Abstract
Purpose
Five-year survival rates for childhood cancer have improved over the past four decades. However, it is unknown whether changes in primary cancer therapy have improved rates of long-term (> 5 years from diagnosis) durable remissions and reduced treatment-related deaths. We investigated changes in patterns of late mortality over time and cause-specific attribution of late-mortality among 5-year survivors.
Patients and Methods
Using data from the Surveillance, Epidemiology and End Results (SEER) population-based registry, we assessed all-cause and cause-specific (recurrence/progression of primary disease, external cause, and nonrecurrence/nonexternal cause) late mortality during four consecutive time periods from 1974 through 2000 among 26,643 5-year survivors of childhood cancer.
Results
All-cause late mortality improved during more recent eras, dropping from 7.1% (95% CI, 6.4% to 7.8%) among children diagnosed during 1974 to 1980 to 3.9% (95% CI, 3.3% to 4.4%) among children diagnosed during 1995 to 2000 (P < .001), largely because of reduced mortality from recurrence or progression. While there was no significant reduction in mortality attributable to other health conditions (including treatment-related health conditions), analysis controlling for demographic characteristics identified a trend toward reduced risk during more recent eras (P = .007). Disparity by race/ethnicity was identified, with higher mortality among non-Hispanic blacks than among non-Hispanic whites for all-cause and nonrecurrence/nonexternal -cause late mortality.
Conclusion
While overall patterns of mortality from other health conditions do not differ over time, adjustment for demographic characteristics provides evidence that risk of treatment-related mortality may be lower in more recent eras. Disparities in health care utilization among survivors should be explored.
INTRODUCTION
Improvements in therapies for childhood cancer over the last four decades have resulted in significant increases in 5-year survival rates for most malignancies. The 5-year overall relative survival rate is 79.4% for patients diagnosed between 1999 and 2005.1 However, long-term survivors of childhood cancer are also at risk of late (> 5 years from diagnosis) mortality.2–8 During more recent decades, risk stratification of therapeutic intensity has guided primary therapy. In general, primary therapeutic regimens have been intensified for patients with poor prognoses in an attempt to reduce recurrence or progression of primary disease and thus improve long-term survival. Likewise, among patients identified as having a good prognosis, efforts have been directed toward reduction in intensity to prevent long-term morbidity and mortality from toxicity.
While detailed assessments of late mortality have been performed in selected cohorts of 5-year survivors, it remains unknown whether late mortality has declined among survivors of childhood cancer treated during more recent eras.3–5 Furthermore, assessment of cause-specific attribution of late mortality may be able to identify specific underlying etiologies of late mortality that have changed during more recent eras. Improvement in late mortality attributable specifically to progression of primary disease versus health conditions, including sequelae of cancer therapy, across consecutive time periods has not been assessed. To evaluate these questions, we used population-based data to describe temporal trends in late mortality over a 27-year period (1974 through 2000). We also report cause-specific mortality in an attempt to explain the contributions of treatment changes to changes in late mortality over time.
PATIENTS AND METHODS
We used data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (http://seer.cancer.gov) for the period 1974 through 2000. Eligibility criteria for inclusion in this analysis were a diagnosis of a malignant neoplasm before age 21 years and survival for 5 years after diagnosis. Diagnostic categories were defined according to the International Classification of Childhood Cancer (ICCC-3). Vital status (as of December 31, 2005) was determined by the National Center for Health Statistics and provided by the SEER program. Underlying cause of death was identified and classified as (1) recurrence/progression of primary malignancy, (2) external causes (eg, injuries, suicide, self-inflicted injury), or (3) nonrecurrence/nonexternal causes (ie, other health conditions, including treatment-related health conditions such as subsequent neoplasms, cardiac conditions, and pulmonary conditions). All cancer deaths were attributed to either primary or subsequent neoplasm. For cumulative mortality analysis, the 27-year period of evaluation was segmented into four consecutive time periods based on date of diagnosis (1974 to 1980, 1981 to 1987, 1988 to 1994, 1995 to 2000). We used Gray's method of accounting for competing risks of death to compare mortality by era.9 To further explore the effect of race/ethnicity on cumulative mortality, multiple pair-wise comparisons were conducted with Bonferroni correction in two-sided tests (P ≤ .005 indicated statistical significance). The effects of covariates such as age, sex, and race on the outcome of interest (all-cause mortality, recurrence/progression, or nonrecurrence/nonexternal cause) were estimated by using Fine and Gray's proportional hazards methods, an extension of the Cox proportional hazards model, to account for competing risks.10 Trend tests were used to assess the change of hazards by consecutive treatment era by incorporating treatment era as a covariate in the model and testing the significance of the regression coefficient. Standardized mortality ratios (SMRs) for 5-year age groups were calculated by using the expected number of deaths based on age-, year-, sex-, and race-specific US mortality rates and the corresponding person-years at risk observed, and then a test of heterogeneity of SMRs was performed.11 Statistical analyses were performed by using SAS 9.1.3 (SAS, Cary, NC) and R 2.8.0 software packages. All statistical tests were two-sided, and P ≤ .05 indicated statistical significance.
RESULTS
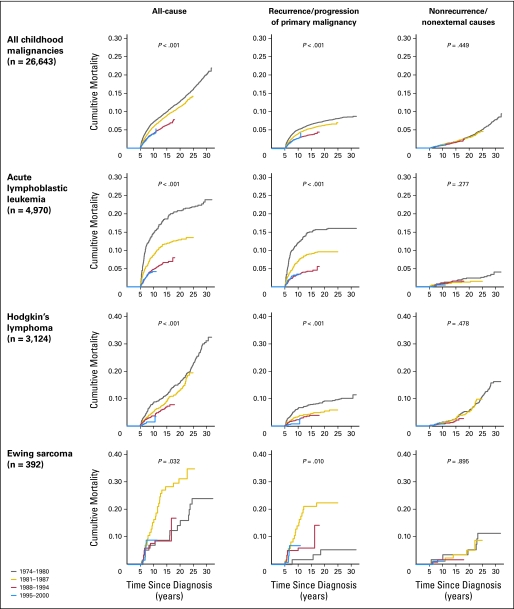
Among 26,643 eligible 5-year survivors (Table 1), there were 2,076 deaths, resulting in 10-, 15-, 20-, and 25-year all-cause cumulative mortality rates of 5.0%, 7.8%, 10.4%, and 13.7%, respectively. The SMR for all-cause mortality was 8.89 (95% CI, 8.52 to 9.29) (Table 2). All-cause mortality at 10 years from diagnosis declined over time from 7.1% (95% CI, 6.4% to 7.8%) among children diagnosed from 1974 through1980 to 3.9% (95% CI, 3.3% to 4.4%) among children diagnosed from 1995 through 2000 (P < .001: Table 3, Fig 1). This improvement in long-term survival by treatment era was true when the primary diagnosis was acute lymphoblastic leukemia (ALL; P < .001), Hodgkin's lymphoma (P < .001), non-Hodgkin's lymphoma (P = .006), or rhabdomyosarcoma (P = .05). In contrast, among long-term survivors of osteosarcoma, Ewing sarcoma, or ependymoma, late-mortality rates appear to have increased over time, although changes were statistically significant only for Ewing sarcoma (Fig 1; Data Supplement).
Table 1.
Life Status of 5-Year Survivors of Childhood Cancer
| Status | No. of Patients |
||
|---|---|---|---|
| Eligible Cohort | Alive | Dead | |
| All patients | 26,643 | 24,567 | 2,076 |
| Sex | |||
| Male | 13,754 | 12,540 | 1,214 |
| Female | 12,889 | 12,027 | 862 |
| Treatment era | |||
| 1974-1980 | 4,631 | 3,806 | 825 |
| 1981-1987 | 5,284 | 4,667 | 617 |
| 1988-1994 | 7,310 | 6,891 | 419 |
| 1995-2000 | 9,418 | 9,203 | 215 |
| Race/ethnicity | |||
| Non-Hispanic white | 18,365 | 16,881 | 1,484 |
| Non-Hispanic black | 2,331 | 2,106 | 225 |
| Hispanic | 3,818 | 3,596 | 222 |
| Non-Hispanic Asian or Pacific Islander | 1,673 | 1,549 | 124 |
| Non-Hispanic American Indian/Alaskan native | 233 | 216 | 17 |
| Age at diagnosis, years | |||
| 0-4 | 7,633 | 7,196 | 437 |
| 5-9 | 4,227 | 3,869 | 358 |
| 10-14 | 4,565 | 4,194 | 371 |
| 15-19 | 8,064 | 7,341 | 723 |
| > 19 | 2,154 | 1,967 | 187 |
| Survival after diagnosis, years | |||
| 5-9 | — | — | 1,125 |
| 10-14 | — | — | 406 |
| 15-19 | — | — | 254 |
| 20-24 | — | — | 180 |
| 25-29 | — | — | 104 |
| 30-34 | — | — | 7 |
| Diagnosis | |||
| Acute lymphoblastic leukemia | 4,970 | 4,570 | 400 |
| Acute myelogenous leukemia | 592 | 549 | 43 |
| Hodgkin's lymphoma | 3,124 | 2,767 | 357 |
| Non-Hodgkin's lymphoma | 1,505 | 1,430 | 75 |
| Astrocytoma | 2,241 | 2,048 | 193 |
| Medulloblastoma | 743 | 643 | 100 |
| Ependymoma | 274 | 226 | 48 |
| Osteosarcoma | 643 | 567 | 76 |
| Ewing sarcoma | 392 | 333 | 59 |
| Rhabdomyosarcoma | 616 | 574 | 42 |
| Neuroblastoma | 1,024 | 973 | 51 |
| Hepatic tumor | 174 | 165 | 9 |
| Renal tumors | 1,242 | 1,203 | 39 |
| Retinoblastoma | 637 | 613 | 24 |
| Thyroid carcinoma | 1,401 | 1,369 | 32 |
| Other cancers | 7,065 | 6,537 | 528 |
Table 2.
All-Cause and Cause-Specific Standardized Mortality Ratios in 5-Year Survivors of Childhood Cancer
| Characteristic | All Causes |
Subsequent Malignancy |
Cardiac Causes |
Pulmonary Causes |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Deaths | SMR | 95% CI | P* | No. of Deaths | SMR | 95% CI | P* | No. of Deaths | SMR | 95% CI | P* | No. of Deaths | SMR | 95% CI | P* | |
| All patients | 2,076 | 8.89 | 8.52 to 9.29 | 205 | 8.02 | 6.96 to 9.19 | 103 | 6.05 | 4.94 to 7.34 | 29 | 7.32 | 4.90 to 10.51 | ||||
| Sex | < .001 | .19 | .89 | .03 | ||||||||||||
| Male | 1,214 | 7.64 | 7.22 to 8.08 | 104 | 8.81 | 7.20 to 10.68 | 68 | 6.12 | 4.75 to 7.75 | 21 | 10.10 | 6.25 to 15.43 | ||||
| Female | 862 | 11.57 | 10.81 to 12.37 | 101 | 7.33 | 5.97 to 8.91 | 35 | 5.93 | 4.13 to 8.25 | 8 | 4.25 | 1.83 to 8.38 | ||||
| Year of diagnosis | < .001 | .04 | .03 | .83 | ||||||||||||
| 1974-1980 | 825 | 7.52 | 7.02 to 8.05 | 121 | 8.18 | 6.79 to 9.77 | 46 | 4.65 | 3.40 to 6.20 | 16 | 7.70 | 4.40 to 12.51 | ||||
| 1981-1987 | 617 | 8.82 | 8.14 to 9.54 | 42 | 6.29 | 4.53 to 8.51 | 40 | 8.72 | 6.23 to 11.88 | 8 | 7.18 | 3.09 to 14.15 | ||||
| 1988-1994 | 419 | 10.32 | 9.36 to 11.36 | 37 | 11.63 | 8.19 to 16.04 | 15 | 7.47 | 4.18 to 12.32 | 3 | 5.04 | 1.01 to 14.72 | ||||
| 1995-2000 | 215 | 16.35 | 14.24 to 18.69 | 5 | 5.40 | 1.74 to 12.60 | 2 | 3.77 | 0.42 to 13.63 | 2 | 11.47 | 1.29 to 41.41 | ||||
| Race/ethnicity | < .001 | < .001 | .15 | .19 | ||||||||||||
| White | 1,699 | 8.92 | 8.50 to 9.36 | 156 | 7.12 | 6.05 to 8.33 | 87 | 6.41 | 5.13 to 7.91 | 22 | 7.27 | 4.55 to 11.00 | ||||
| Black | 229 | 6.67 | 5.84 to 7.59 | 29 | 10.72 | 7.18 to 15.40 | 11 | 3.71 | 1.85 to 6.65 | 4 | 5.01 | 1.35 to 12.83 | ||||
| Asian, Pacific Islander, American Indian, Alaskan native | 144 | 16.60 | 14.00 to 19.55 | 20 | 20.73 | 12.66 to 32.02 | 4 | 8.31 | 2.24 to 21.28 | 3 | 22.25 | 4.47 to 65.02 | ||||
NOTE. As of December 31, 2005. All SMRs were age-, sex-, and race-standardized according to the US mortality rates from the SEER Program. “Subsequent malignancy” refers to a new neoplasm. Cancer deaths resulting from progression of the original cancer are not included in the observed number of events.
Abbreviations: SMR, standardized mortality ratio; SEER, Surveillance, Epidemiology, and End Results.
P from test for heterogeneity.
Table 3.
10-Year Cumulative Incidence of Mortality Among 5-Year Survivors of Childhood Cancer
| Diagnosis | 1974-1980 |
1981-1987 |
1988-1994 |
1995-2000 |
P* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Cumulative Incidence | 95% CI | No. | Cumulative Incidence | 95% CI | No. | Cumulative Incidence | 95% CI | No. | Cumulative Incidence | 95% CI | ||
| All-cause | |||||||||||||
| All diagnoses | 4,266 | 7.1 | 6.4 to 7.8 | 4,958 | 5.8 | 5.2 to 6.5 | 6,809 | 4.0 | 3.6 to 4.5 | 2,311 | 3.9 | 3.3 to 4.4 | < .001 |
| All leukemias | 673 | 15.1 | 12.6 to 17.6 | 920 | 9.7 | 7.9 to 11.5 | 1,535 | 5.0 | 4.0 to 6.1 | 543 | 4.1 | 3.0 to 5.2 | < .001 |
| Acute lymphoblastic leukemia | 555 | 14.8 | 12.0 to 17.5 | 805 | 8.7 | 6.8 to 10.6 | 1,306 | 4.8 | 3.7 to 5.9 | 467 | 4.2 | 3.0 to 5.4 | < .001 |
| Acute myeloid leukemia | 59 | 10.5 | 3.1 to 17.9 | 72 | 10.1 | 3.4 to 16.8 | 153 | 4.9 | 1.6 to 8.2 | 59 | 4.7 | 0.8 to 8.6 | .19 |
| Hodgkin's lymphoma | 661 | 8.4 | 6.3 to 10.4 | 647 | 5.1 | 3.5 to 6.8 | 762 | 3.7 | 2.4 to 5.0 | 220 | 1.5 | 0.5 to 2.4 | < .001 |
| Non-Hodgkin's lymphoma | 177 | 5.9 | 2.5 to 9.2 | 300 | 3.6 | 1.5 to 5.7 | 399 | 1.5 | 0.3 to 2.7 | 145 | 1.2 | 0.0 to 2.4 | .006 |
| All CNS tumors | 539 | 9.0 | 6.7 to 11.3 | 679 | 7.6 | 5.6 to 9.5 | 1,040 | 5.4 | 4.1 to 6.7 | 348 | 7.1 | 5.0 to 9.2 | .17 |
| Astrocytoma | 339 | 6.3 | 3.8 to 8.8 | 411 | 5.1 | 3.0 to 7.2 | 631 | 2.8 | 1.5 to 4.0 | 198 | 5.9 | 3.0 to 8.7 | .13 |
| Medulloblastoma | 80 | 17.6 | 9.9 to 25.2 | 112 | 12.7 | 6.8 to 18.5 | 185 | 7.5 | 3.8 to 11.2 | 58 | 8.7 | 4.2 to 13.2 | .11 |
| Ependymoma | 28 | 10.1 | 0.0 to 21.2 | 40 | 16.9 | 6.1 to 27.6 | 66 | 13.9 | 6.2 to 21.5 | 35 | 13.4 | 4.8 to 21.9 | .61 |
| Osteosarcoma | 98 | 4.8 | 0.7 to 8.9 | 108 | 8.4 | 3.4 to 13.5 | 166 | 11.3 | 6.7 to 15.9 | 59 | 12.3 | 6.0 to 18.6 | .71 |
| Ewing sarcoma | 52 | 7.1 | 0.3 to 13.8 | 73 | 16.5 | 8.5 to 24.4 | 104 | 8.6 | 3.5 to 13.7 | 26 | 8.9 | 2.9 to 14.9 | .03 |
| Rhabdomyosarcoma | 96 | 6.8 | 1.9 to 11.7 | 126 | 6.6 | 2.4 to 10.8 | 165 | 1.8 | 0.0 to 3.8 | 61 | 3.7 | 0.7 to 6.7 | .05 |
| Neuroblastoma | 160 | 4.1 | 1.1 to 7.1 | 191 | 3.5 | 1.0 to 6.1 | 284 | 3.6 | 1.5 to 5.7 | 91 | 4.9 | 1.9 to 7.9 | .65 |
| Hepatic tumors | 12 | 7.1 | 0.0 to 21.1 | 36 | 5.5 | 0.0 to 13.0 | 47 | 2.0 | 0.0 to 5.8 | 16 | 2.6 | 0.0 to 7.6 | .70 |
| Renal tumors | 202 | 1.0 | 0.0 to 2.3 | 263 | 1.1 | 0.0 to 2.4 | 348 | 1.6 | 0.3 to 2.9 | 89 | 1.6 | 0.0 to 3.2 | .49 |
| Retinoblastoma | 105 | 1.9 | 0.0 to 4.5 | 120 | 4.0 | 0.5 to 7.4 | 176 | 1.0 | 0.0 to 2.5 | 47 | 0.9 | 0.0 to 2.8 | .75 |
| Recurrence/progression | |||||||||||||
| All diagnoses | 4,266 | 5.1 | 4.5 to 5.8 | 4,958 | 4.1 | 3.5 to 4.6 | 6,809 | 2.8 | 2.4 to 3.2 | 2,311 | 2.8 | 2.3 to 3.3 | < .001 |
| All leukemias | 673 | 12.5 | 10.2 to 14.8 | 920 | 7.7 | 6.1 to 9.4 | 1,535 | 3.6 | 2.7 to 4.5 | 543 | 3.3 | 2.3 to 4.3 | < .001 |
| Acute lymphoblastic leukemia | 555 | 12.0 | 9.5 to 14.5 | 805 | 7.0 | 5.3 to 8.7 | 1,306 | 3.4 | 2.4 to 4.3 | 467 | 3.5 | 2.4 to 4.5 | < .001 |
| Acute myeloid leukemia | 59 | 9.0 | 2.1 to 15.9 | 72 | 6.3 | 0.9 to 11.7 | 153 | 4.3 | 1.2 to 7.4 | 59 | 3.0 | 0.0 to 6.4 | .36 |
| Hodgkin's lymphoma | 661 | 6.3 | 4.5 to 8.0 | 647 | 3.4 | 2.0 to 4.7 | 762 | 2.3 | 1.2 to 3.3 | 220 | 0.8 | 0.1 to 1.4 | < .001 |
| Non-Hodgkin's lymphoma | 177 | 2.1 | 0.1 to 4.2 | 300 | 1.6 | 0.2 to 3.0 | 399 | 0.2 | 0.0 to 0.7 | 145 | 0.4 | 0.0 to 1.0 | .15 |
| All CNS tumors | 539 | 6.8 | 4.7 to 8.8 | 679 | 5.2 | 3.6 to 6.8 | 1,040 | 4.4 | 3.2 to 5.6 | 348 | 5.4 | 3.6 to 7.3 | .17 |
| Astrocytoma | 339 | 4.9 | 2.7 to 7.2 | 411 | 3.0 | 1.4 to 4.7 | 631 | 2.3 | 1.1 to 3.4 | 198 | 4.3 | 1.7 to 6.8 | .08 |
| Medulloblastoma | 80 | 13.4 | 6.6 to 20.3 | 112 | 11.1 | 5.6 to 16.6 | 185 | 7.5 | 3.8 to 11.2 | 58 | 6.8 | 2.9 to 10.8 | .18 |
| Ependymoma | 28 | 6.7 | 0.0 to 15.7 | 40 | 12.6 | 3.1 to 22.2 | 66 | 10.1 | 3.4 to 16.8 | 35 | 10.1 | 2.7 to 17.5 | .56 |
| Osteosarcoma | 98 | 2.9 | 0.0 to 6.1 | 108 | 5.9 | 1.6 to 10.1 | 166 | 7.5 | 3.7 to 11.3 | 59 | 8.0 | 3.2 to 12.9 | .78 |
| Ewing sarcoma | 52 | 1.8 | 0.0 to 5.3 | 73 | 14.1 | 6.7 to 21.6 | 104 | 6.0 | 1.7 to 10.4 | 26 | 6.9 | 1.5 to 12.3 | .01 |
| Rhabdomyosarcoma | 96 | 4.9 | 0.7 to 9.0 | 126 | 4.4 | 0.9 to 7.8 | 165 | 1.2 | 0.0 to 2.8 | 61 | 2.9 | 0.3 to 5.4 | .17 |
| Neuroblastoma | 160 | 1.8 | 0.0 to 3.7 | 191 | 2.0 | 0.1 to 4.0 | 284 | 2.9 | 1.0 to 4.8 | 91 | 4.9 | 1.9 to 7.9 | .06 |
| Hepatic tumors | 12 | 7.1 | 0.0 to 21.1 | 36 | 2.7 | 0.0 to 8.0 | 47 | 2.0 | 0.0 to 5.8 | 16 | 2.6 | 0.0 to 7.6 | .30 |
| Renal tumors | 202 | 1.0 | 0.0 to 2.3 | 263 | 0.7 | 0.0 to 1.8 | 348 | 1.4 | 0.2 to 2.6 | 89 | 1.3 | 0.0 to 2.8 | .58 |
| Retinoblastoma | 105 | 0.0 | 120 | 1.6 | 0.0 to 3.8 | 176 | 0.0 | 47 | 0.0 | .29 | |||
| Nonrecurrence/nonexternal cause | |||||||||||||
| All diagnoses | 4,266 | 1.0 | 0.7 to 1.3 | 4,958 | 1.0 | 0.8 to 1.3 | 6,809 | 0.7 | 0.5 to 0.9 | 2,311 | 0.8 | 0.5 to 1.0 | .45 |
| All leukemias | 673 | 1.1 | 0.4 to 1.9 | 920 | 1.0 | 0.4 to 1.6 | 1,535 | 0.9 | 0.4 to 1.3 | 543 | 0.6 | 0.2 to 1.0 | .39 |
| Acute lymphoblastic leukemia | 555 | 1.1 | 0.3 to 1.9 | 805 | 0.8 | 0.2 to 1.4 | 1,306 | 0.8 | 0.3 to 1.3 | 467 | 0.5 | 0.1 to 0.9 | .28 |
| Acute myeloid leukemia | 59 | 1.5 | 0.0 to 4.4 | 72 | 1.3 | 0.0 to 3.7 | 153 | 0.6 | 0.0 to 1.8 | 59 | 1.7 | 0.0 to 3.7 | .24 |
| Hodgkin's lymphoma | 661 | 1.4 | 0.5 to 2.3 | 647 | 1.2 | 0.4 to 2.0 | 762 | 0.8 | 0.2 to 1.4 | 220 | 0.7 | 0.0 to 1.4 | .48 |
| Non-Hodgkin's lymphoma | 177 | 0.5 | 0.0 to 1.6 | 300 | 1.0 | 0.0 to 2.1 | 399 | 1.2 | 0.2 to 2.3 | 145 | 0.5 | 0.0 to 1.1 | .93 |
| All CNS tumors | 539 | 1.9 | 0.8 to 3.0 | 679 | 1.7 | 0.7 to 2.6 | 1,040 | 0.5 | 0.1 to 1.0 | 348 | 1.2 | 0.5 to 2.0 | .94 |
| Astrocytoma | 339 | 1.1 | 0.0 to 2.2 | 411 | 1.6 | 0.4 to 2.8 | 631 | 0.2 | 0.0 to 0.4 | 198 | 1.0 | 0.2 to 1.9 | .80 |
| Medulloblastoma | 80 | 3.1 | 0.0 to 6.6 | 112 | 1.6 | 0.0 to 3.8 | 185 | 0.0 | 58 | 1.4 | 0.0 to 3.5 | .76 | |
| Ependymoma | 28 | 3.5 | 0.0 to 10.2 | 40 | 0.0 | 66 | 2.5 | 0.0 to 5.8 | 35 | 3.3 | 0.0 to 8.0 | .82 | |
| Osteosarcoma | 98 | 1.0 | 0.0 to 2.8 | 108 | 2.6 | 0.0 to 5.4 | 166 | 1.1 | 0.0 to 2.6 | 59 | 1.5 | 0.0 to 4.5 | .97 |
| Ewing sarcoma | 52 | 1.8 | 0.0 to 5.2 | 73 | 1.2 | 0.0 to 3.5 | 104 | 1.7 | 0.0 to 4.1 | 26 | 1.2 | 0.0 to 3.6 | .90 |
| Rhabdomyosarcoma | 96 | 1.9 | 0.0 to 4.6 | 126 | 1.5 | 0.0 to 3.5 | 165 | 0.6 | 0.0 to 1.8 | 61 | 0.0 | .42 | |
| Neuroblastoma | 160 | 1.8 | 0.0 to 3.8 | 191 | 1.0 | 0.0 to 2.4 | 284 | 0.7 | 0.0 to 1.6 | 91 | 0.0 | .31 | |
| Hepatic tumors | 12 | 0.0 | 36 | 2.8 | 0.0 to 8.2 | 47 | 0.0 | 16 | 0.0 | .34 | |||
| Renal tumors | 202 | 0.0 | 263 | 0.0 | 348 | 0.0 | 89 | 0.3 | 0.0 to 0.9 | .21 | |||
| Retinoblastoma | 105 | 0.9 | 0.0 to 2.8 | 120 | 0.8 | 0.0 to 2.3 | 176 | 0.5 | 0.0 to 1.5 | 47 | 0.9 | 0.0 to 2.8 | .76 |
Based on the comparison of cumulative mortality curves for the four time periods.
Fig 1.
Cumulative incidence of all-cause late mortality, recurrence/progression-related late mortality, and nonrecurrence/nonexternal-cause late mortality for all 5-year survivors and for 5-year survivors of acute lymphoblastic leukemia, Hodgkin's lymphoma, or Ewing sarcoma.
There were 1,147 deaths attributable to primary disease recurrence/progression, 139 deaths from external causes, and 613 deaths from nonrecurrence/nonexternal causes. The 10-year cumulative incidence of mortality attributable to recurrence/progression across treatment eras was 5.1%, 4.1%, 2.8%, and 2.8% (P < .001; Table 3). Five-year survivors of ALL or Hodgkin's lymphoma experienced a statistically significant reduction in death rates due to primary malignancy across these time periods (Table 3). The cumulative incidence of mortality attributable to recurrence/progression of primary disease increased over the same periods for patients with osteosarcoma, Ewing sarcoma, ependymoma, and neuroblastoma, although this increase was statistically significant only in Ewing sarcoma (P = .01).
No significant decline in late mortality attributable to nonrecurrence/nonexternal causes was identified either in the overall survivor population (1.0%, 1.0%, 0.7%, and 0.8%; P = .45) or in any specific diagnostic group (Table 3). While monotonic trends in nonrecurrence/nonexternal cause mortality were seen for survivors of ALL (1.1%, 0.8%, 0.8%, and 0.5%; P = .28) or Hodgkin's lymphoma (1.4%, 1.2%, 0.8%, and 0.7%; P = .48), these improvements did not reach statistical significance.
Multivariable analyses incorporating demographic factors that may be associated with mortality were conducted (Table 4). Male sex was associated with an increased absolute risk of all-cause mortality (hazard ratio [HR], 1.43; 95% CI, 1.31 to 1.56) and of death from both recurrence/progression (HR, 1.34; 95% CI, 1.19 to 1.51) and nonrecurrence/nonexternal causes (HR, 1.36; 95% CI, 1.16 to 1.60). Compared with non-Hispanic whites, non-Hispanic blacks had an almost two-fold increased risk of death due to nonrecurrence/nonexternal causes (HR, 1.88; 95% CI, 1.49 to 2.37) but not for recurrence/progression (HR, 1.12; 95% CI, 0.91 to 1.37), resulting in an increased all-cause mortality for blacks (HR, 1.38; 95% CI, 1.20 to 1.59). Older age at diagnosis conferred greater risk of mortality from any cause. No interaction between treatment era and race, sex, or age at diagnosis was identified, with the exception of a significant interaction between age at diagnosis and treatment era for recurrence/progression-related mortality (P = .01). Of note, however, when treatment era was included in the multivariable model, there was evidence of a decreasing hazard with more modern treatment eras not only for all-cause and recurrence/progression mortality (P for trend < .001) but also for nonrecurrence/nonexternal cause mortality (P for trend = .007).
Table 4.
Hazard Ratios for Mortality Among 5-Year Survivors of Childhood Cancer
| Characteristic | All Cause |
Recurrence/Progression of Primary Malignancy |
Nonrecurrence/Nonexternal Cause |
|||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age* | 1.02 | 1.01 to 1.02 | 1.00 | 1.00 to 1.01 | 1.04 | 1.03 to 1.05 |
| Sex | ||||||
| Female | 1.0 | 1.0 | 1.0 | |||
| Male | 1.43 | 1.31 to 1.56 | 1.34 | 1.19 to 1.51 | 1.36 | 1.16 to 1.60 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1.0 | 1.0 | 1.0 | |||
| Non-Hispanic black† | 1.38 | 1.20 to 1.59 | 1.12 | 0.91 to 1.37 | 1.88 | 1.49 to 2.37 |
| Non-Hispanic American Indian/Alaskan native† | 1.08 | 0.67 to 1.74 | 1.18 | 0.65 to 2.13 | 0.71 | 0.23 to 2.17 |
| Non-Hispanic Asian or Pacific Islander† | 1.20 | 1.00 to 1.44 | 1.11 | 0.87 to 1.42 | 1.42 | 1.02 to 1.97 |
| Hispanic† | 1.27 | 1.10 to 1.47 | 1.30 | 1.08 to 1.55 | 1.09 | 0.81 to 1.48 |
| Year of diagnosis | ||||||
| 1974-1980 | 1.0 | 1.0 | 1.0 | |||
| 1981-1987 | 0.80 | 0.76 to 0.84 | 0.78 | 0.73 to 0.83 | 0.87 | 0.79 to 0.96 |
| 1988-1994 | 0.64 | 0.58 to 0.70 | 0.61 | 0.54 to 0.69 | 0.76 | 0.63 to 0.93 |
| 1995-2000 | 0.51 | 0.44 to 0.59 | 0.48 | 0.40 to 0.57 | 0.67 | 0.50 to 0.89 |
| Treatment era test for trend, P | < .001 | < .001 | .007 | |||
Age is a continuous variable and hazard ratios are based on 1-year increase in age.
Reference group is non-Hispanic white.
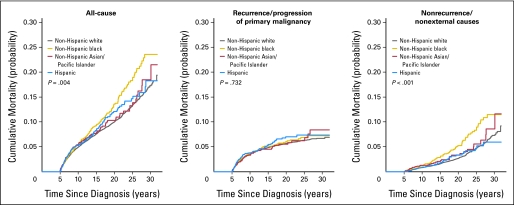
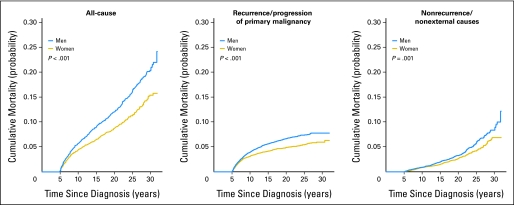
Males had a significantly greater cumulative incidence of all-cause and recurrence/progression mortality than did females (P < .001). At 10 years from diagnosis, there was no obvious difference between males and females in the incidence of nonrecurrence/nonexternal-cause mortality; however, with continued follow-up, males showed an increasing and significantly greater risk (Appendix Fig A1 and Table A1, online only). Within specific diagnostic groups, no statistically significant sex differences were identified, but sample sizes were small for many assessments. Additionally, non-Hispanic blacks showed increased all-cause and nonrecurrence/nonexternal-cause mortality at 25 years from diagnosis (Fig 2; Table 5). When standardized for the background rate of death in the general population, whites had a higher all-cause SMR than did blacks (8.92 v 6.67; Table 2) but a lower SMR for deaths attributable to subsequent malignant neoplasms (7.12 v 10.7; P < .001), the most common type of nonrecurrence/nonexternal-cause death.
Fig 2.
Cumulative all-cause and cause-specific mortality by race/ethnicity; non-Hispanic white (n = 18,365), non-Hispanic black (n = 2,331), non-Hispanic Asian/Pacific Islander (n = 1,673), and Hispanic (n = 3,818).
Table 5.
Mortality Among 5-Year Survivors of Childhood Cancer by Ethnicity
| Mortality by Ethnicity | Years After Diagnosis |
||
|---|---|---|---|
| 10 | 20 | 30 | |
| All cause | |||
| White | |||
| Cumulative incidence | 5.0 | 10.0 | 17.3 |
| 95% CI | 4.6 to 5.3 | 9.4 to 10.5 | 16.1 to 18.5 |
| Black | |||
| Cumulative incidence | 5.5 | 13.6 | 23.6 |
| 95% CI | 4.5 to 6.5 | 11.6 to 15.6 | 19.6 to 27.5 |
| American Indian | |||
| Cumulative incidence | 4.6 | 9.5 | 18.0 |
| 95% CI | 1.6 to 7.6 | 4.5 to 14.5 | 5.7 to 30.2 |
| Asian | |||
| Cumulative incidence | 5.5 | 10.3 | 18.5 |
| 95% CI | 4.3 to 6.7 | 8.3 to 12.4 | 13.4 to 23.5 |
| Hispanic | |||
| Cumulative incidence | 5.3 | 12.1 | 18.3 |
| 95% CI | 4.4 to 6.1 | 10.1 to 14.1 | 13.8 to 22.7 |
| Recurrence/progression | |||
| White | |||
| Cumulative incidence | 3.6 | 5.7 | 6.7 |
| 95% CI | 3.3 to 3.9 | 5.3 to 6.1 | 6.2 to 7.2 |
| Black | |||
| Cumulative incidence | 3.7 | 6.1 | 7.2 |
| 95% CI | 2.9 to 4.5 | 4.8 to 7.3 | 5.6 to 8.9 |
| American Indian | |||
| Cumulative incidence | 3.1 | 6.0 | 10.1 |
| 95% CI | 0.6 to 5.6 | 2.3 to 9.7 | 1.3 to 18.9 |
| Asian | |||
| Cumulative incidence | 3.3 | 5.5 | 8.4 |
| 95% CI | 2.4 to 4.3 | 4.1 to 7.0 | 5.4 to 11.4 |
| Hispanic | |||
| Cumulative incidence | 3.8 | 7.0 | 7.4 |
| 95% CI | 3.1 to 4.5 | 5.6 to 8.5 | 5.8 to 9.0 |
| Nonrecurrence/nonexternal cause | |||
| White | |||
| Cumulative incidence | 0.8 | 2.7 | 7.3 |
| 95% CI | 0.7 to 1.0 | 2.4 to 3.0 | 6.4 to 8.2 |
| Black | |||
| Cumulative incidence | 1.2 | 5.3 | 11.5 |
| 95% CI | 0.7 to 1.7 | 3.9 to 6.6 | 8.5 to 11.4 |
| American Indian | |||
| Cumulative incidence | 0.5 | 2.0 | 6.3 |
| 95% CI | 0 to 1.6 | 0 to 5.0 | 0 to 15.2 |
| Asian | |||
| Cumulative incidence | 1.3 | 3.3 | 8.6 |
| 95% CI | 0.7 to 1.9 | 2.0 to 4.6 | 4.5 to 12.7 |
| Hispanic | |||
| Cumulative incidence | 0.8 | 3.4 | 6.0 |
| 95% CI | 0.5 to 1.2 | 2.2 to 4.6 | 3.5 to 8.4 |
DISCUSSION
These population-based analyses demonstrate a decrease in the cumulative incidence of all-cause mortality over time among children who survive 5 years after their original cancer diagnosis. These findings are consistent with three previous investigations3,5,8 that explored late mortality in earlier treatment eras (1960-1995). In a population-based study in five Nordic countries, Möller et al5 reported that patients diagnosed between 1980 and 1989 were at less risk of late mortality (HR, 0.61; 95% CI, 0.54 to 0.70) than those diagnosed between 1960 and 1979. Likewise, in an institution-based study, Hudson et al3 noted higher mortality rates at 15 years after diagnosis among children diagnosed between 1962 and 1970 compared with those diagnosed between 1971 and 1983 (19.6% v 10.5%). Our data add to this evidence and further suggest that modern therapy has improved late mortality for children with the most common cancer types (ALL, Hodgkin's and non-Hodgkin's lymphoma, rhabdomyosarcoma) but not for children with osteosarcoma, Ewing sarcoma, or ependymoma.
Reduction in late mortality appears to be largely due to reduction in deaths attributable to recurrence/progression of primary disease. This fact suggests that treatments that have improved 5-year survival in more recent eras have also improved durable remission of primary disease beyond the 5-year time point. However, notable exceptions exist. Patients with osteosarcoma, Ewing sarcoma, ependymoma, or neuroblastoma experienced worsening late mortality rates attributable to their primary malignancy over time. It is possible that more intensive primary therapy prolongs initial disease control or that improvements in salvage (after primary progression) therapy may have extended survival beyond the 5-year time point, thus delaying, but not preventing, death resulting from primary disease.
In recent decades, the general philosophy in the design of treatment regimens for many pediatric malignancies has been to reduce the potential for long-term toxicities while not compromising therapeutic benefit. Examples of this approach include reduction in CNS-directed radiation for ALL,12,13 multimodal therapy with generalized reduction in radiation doses for patients with Hodgkin's lymphoma, and lower cumulative doses of anthracycline to reduce cardiac toxicity.14,15 Given these changes, one would anticipate that more recently treated patient populations should experience a lower rate of late treatment–related mortality. In the current analysis, we did not observe a significant difference by treatment era when assessing cumulative incidence of mortality attributed to nonrecurrence/nonexternal causes (Fig 1). However, analyses controlling for demographic characteristics of the treatment era cohorts did provide results showing a reduction in the HRs, with a statistically significant test for trend (P = .007). Thus, the strategy of reducing late toxicity seems to be having an effect because a 33% reduction in risk of death due to nonrecurrence/nonexternal causes was present when comparing the 1974 to 1980 cohort with the 1995 to 2000 cohort. The contrasting conclusions between the cumulative incidence and the adjusted risk estimates may well be related to the changing demographics of the SEER population between 1974 and 2000 (eg, 20% non-white and 52% female in 1974-1980 v 40% non-white and 47% female in 1995-2000), coupled with the observed demographic-specific risks for late mortality (eg, higher late mortality in non-whites and higher mortality in females). While the observed lower mortality in the more recently treated cohorts is encouraging, it will be important to continue to follow this trend and document that changes in therapy are preventing the occurrence of late mortality, as opposed to delaying the occurrence of fatal late toxicities.
In a population-based study, Howell et al16 demonstrated higher mortality rates for blacks than for whites at an early time period following treatment (median follow-up, 25 months). In addition to previous analyses of SEER data showing lower 5-year survival rates for blacks than whites,1 several studies17–20 have established a worse survival rate for blacks with ALL or acute myeloid leukemia. However, we have now shown that beyond the 5-year time point, blacks are at a greater absolute risk of mortality than are whites. This difference is not due to recurrence/progression of primary disease. Rather, blacks have significantly higher mortality from nonrecurrence/nonexternal causes. This diagnostic group represents medical causes of death largely due, in this young age group, to complications of cancer therapy.21 We speculate that these findings may suggest disparity in access to, utilization of, and knowledge about long-term medical care after cancer within the black population. Such disparities are well established in the general population.22–29 It has been demonstrated that given equal access to effective antileukemic therapy, black and white children can expect the same event-free and overall survival rates.30 Previous assessment of health care utilization by minority cancer survivors has not shown major disparities; however, these data were collected from a large retrospective cohort study (the Childhood Cancer Survivor Study [CCSS]), which may have lower rates of minority participation and thus may be biased toward lower mortality rates among participating minorities.31,32
Sex-based differences in mortality have been previously reported. Males have a greater absolute risk of death, but when data are standardized for the background rate of mortality in the population, female survivors of childhood cancer have a higher standardized mortality rate than males.2,4,5,33 Our population-based analysis has identified a higher incidence of mortality among males similar to that identified by the CCSS,4 with one exception. While our study identified greater male risk of nonrecurrence/nonexternal-cause death beyond the 10-year time point, the CCSS identified females as being at higher risk, likely because of high rates of breast cancer in the 1970 to 1986 time period. The rates of breast cancer may have been lower in patients treated during the more recent period of our study.
One limitation of this population-based study is that there is insufficient information on cause of death among those in the nonrecurrence/nonexternal-cause category to further classify them as treatment-related or nontreatment-related. Nontreatment-related causes of death in this population may include HIV, diabetes, influenza, and asthma, among other diagnoses. However, in this cohort with a median age of 24.5 years at the time of ascertainment of vital status, it is likely that the majority of medical deaths are attributable to treatment-related causes, as was demonstrated in the CCSS.21 In addition, because SEER does not have complete and detailed information on cancer treatment, it is not possible to accurately evaluate associations between specific treatment exposures and late mortality. Therefore, we can only hypothesize that improved rates of mortality attributable to recurrence/progression of primary disease are due to increased intensity of treatment regimens among high-risk patients, while improved rates of mortality from nonrecurrence/nonexternal causes are associated with decreased exposure among low-risk populations. However, use of the SEER population-based data set for this analysis provides mortality data on a population diagnosed across a broader time range (1974-2000) than that of the CCSS (1970-1986), thus improving the ability to assess temporal trends in mortality. In addition, findings from this population-based study may be more generalizable to the broad population of cancer survivors than those from the CCSS, which is a hospital-based cohort with a narrower spectrum of diagnoses. Future investigations using established cohorts with comprehensive assessment of therapy, such as the CCSS,32 will allow more detailed assessment of relationships between radiation therapy, chemotherapy doses, and mortality, providing increased sensitivity to determine whether reductions in dose and intensity of therapies over time improve late mortality. The current expansion of the CCSS cohort will ultimately include patients diagnosed between 1970 and 1999, thus allowing a comprehensive assessment of these remaining questions.
Supplementary Material
Appendix
Fig A1.
Cumulative all-cause and cause-specific mortality by sex.
Table A1.
Mortality Among 5-Year Survivors of Childhood Cancer by Sex
| Mortality by Sex | Years After Diagnosis |
||
|---|---|---|---|
| 10 | 20 | 30 | |
| All cause | |||
| Male | |||
| Cumulative incidence | 5.7 | 12.0 | 20.4 |
| 95% CI | 5.2 to 6.1 | 11.3 to 12.8 | 18.8 to 22.0 |
| Female | |||
| Cumulative incidence | 4.4 | 8.8 | 15.4 |
| 95% CI | 4.0 to 4.8 | 8.1 to 9.4 | 13.9 to 16.9 |
| Recurrence/progression | |||
| Male | |||
| Cumulative incidence | 4.0 | 6.7 | 7.8 |
| 95% CI | 3.6 to 4.3 | 6.1 to 7.2 | 7.1 to 8.5 |
| Female | |||
| Cumulative incidence | 3.2 | 4.9 | 6.0 |
| 95% CI | 2.8 to 3.5 | 4.4 to 5.4 | 5.3 to 6.6 |
| Nonrecurrence/nonexternal cause | |||
| Male | |||
| Cumulative incidence | 0.9 | 3.3 | 8.4 |
| 95% CI | 0.7 to 1.1 | 2.8 to 3.7 | 7.2 to 9.5 |
| Female | |||
| Cumulative incidence | 0.9 | 2.7 | 6.9 |
| 95% CI | 0.7 to 1.0 | 2.3 to 3.0 | 5.7 to 8.0 |
Footnotes
Supported by the American Lebanese Syrian Associated Charities.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Gregory T. Armstrong, Zhenyu Pan, Kirsten K. Ness, Deokumar Srivastava, Leslie L. Robison
Administrative support: Gregory T. Armstrong, Zhenyu Pan
Collection and assembly of data: Gregory T. Armstrong, Zhenyu Pan, Deokumar Srivastava
Data analysis and interpretation: Gregory T. Armstrong, Zhenyu Pan, Kirsten K. Ness, Deokumar Srivastava, Leslie L. Robison
Manuscript writing: Gregory T. Armstrong, Deokumar Srivastava, Leslie L. Robison
Final approval of manuscript: Gregory T. Armstrong, Zhenyu Pan, Kirsten K. Ness, Deokumar Srivastava, Leslie L. Robison
REFERENCES
- 1.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review. 2005:1975–2004. [Google Scholar]
- 2.Lawless SC, Verma P, Green DM, et al. Mortality experiences among 15+ year survivors of childhood and adolescent cancers. Pediatr Blood Cancer. 2007;48:333–338. doi: 10.1002/pbc.20723. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Jones D, Boyett J, et al. Late mortality of long-term survivors of childhood cancer. J Clin Oncol. 1997;15:2205–2213. doi: 10.1200/JCO.1997.15.6.2205. [DOI] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Möller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 6.Li FP, Myers MH, Heise HW, et al. The course of five-year survivors of cancer in childhood. J Pediatr. 1978;93:185–187. doi: 10.1016/s0022-3476(78)80492-2. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur AC, Spinelli JJ, Rogers PC, et al. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer. 2007;48:460–467. doi: 10.1002/pbc.20922. [DOI] [PubMed] [Google Scholar]
- 9.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 11.Breslow NE, Day NE. Lyon, France: IARC; 1987. Statistical Methods in Cancer Research Volume II: The Design and Analysis of Cohort Studies—IARC Scientific Publications No. 82. [PubMed] [Google Scholar]
- 12.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aur RJ, Simone JV, Hustu HO, et al. A comparative study of central nervous system irradiation and intensive chemotherapy early in remission of childhood acute lymphocytic leukemia. Cancer. 1972;29:381–391. doi: 10.1002/1097-0142(197202)29:2<381::aid-cncr2820290219>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Hudson MM, Donaldson SS. Treatment of pediatric Hodgkin's lymphoma. Semin Hematol. 1999;36:313–323. [PubMed] [Google Scholar]
- 15.Donaldson SS, Link MP. Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin's disease. J Clin Oncol. 1987;5:742–749. doi: 10.1200/JCO.1987.5.5.742. [DOI] [PubMed] [Google Scholar]
- 16.Howell DL, Ward KC, Austin HD, et al. Access to pediatric cancer care by age, race, and diagnosis, and outcomes of cancer treatment in pediatric and adolescent patients in the state of Georgia. J Clin Oncol. 2007;25:4610–4615. doi: 10.1200/JCO.2006.07.6992. [DOI] [PubMed] [Google Scholar]
- 17.Schiffer CA. Differences in outcome in adolescents with acute lymphoblastic leukemia: A consequence of better regimens? Better doctors? Both? J Clin Oncol. 2003;21:760–761. doi: 10.1200/JCO.2003.11.116. [DOI] [PubMed] [Google Scholar]
- 18.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: A Pediatric Oncology Group Study. J Clin Oncol. 2000;18:813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia S. Influence of race and socioeconomic status on outcome of children treated for childhood acute lymphoblastic leukemia. Curr Opin Pediatr. 2004;16:9–14. doi: 10.1097/00008480-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Davies SM, Robison LL, Buckley JD, et al. Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia. J Clin Oncol. 2001;19:1279–1287. doi: 10.1200/JCO.2001.19.5.1279. [DOI] [PubMed] [Google Scholar]
- 21.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 22.Weinick RM, Zuvekas SH, Cohen JW. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev. 2000;57(suppl 1):36–54. doi: 10.1177/1077558700057001S03. [DOI] [PubMed] [Google Scholar]
- 23.Wagner TH, Guendelman S. Healthcare utilization among Hispanics: Findings from the 1994 Minority Health Survey. Am J Manag Care. 2000;6:355–364. [PubMed] [Google Scholar]
- 24.Guendelman S, Schwalbe J. Medical care utilization by Hispanic children: How does it differ from black and white peers? Med Care. 1986;24:925–940. doi: 10.1097/00005650-198610000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Cornelius LJ. Barriers to medical care for white, black, and Hispanic American children. J Natl Med Assoc. 1993;85:281–288. [PMC free article] [PubMed] [Google Scholar]
- 26.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(suppl 1):108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 27.Carrasquillo O, Himmelstein DU, Woolhandler S, et al. Going bare: Trends in health insurance coverage, 1989 through 1996. Am J Public Health. 1999;89:36–42. doi: 10.2105/ajph.89.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrasquillo O, Himmelstein DU, Woolhandler S, et al. Trends in health insurance coverage, 1989-1997. Int J Health Serv. 1999;29:467–483. doi: 10.2190/1AV3-E901-TN3D-3H38. [DOI] [PubMed] [Google Scholar]
- 29.Valdez RB, Giachello A, Rodriguez-Trias H, et al. Improving access to health care in Latino communities. Public Health Rep. 1993;108:534–539. [PMC free article] [PubMed] [Google Scholar]
- 30.Pui CH, Sandlund JT, Pei D, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290:2001–2007. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 31.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. J Clin Oncol. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 32.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.