta-Analytic Review of Obesity Prevention Programs for Children and Adolescents: The Skinny on Interventions that Work. Psychological bulletin. 2006;132(5):667. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A, Shiffman S, Atienza AA, Nebeling L, editors. The Science of real-time data capture: Self-reports in health research. New York: Oxford University Press; 2007. [Google Scholar]
- Story M, Kaphingst KM, Robinson-O'Brien R, Glanz K. Creating healthy food and eating environments: policy and environmental approaches. Annual Review of Public Health. 2008;29:253–272. doi: 10.1146/annurev.publhealth.29.020907.090926. [DOI] [PubMed] [Google Scholar]
- Story M, Nanney M, Schwartz M. Schools and obesity prevention: creating school environments and policies to promote healthy eating and physical activity. The Milbank Quarterly. 2009;87(1):71–100. doi: 10.1111/j.1468-0009.2009.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986–1998. Jama. 2001;286(22):2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- Strauss RS, Pollack HA. Social marginalization of overweight children. Archives of Pediatrics & Adolescent Medicine. 2003;157(8):746–752. doi: 10.1001/archpedi.157.8.746. [DOI] [PubMed] [Google Scholar]
- Sturm R. The Effects Of Obesity, Smoking, And Drinking On Medical Problems And Costs. Health Affairs. 2002;21(2):245–253. doi: 10.1377/hlthaff.21.2.245. [DOI] [PubMed] [Google Scholar]
- Sturm R, Datar A. Body mass index in elementary school children, metropolitan area food prices and food outlet density. Public Health. 2005;119(12):1059–1068. doi: 10.1016/j.puhe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, et al. Using Intake Biomarkers to Evaluate the Extent of Dietary Misreporting in a Large Sample of Adults: The OPEN Study. Am. J. Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- Summerbell CD, Waters E, Edmunds LD, Kelly S, Brown T, Campbell KJ. Interventions for preventing obesity in children. Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD001871.pub2. [update of Cochrane Database Syst Rev. PMID: 12076426]. CD001871. [DOI] [PubMed] [Google Scholar]
- Sung RY, Lau P, Yu CW, Lam PK, Nelson EA. Measurement of body fat using leg to leg bioimpedance. Archives of Disease in Childhood. 2001;85(3):263–267. doi: 10.1136/adc.85.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svec F, Nastasi K, Hilton C, Bao W, Srinivasan SR, Berenson GS. Black-White contrasts in insulin levels during pubertal development. Diabetes. 1992;41:313–317. doi: 10.2337/diab.41.3.313. [DOI] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short Sleep Duration is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLOS MEDICINE. 2004;1(3):210. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte G, Li M, Emken A, Mitra U, Narayanan S, Annavaram M, et al. Energy-efficient multihypothesis activity-detection for health-monitoring applications. Conf Proc IEEE Eng Med Biol Soc. 2009;1:4678–4681. doi: 10.1109/IEMBS.2009.5334222. [DOI] [PubMed] [Google Scholar]
- Thune I, Brenn T, Lund E, Gaard M. Physical Activity and the Risk of Breast Cancer. N Engl J Med. 1997;336(18):1269–1275. doi: 10.1056/NEJM199705013361801. [DOI] [PubMed] [Google Scholar]
- Tiggemann M, Anesbury T. Negative stereotyping of obesity in children: The role of controllability beliefs. Journal of Applied Social Psychology. 2000;30(9):1977–1993. doi: 10.1093/her/15.2.145. [DOI] [PubMed] [Google Scholar]
- Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast Eating and Weight Change in a 5-Year Prospective Analysis of Adolescents: Project EAT (Eating Among Teens) Pediatrics. 2008;121(3):e638–e645. doi: 10.1542/peds.2007-1035. [DOI] [PubMed] [Google Scholar]
- Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and tanner stage differences in body composition and insulin sensitivity in early pubertal children. Journal of Clinical Endrocrinology & Metabolism. 1995;80(1):172–178. doi: 10.1210/jcem.80.1.7829608. [DOI] [PubMed] [Google Scholar]
- Treiber F, Harshfield G, Davis H, Kapuku G, Moore D. Stress responsivity and body fatness: links between socioeconomic status and cardiovascular risk factors in youth. Ann.N.Y. Acad.Sci. 1999;896:435. doi: 10.1111/j.1749-6632.1999.tb08163.x. [DOI] [PubMed] [Google Scholar]
- Troiano RP. A timely meeting: objective measurement of physical activity. Medicine & Science in Sports & Exercise. 2005;37(11) Suppl:S487–S489. doi: 10.1249/01.mss.0000185473.32846.c3. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Twenge JM. The age of anxiety? Birth cohort change in anxiety and neuroticism, 1952–1993. Journal of Personality & Social Psychology. 2000;79(6):1007–1021. doi: 10.1037//0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity 2001. Rockville, MD: 2001. [PubMed] [Google Scholar]
- Valente TW, Fujimoto K, Chou CP, Spruijt-Metz D. Adolescent affiliations and adiposity: a social network analysis of friendships and obesity. Journal of Adolescent Health. 2009;45(2):202–204. doi: 10.1016/j.jadohealth.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E. Sleep, awakenings, and insulin-like growth factor-I modulate the growth hormone (GH) secretory response to GH-releasing hormone. Journal of Clinical Endocrinology & Metabolism. 1992;74(6):1451–1459. doi: 10.1210/jcem.74.6.1592893. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, et al. Impact of Sleep and Sleep Loss on Neuroendocrine and Metabolic Function. Horm Res. 2007;67:2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- Van den Bulck J. Adolescent use of mobile phones for calling and for sending text messages after lights out: results from a prospective cohort study with a one-year follow-up. Sleep. 2007;30(9):1220. doi: 10.1093/sleep/30.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst K, Oenema A, Ferreira I, Wendel-Vos W, Giskes K, van Lenthe F, et al. A systematic review of environmental correlates of obesity-related dietary behaviors in youth. Health Education Research. 2007;22(2):203–226. doi: 10.1093/her/cyl069. [DOI] [PubMed] [Google Scholar]
- van Sluijs EMF, McMinn AM, Griffin SJ. Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials. BMJ. 2007;335(7622):703. doi: 10.1136/bmj.39320.843947.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura E, Davis J, Byrd-Williams C, Alexander K, McClain A, Lane CJ, et al. Reduction in risk factors for type 2 diabetes mellitus in response to a low-sugar, high-fiber dietary intervention in overweight Latino adolescents. Archives of Pediatrics and Adolescent Medicine. 2009;163(4):320–327. doi: 10.1001/archpediatrics.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ver Ploeg M, Breneman V, Farrigan T, Hamrick K, Hopkins D, Kaufman P, et al. Access to affordable and nutritious foodómeasuring and understanding food deserts and their consequences: report to Congress. US Department of Agriculture. 2009 [Google Scholar]
- Verloop J, Rookus MA, van der Kooy K, van Leeuwen FE. Physical activity and breast cancer risk in women aged 20–54 years. Journal of the National Cancer Institute. 2000;92(2):128–135. doi: 10.1093/jnci/92.2.128. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. American Journal of Clinical Nutrition. 2002;75(6):971–977. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]
- Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom.Res. 2000;48(2):195. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- Weigensberg MJ, Lane CJ, Winners O, Wright T, Nguyen-Rodriguez S, Goran MI, et al. Acute effects of stress-reduction Interactive Guided Imagery(SM) on salivary cortisol in overweight Latino adolescents. Journal of Alternative and Complementary Medicine. 2009;15(3):297–303. doi: 10.1089/acm.2008.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting Obesity in Young Adulthood from Childhood and Parental Obesity. New England Journal of Medicine. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1464–R1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White House Task Force on Childhood Obesity. Solving the problem of childhood obesity within a generation. Washington DC: 2010. [DOI] [PubMed] [Google Scholar]
- Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann.N.Y.Acad.Sci. 1999;896:173. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2004;286(1):G7–G13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16(10):894–902. doi: 10.1016/s0899-9007(00)00454-8. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ. Regulation of appetite, satiety and energy metabolism. In: Antel J, Finer N, Heal D, Krause G, editors. Obesity and metabolic disorders. Amsterdam: IOS Press; 2005. [Google Scholar]
- World Health Organization. Geneva: World Health Organization; Obesity: Preventing and managing the Global Epidemic - Report of a WHO Consultation on Obesity, 3–5 June 1997. 1997 (No. WHO/NUT/NCD/98) [PubMed]
- Wright J, Wang C, Kennedy-Stephenson J, Ervin R. Dietary intake of ten key nutrients for public health, United States: 1999ñ2000. Advance Data. 2003;334(17):1ñ4. [PubMed] [Google Scholar]
- Yoshii H, Lam TK, Gupta N, Goh T, Haber CA, Uchino H, et al. Effects of portal free fatty acid elevation on insulin clearance and hepatic glucose flux. Am J Physiol Endocrinol Metab. 2006;290(6):E1089–E1097. doi: 10.1152/ajpendo.00306.2005. [DOI] [PubMed] [Google Scholar]
- Young-Hyman D, Schlundt DG, Herman L, De Luca F, Counts D. Evaluation of the insulin resistance syndrome. Diabetes Care. 2001;24(8):1359–1364. doi: 10.2337/diacare.24.8.1359. [DOI] [PubMed] [Google Scholar]