Abstract
Human mast cells express the G protein coupled receptor (GPCR) for C5a (CD88). Previous studies indicated that C5a could cause mast cell degranulation, at least in part, via a mechanism similar to that proposed for basic neuropeptides such as substance P, possibly involving Mas-related gene 2 (MrgX2). We therefore sought to more clearly define the receptor specificity for C5a-induced mast cell degranulation. We found that LAD2, a human mast cell line, and CD34+ cell-derived primary mast cells express functional MrgX1 and MrgX2 but the immature human mast cell line HMC-1 does not. A potent CD88 antagonist, PMX-53 (10 nM) inhibited C5a-induced Ca2+ mobilization in HMC-1 cells, but at higher concentrations (≥30 nM) it caused degranulation in LAD2 mast cells, CD34+ cell-derived mast cells, and RBL-2H3 cells stably expressing MrgX2. PMX-53 did not, however, activate RBL-2H3 cells expressing MrgX1. Although C5a induced degranulation in LAD2 and CD34+ cell-derived mast cells, it did not activate RBL-2H3 cells expressing MrgX1 or MrgX2. Replacement of Trp with Ala and Arg with dArg abolished the ability of PMX-53 to inhibit C5a-induced Ca2+ mobilization in HMC-1 cells and to cause degranulation in RBL-2H3 cells expressing MrgX2. These findings demonstrate that C5a does not use MrgX1 or MrgX2 for mast cell degranulation. Moreover, it reveals the novel finding that PMX-53 functions as a potent CD88 antagonist and a low-affinity agonist for MrgX2. Furthermore, Trp and Arg residues are required for the ability of PMX53 to act as both a CD88 antagonist and a MrgX2 agonist.
Introduction
The anaphylatoxin C5a is generated as a byproduct of complement activation, which interacts with its cognate cell surface G protein-coupled receptor (GPCR; CD88) to activate neutrophils and macrophages (Tomhave et al., 1994; Guo and Ward, 2005). C5a induces chemotaxis of a human mast cell line, HMC-1 via a pertussis toxin-sensitive G protein (Nilsson et al., 1996; Hartmann et al., 1997). In purified human skin mast cells and a subpopulation of human lung mast cells, C5a induces degranulation (Oskeritzian et al., 2005). C5a also causes degranulation and chemokine expression in LAD2 cells, a newly developed human mast cell line (Venkatesha et al., 2005). Although CD88 are expressed in human mast cells, previous studies suggested that effects of C5a on mast cell degranulation may involve pathways independent of cell surface receptors (el-Lati et al., 1994; Oskeritzian et al., 2005).
Human C5a is a 74-residue glycopolypeptide that consists of two distinct structural domains, the N-terminal core (residues 1–63) that promotes CD88 recognition and the C-terminal region (residues 65–74) that constitutes the receptor activation domain. A large number of peptide CD88 agonists and antagonists have recently been synthesized and tested both in vitro and in vivo. A cyclic hexapeptide, Ac-Phe-[Orn-Pro-dCha-Trp-Arg], based on the terminal amino acid sequence of C5a is a potent CD88 antagonist. It inhibits C5a-induced responses in human neutrophil and monocytes/macrophages in vitro (Haynes et al., 2000; Woodruff et al., 2001, 2004) and protects rodents from a number of experimental inflammatory diseases such as ischemia reperfusion injury, neurodegeneration, arthritis, and immune-complex-mediated inflammation (Woodruff et al., 2004, 2006; Köhl, 2006; Qu et al., 2009). Surprisingly, the effects of these peptides on human mast cells have not been determined.
Polybasic molecules such as compound 48/80, substance P, and mastoparan induce substantial degranulation in mast cells. Previous studies indicated that the mechanism of action of basic secretagogs involves their insertion into plasma membrane and direct activation of G proteins (Mousli et al., 1994; Ferry et al., 2002). Studies with human skin mast cells indicated that C5a-induced mast cell degranulation involve direct activation of G proteins similar to that proposed for polybasic compounds (el-Lati et al., 1994).
A large family of GPCRs called Mas-related genes (Mrgs; also known as sensory neuron-specific receptors) has been identified in rodents (Dong et al., 2001; Lembo et al., 2002). These receptors are selectively expressed in small-diameter sensory neurons of dorsal root ganglia and are thought to be involved in the sensation and modulation of pain. On the basis of homology analysis the ∼50 mouse Mrg receptors have been subdivided into MrgD and three subfamilies termed MrgA, MrgB, and MrgC (Dong et al., 2001; Lembo et al., 2002). However, no information is available regarding which of these Mrg receptors are expressed in murine mast cells. A subgroup of these receptors (MrgX1–MrgX4) are expressed in human neurons (Dong et al., 2001; Burstein et al., 2006). It is noteworthy that there is very little sequence homology between the human and mouse receptors. Tatemoto et al. (2006) recently showed that MrgX1 and MrgX2 are expressed in human cord blood-derived mast cells (CBMC) and that compound 48/80 as well as substance P activate MrgX2 but not MrgX1. These findings raise the interesting possibility that C5a could activate human mast cells, at least in part, via MrgX2.
The purpose of this study was to determine the receptor specificity of C5a-induced mast cell degranulation. To achieve this objective, we used two human mast cell lines, primary CD34+ cell-derived mast cells, murine mast cells, and transfected RBL-2H3 cells, as well as C5a and AcPhe(ornithine-Pro-cyclohexylamine-Trp-Arg) (PMX-53), a potent antagonist of CD88. Using these systems, we demonstrate that C5a does not use MrgX1 or MrgX2 for mast cell degranulation, but PMX-53 acts as a dual CD88 antagonist and an MrgX2 agonist. Studies with PMX-53 allowed us to identify specific amino acid residues within PMX-53 that are required for CD88 antagonist and MrgX2 agonist activities.
Materials
All cell culture reagents and pertussis toxin were purchased from Invitrogen (Carlsbad, CA). Human IgE was purchased from EMD Biosciences (San Diego, CA). Monoclonal anti-DNP specific IgE and anti-human IgE were purchased from Sigma-Aldrich (St. Louis, MO). Amaxa cell transfection kits and reagents were purchased from Lonza Walkersville, Inc. (Walkersville, MD). Plasmids encoding hemagglutinin (HA)-tagged human MrgX1, and MrgX2 in pReceiver-M06 vector were obtained from GeneCopeia (Rockville, MD). All recombinant human cytokines were purchased from Peprotech (Rocky Hill, NJ). G Protein Antagonist 2 was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY). Cortistatin-14 (CST) and bovine adrenal medulla docosapeptide (BAM-22P) were obtained from American Peptide (Vista, CA). Native complement C5a was from Complement Technology (Tyler, TX).
Synthesis and Purification of CD88 Peptides.
Linear peptides were synthesized on a peptide synthesizer using Fmoc chemistry (433A; Applied Biosystems, Foster City, CA). If necessary, a lactam bridge was formed in solution as described previously (Finch et al., 1999). All peptides were purified using reversed-phase high-performance liquid chromatography. Their mass was confirmed by using matrix-assisted laser desorption ionization/time-of-flight mass spectrometry.
Differentiation of Human Mast Cells from CD34+ Progenitors and Culture of Mast Cell Lines.
Human CD34+ progenitors were cultured in StemPro-34 medium supplemented with 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 100 ng/ml rhSCF, 100 ng/ml rhIL-6, and 30 ng/ml rhIL-3 (first week only). Half the cell culture medium was replaced weekly with media containing 100 ng/ml rhSCF and 100 ng/ml rhIL-6. Cells were used for experiments after 7 to 10 weeks in culture (Venkatesha et al., 2005; Rådinger et al., 2010). LAD2 cells were maintained in StemPro-34 medium containing nutrient supplements (Invitrogen) supplemented with 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 100 ng/ml rhSCF (Kirshenbaum et al., 2003; Rådinger et al., 2010). Half of the cell culture medium was replaced weekly with fresh culture medium (Kirshenbaum et al., 2003). Human mast cell line, HMC-1 cells were cultured in Iscove's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. RBL-2H3 cells were maintained as monolayer cultures in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine, l-glutamine (2 mM), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (Ali et al., 1994).
Stable Transfection of RBL-2H3 Cells.
RBL-2H3 cells were detached with versene, washed twice with Dulbecco's modified Eagle's medium, and 106 cells were transfected with plasmids encoding HA-tagged MrgX1 or MrgX2, using the Amaxa nucleofector device and Amaxa kit V according to the manufacturer's protocol. After nucleofection, cells were cultured in the presence of G418 (1 mg/ml) and cells expressing equivalent receptors were sorted using an anti-HA-specific antibody 12CA5/fluorescein isothiocyanate-conjugated anti-mouse-IgG and used for studies on degranulation and Ca2+ mobilization.
Culture of Murine Bone Marrow-Derived Mast Cells and Isolation of Peritoneal Mast Cells.
Murine bone marrow-derived mast cells (BMMC) were obtained by flushing bone marrow cells from the femurs of C57BL6 mice and were cultured for 4 to 6 weeks in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 units/ml penicillin, 100 mg/ml streptomycin, and 10 ng/ml rmIL-3. The homogeneity of the mast cells was confirmed by acid toluidine blue staining. More than 95% pure BMMC population was used for these studies. For peritoneal mast cells, mixed population of peritoneal cells were obtained by lavage, incubated with IgE for 16 h, and used for degranulation studies.
RT-PCR.
Total RNA from mast cells was extracted using TRIzol (Invitrogen), treated with DNase I, and reverse transcribed to cDNA using first-strand cDNA synthesis kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The cDNAs were amplified with primers specific for MrgX1 and MrgX2 (Origene, Rockville, MD). Human β-actin primers were used as an internal control for the PCR.
Calcium Mobilization.
Ca2+ mobilization was determined as described previously (Ali et al., 1993, 2000). In brief, cells (0.2 × 106 human mast cells and 106 RBL-2H3 and murine bone marrow-derived mast cells) were loaded with 1 μM indo-1 acetoxymethyl ester in the presence of 1 μM Pluronic acid F-127 for 30 min at room temperature. Cells were washed and resuspended in 1.5 ml of HEPES-buffered saline. Ca2+ mobilization was measured in a spectrophotometer (F-2500; Hitachi, Yokohama, Japan) with an excitation wavelength of 355 nm and an emission wavelength of 410 nm (Ali et al., 2000).
Degranulation Assay.
CD34+-derived mast cells, LAD2 cells (5 × 103) and RBL-2H3 cells, and murine mast cells (5 × 104) were seeded into 96-well plates overnight in the presence of human IgE (1 μg/ml) or DNP-specific mouse IgE (1 μg/ml), respectively. The following day, cells were washed and incubated in a total volume of 50 μl of buffer containing 0.1% BSA and exposed to anti-human IgE (human mast cells), DNP-BSA (RBL-2H3, murine mast cells), or different concentrations of peptides. For total β-hexosaminidase release, control cells were lysed in 50 μl of 0.1% Triton X-100. Aliquots (20 μl) of supernatants or cell lysates were incubated with 20 μl of 1 mM p-nitrophenyl-N-acetyl-β-d-glucosamine for 1.5 h at 37°C. Reaction was stopped by adding 250 μl of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer, and absorbance was measured at 405 nm (Ali et al., 1994).
Results
C5a and CD88 Antagonist PMX-53 Induce Degranulation in Human LAD2 Mast Cells.
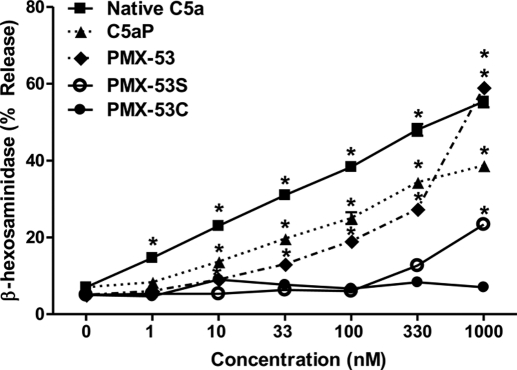
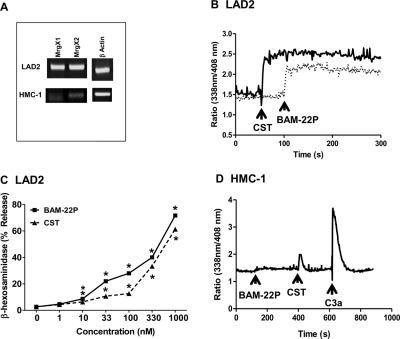
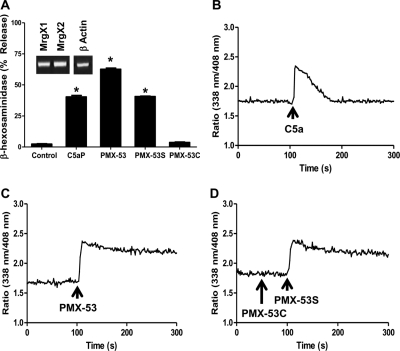
C5a induces degranulation in human skin mast cells, a subpopulation of lung mast cells, and LAD2 cells (Oskeritzian et al., 2005; Venkatesha et al., 2005). For our initial studies, we tested the effects of purified C5a and a synthetic CD88 agonist peptide C5aP (Table 1) on degranulation in LAD2 mast cells. As shown in Fig. 1, both C5a and C5aP induced mast cell degranulation in a dose-dependent manner. We were surprised to find that CD88 antagonist PMX-53 caused degranulation in LAD2 mast cells, and this response was dose-dependent, reaching a maximum of ∼60% at a concentration of 1 μM. A scrambled linear PMX-53 peptide (PMX-53S) also induced degranulation in LAD2 mast cells, but the magnitude of the response was lower (Fig. 1). It is noteworthy that a scrambled control peptide in which the Trp-Arg was replaced with Ala-dArg (PMX-53C) did not induce degranulation even at a concentration of 1 μM (Fig. 1). Consistent with degranulation, PMX-53 (Fig. 2A) and PMX-53S (Fig. 2B) induced Ca2+ mobilization at concentrations of 100 nM and 1 μM. Not surprisingly, 100 nM PMX-53C was inactive and 1 μM PMX-53C induced only a small and variable response (Fig. 2C). These findings suggest that PMX-53 (Figs. 1 and 2) can activate human mast cells and requires the presence of Trp-Arg residues for activity (Table 1).
TABLE 1.
Amino acid sequences of the peptides used
| Peptide | Amino Acid Sequence |
|---|---|
| C5aP | Tyr-Ser-Phe-Lys-Pro-Met-Pro-Leu-dAla-Arg |
| PMX-53 | Ac-Phe-[Orn-Pro-dCha-Trp-Arg] |
| PMX-53C | Ac-Phe-[Orn-Pro-dCha-Ala-dArg] |
| PMX-53S | Ac-dCha-Pro-Trp-Phe-Arg-Orn-NH2 |
| BAM-22P | Tyr-Gly-Gly-Phe-Met-Arg-Arg-Val-Gly-Arg-Pro-Glu-Trp-Trp-Met-Asp-Tyr-Gln-Lys-Arg-Tyr-Gly |
| CST | Pro-[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Ser-Ser-Cys]-Lys |
| Substance P | Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
Fig. 1.
C5a, C5aP, and CD88 antagonist PMX-53 induce degranulation in human mast cells. LAD2 mast cells were stimulated with different concentrations of native C5a, C5aP, PMX-53, PMX-53S, or PMX-53C and percentage degranulation (β-hexosaminidase release) was determined. Data are mean ± S.E.M. of n = 3. Statistical significance was determined by two-way ANOVA with Bonferroni's post test. *, p < 0.05.
Fig. 2.
CD88 antagonist PMX-53 induces Ca2+ mobilization in LAD2 mast cells. Cells were incubated with Indo-1AM and stimulated with increasing concentrations of PMX-53 (A), PMX-53S (B), and PMX-53C (C) and intracellular Ca2+ mobilization was determined. Data shown are representative of 3 similar experiments.
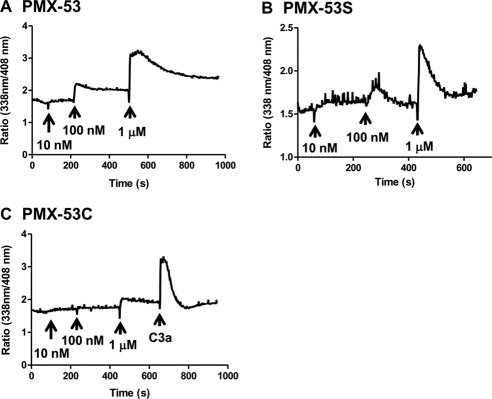
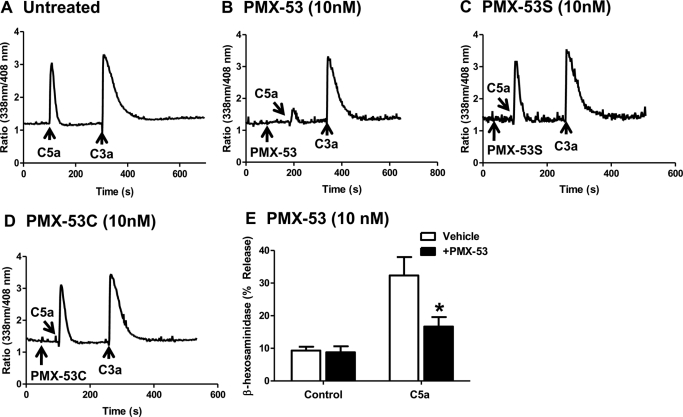
PMX-53 is a potent CD88 antagonist and inhibits C5a-induced neutrophil myeloperoxidase release and chemotaxis with IC50 values of 22 and 75 nM, respectively (Haynes et al., 2000; March et al., 2004). We therefore sought to determine whether the PMX-53 used in the present study displayed CD88 inhibitory activity. Given that PMX-53 induced signaling and degranulation in the mature mast cell line LAD2 cells, we tested its effects on C5a-induced Ca2+ mobilization in HMC-1 cells. In untreated cells, C5a (10 nM) and C3a (10 nM) induced transient Ca2+ mobilization responses of similar magnitude (Fig. 3A). Preincubation with PMX-53 (10 nM) almost completely inhibited Ca2+ response to C5a but had no effect on C3a-induced response (Fig. 3B). By contrast, PMX-53C (Fig. 3C) and PMX-53S (Fig. 3D) had no effect on either C5a or C3a-induced responses. Consistent with these results, PMX-53 (10 nM) also inhibited C5a-induced degranulation response in RBL-2H3 cells expressing CD88 (Fig. 3E). These findings demonstrate that the ability of PMX-53 to induce degranulation in LAD2 mast cells (Fig. 1) is independent of CD88.
Fig. 3.
PMX-53 inhibits C5a-induced responses in HMC-1 and RBL-2H3 cells expressing CD88. A, HMC-1 cells were incubated with Indo-1AM and stimulated sequentially with 10 nM C5a and 10 nM C3a, and intracellular Ca2+ mobilization was determined. Cells were exposed to 10 nM PMX-53 (B), PMX-53C (C), or PMX-53S (D) 100 s before stimulation with C5a and C3a, and Ca2+ mobilization was determined. E, RBL-2H3 cells stably expressing CD88 were pretreated with vehicle or 10 nM PMX-53 and exposed to 1 nM C5a, and percentage degranulation (β-hexosaminidase release) was determined. Data shown are representative of three similar experiments. Data are mean ± S.E.M. of n = 3. Statistical significance was determined by two-way ANOVA with Bonferroni's post test. *, p < 0.05.
PMX-53 and C5a Activate Human Mast Cells via Cell Surface GPCR.
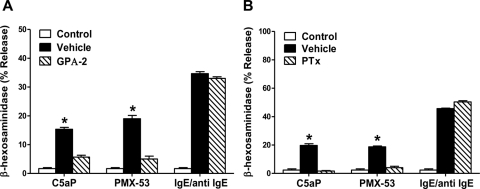
We used two approaches to determine whether PMX-53-induced mast cell degranulation occurs via a GPCR or by the direct activation of G proteins. A substance P-related peptide [G protein antagonist 2 (GPA-2); pGlu-Gln-dTrp-Phe-dTrp-dTrp-Met-NH2] inhibits M2 muscarinic cholinergic receptor-mediated G protein Gαo/Gαi activation in vesicles containing purified proteins (Mukai et al., 1992). This peptide does not directly inhibit G protein activity but blocks the ability of the receptor to activate G proteins (Mukai et al., 1992). We found that 1 μM GPA-2 caused a substantial inhibition of C5a and PMX-53-induced degranulation in LAD2 cells but had little or no effect on IgE-mediated response (Fig. 4A). Unlike GPA-2, pertussis toxin (PTx) covalently modifies the Gαo/Gαi family of G proteins to prevent their activation by GPCRs. We found that PTx also inhibited C5a- and PMX-53-mediated degranulation but not IgE-mediated degranulation (Fig. 4B). These findings in total suggest that C5a and PMX-53 activate PTx-sensitive GPCRs to induce degranulation in human LAD2 mast cells.
Fig. 4.
PMX-53 causes degranulation in human mast cells via GPCR. LAD2 mast cells were pretreated with vehicle, GPA-2 (1 μM, 30 min; A), or PTx (100 ng/ml, 16 h; B). Cells were stimulated with 10 nM C5aP and 100 nM PMX-53, and degranulation was determined. In all experiments, LAD2 cells were also stimulated by IgE/anti-IgE as a non-GPCR control. Data are mean ± S.E.M. of n = 3. Statistical significance was determined by one-way ANOVA with Dunnett's post test to compare differences between vehicle and GPA-2 or PTx treatment. *, p < 0.05.
PMX-53 Activates Human Mast Cells via MrgX2 but C5a Does Not.
GPCRs constitute the largest family of receptors, with ∼1000 members, and ∼50% have no known ligands and are classified as orphan receptors. Ligands for the previously orphan GPCRs MrgX1 and MrgX2 have recently been identified as neuropeptides BAM-22P and the cyclic neuropeptide CST (Table 1) respectively. Emerging evidence suggests that MrgX2 is activated by short peptides containing amino acids Pro, Phe, Trp, and Arg/Lys, but the presence of negatively charged residues such as Asp and Glu results in loss of activity (Robas et al., 2003; Nothacker et al., 2005). Based on the sequence comparison of PMX-53 with BAM-22P and CST (Table 1), we postulated that PMX-53 could activate human mast cells via MrgX2. Human CBMCs express MrgX1 and MrgX2 (Tatemoto et al., 2006). Because LAD2 cells display a mature phenotype similar to that of CBMCs (Kirshenbaum et al., 2003), we suspected that this cell could also express MrgX1 and MrgX2. Indeed, we found that mRNA for MrgX1 and MrgX2 are expressed in LAD2 mast cells (Fig. 5A). By contrast, the immature human mast cell line HMC-1 expressed MrgX1 and MrgX2 at low levels (Fig. 5A). This difference reflected the abilities of known ligands for MrgX1 and MrgX2 to activate LAD2 and HMC-1 cells. Thus, although BAM-22P and CST (ligands for MrgX1 and MrgX2, respectively) caused sustained Ca2+ mobilization and degranulation in LAD2 cells, they were essentially without effect in HMC-1 cells (Fig. 5, B–D).
Fig. 5.
LAD2 cells express functional MrgX1 and MrgX2 receptors but HMC-1 cells do not. A, MrgX1 and MrgX2 receptor expression was determined in LAD2 and HMC-1 cells by RT-PCR. Ligands for MrgX1 (BAM-22P; 1 μM) and MrgX2 (CST; 1 μM), induced sustained Ca2+ mobilization (B) and degranulation (C) in LAD2 mast cells. Statistical significance was determined by two-way ANOVA with Bonferroni's post test. *, p < 0.05. D, BAM-22P did not induce Ca2+ mobilization, and CST induced modest Ca2+ mobilization in HMC-1 cells. 10 nM C3a was used as a positive control. Data shown are representative of three similar experiments.
To determine the relevance of studies using human LAD2 cells, we performed selected confirmatory experiments in primary human CD34+-derived mast cells. Transcripts for MrgX1 and MrgX2 are expressed in CD34+-derived mast cells (Fig. 6A, inset). Furthermore, PMX-53 and PMX-53S caused substantial degranulation in CD34+ mast cells (Fig. 6A). As in LAD2 mast cells, although C5a caused a transient Ca2+ mobilization, PMX-53 (Fig. 6B) and PMX-53S (Fig. 6C) promoted sustained Ca2+ responses. Again, as in LAD2 cells, PMX-53C did not induce Ca2+ mobilization and degranulation in CD34+ mast cells (Fig. 6, A and D). These findings clearly demonstrate that the data obtained in LAD2 mast cell line is biologically relevant and does not reflect an artifact of immortalization.
Fig. 6.
Human CD34+-derived primary mast cells express MrgX1 and MrgX2 and respond to PMX-53 for degranulation and Ca2+ mobilization. Human CD34+-derived primary mast cells were stimulated with C5aP, PMX-53, PMX-53S, and PMX-53C (1 μM), and percentage degranulation (β-hexosaminidase release) was determined (A). MrgX1 and MrgX2 receptor expression was determined in CD34+-derived primary mast cells by RT-PCR (A, inset). C5a (B), PMX-53 (C), and PMX-53S but not PMX-53C (D) induced Ca2+ mobilization in CD34+-derived mast cells. Data shown are representative of three similar experiments. Statistical significance was determined by one-way ANOVA with Dunnett's post test. *, p < 0.05.
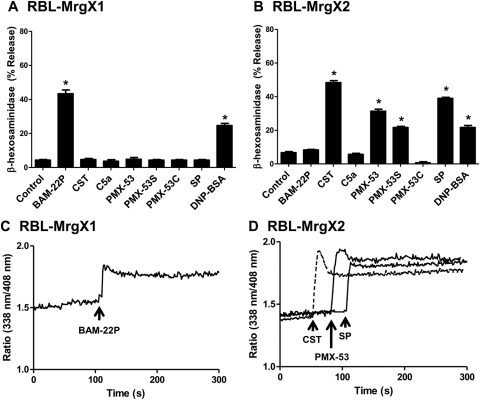
The rodent mast cell line RBL-2H3 did not respond to BAM-22P and CST (data not shown), indicating that MrgX1 and MrgX2 are not expressed in these cells. To precisely determine the role of MrgX receptors on C5a- and PMX-53-induced mast cell degranulation, we generated stable transfectants in RBL-2H3 cells expressing HA-tagged MrgX2 and for control MrgX1 (Fig. 7, A and B). We used a HA-specific antibody to determine cell surface receptor expression. Cells expressing equivalent MrgX1 and MrgX2 were used for degranulation and signaling studies. Cells expressing MrgX2 responded to CST, PMX-53, PMX-53S, and substance P for degranulation, but PMX-53C had no effect (Fig. 7B). PMX-53 caused a sustained Ca2+ mobilization in RBL-2H3 cells expressing MrgX2 that was similar in magnitude and duration to that observed in LAD2 cells and CD34+-derived mast cells (Fig. 7C). The effects of PMX-53, PMX-53S, and substance P were specific for MrgX2. Thus, RBL-2H3 cells expressing MrgX1 responded to its known ligand BAM-22P for Ca2+ mobilization and degranulation but PMX-53, PMX-53S, or substance P had no effect (Fig. 7, A and C). It is noteworthy that 1 μM C5a, which induced ∼60% degranulation in LAD2 mast cells, caused no noticeable degranulation in RBL-2H3 cells expressing MrgX1 or MrgX2 (Fig. 7, A and B).
Fig. 7.
PMX-53 uses MrgX2 to induce mast cell degranulation. RBL-2H3 cells stably expressing MrgX1 (A) or MrgX2 (B) were incubated with anti-DNP specific IgE (1 μg/ml, 16 h) and stimulated with the indicated peptides (1 μM), substance P (SP; 1 μM), or antigen (DNP-BSA; 30 ng/ml) for 30 min, and β-hexosaminidase release was measured. C and D, MrgX1 and MrgX2-expressing cells were also incubated with Indo-1AM and stimulated with selected peptides (1 μM), and intracellular calcium mobilization was determined. Data shown are representative of three similar experiments. Statistical significance was determined by one-way ANOVA with Dunnett's post test. *, p < 0.05.
Murine Peritoneal and Bone Marrow-Derived Mast Cells Do Not Respond to PMX-53.
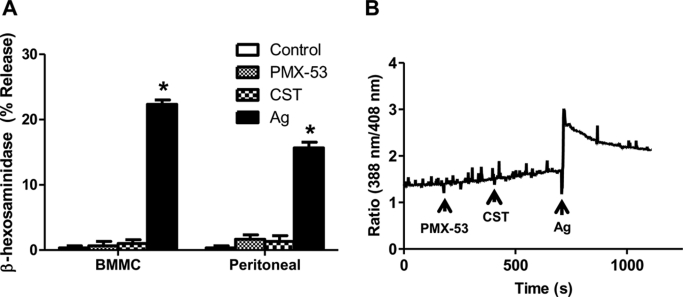
In contrast to human, ∼50 Mrg sequences have been identified in the mouse (Dong et al., 2001; Lembo et al., 2002). It is noteworthy that there is very little sequence homology between the human and mouse receptors. Furthermore, there is no information available as to which of these Mrg receptors are expressed in murine mast cells. We therefore sought to determine whether agonists for human MrgX2 could activate murine mast cells. For these experiments, we used murine BMMC and murine peritoneal mast cells. As shown in Fig. 8A, although antigen/IgE caused degranulation in BMMC and peritoneal mast cells, PMX-53 and CST (1 μM) had no effect. Likewise, BMMCs did not respond to PMX-53 or CST (1 μM) for Ca2+ mobilization, but antigen/IgE caused a robust response (Fig. 8B).
Fig. 8.
PMX-53 and CST do not activate murine peritoneal and bone marrow-derived mast cells. A, murine bone marrow-derived and peritoneal mast cells were incubated with DNP-specific mouse IgE (1 μg/ml, 16 h). Cells were exposed to buffer (control), 1 μM PMX-53, 1 μM CST, or 30 ng/ml DNP-BSA (Ag) for 30 min, and β-hexosaminidase release was measured. Data are mean ± S.E.M. of n = 3. B, murine bone marrow-derived mast cells were incubated with DNP-specific mouse IgE (1 μg/ml, 16 h). Cells were incubated with Indo-1AM and were exposed to 1 μM PMX-53, 1 μM CST, or 100 ng/ml DNP-BSA, and intracellular Ca2+ mobilization was determined. Data shown are representative of three similar experiments. Statistical significance was determined by one-way ANOVA with Dunnett's post test. *, p < 0.05.
Discussion
Previous studies indicated that C5a could induce mast cell degranulation, at least in part, via a CD88-independent pathway, possibly involving MrgX2 (el-Lati et al., 1994; Tatemoto et al., 2006). In the present study, we used a potent CD88 antagonist, CD34+-derived primary mast cells, two human mast cell lines, primary murine mast cells, as well as RBL-2H3 cells stably expressing human CD88, MrgX1, and MrgX2 to determine the receptor specificity of C5a-induced mast cell degranulation. We found that although C5a does not use MrgX1/MrgX2 for mast cell degranulation, PMX-53 functions as both a potent CD88 antagonist and a low-affinity ligand for MrgX2. The effect of PMX-53 on mast cell activation seems to be specific for human mast cells; murine mast cells, which do not express MrgX2 are unresponsive to activation by PMX-53. Our studies with PMX-53 analogs demonstrate that Trp and Arg residues are required for its activity as both a CD88 antagonist and a MrgX2 agonist.
PMX-53 is a potent CD88 antagonist in human neutrophils and macrophages in vitro and protects rodents from a number of experimental inflammatory diseases (Strachan et al., 2001; Arumugam et al., 2002, 2004; Woodruff et al., 2002, 2006). PMX-53 is currently undergoing clinical trials for treatment of osteoarthritis. Human mast cells endogenously express CD88 and respond to C5a for signaling and mediator release (Oskeritzian et al., 2005). Using HMC-1 cells and RBL-2H3 cells expressing CD88, we have shown that PMX-53 (10 nM) selectively inhibits C5a-induced, but not C3a-induced, responses. However, utilization of LAD2 cells, CD34+ mast cells, and MrgX transfected RBL-2H3 cells clearly demonstrated that PMX-53, at concentrations higher than that required to inhibit CD88, causes mast cell degranulation via MrgX2. MrgX2 is a low-affinity receptor and responds to CST and substance P for mast cell degranulation and reporter gene activity in transfected Pch12 cells with EC50 values of ≥30 nM (Robas et al., 2003; Tatemoto et al., 2006). Our finding that PMX-53 promotes degranulation in human mast cells at concentrations of ≥30 nM is consistent with MrgX2 activation. This was confirmed in studies with RBL-2H3 cells showing that PMX-53 activated MrgX2 and not MrgX1. These findings suggest that PMX-53 is a dual action molecule; it is a high-affinity antagonist of CD88 but a low-affinity agonist of MrgX2. It is noteworthy that there is very little sequence homology between human MrgX2 and any of the known mouse Mrg receptors (Dong et al., 2001; Lembo et al., 2002). Accordingly, we found that CST, a known ligand for MrgX2 and PMX-53, failed to induce degranulation in murine BMMC and peritoneal mast cells. Thus, the effect of PMX53 seems to be specific for human MrgX2.
An important shared property of the peptides that activate mast cells via MrgX2 is that they possess amino acids with hydrophobic and positively charged side chains (Table 1). Emerging evidence suggests that MrgX2 is activated by short peptides containing four amino acids (Pro, Phe, Trp and Arg/Lys) (Robas et al., 2003; Nothacker et al., 2005). A scrambled linear PMX-53 that has no antagonist activity against CD88 caused mast cell degranulation, albeit at higher concentration, which suggests that the cyclic nature of the peptide is not essential for its agonist activity toward MrgX2. In addition, we found that replacement of Trp-Arg with Ala-dArg in a cyclic PMX-53 peptide resulted in loss of the abilities to inhibit C5a-induced Ca2+ mobilization in HMC-1 cells and to induce degranulation in LAD2 mast cells. This study thus identified the hexapeptide PMX-53 as an agonist for MrgX2, which requires Trp and Arg residues for activity. It is unclear why PMX-53 did not induce degranulation in RBL-2H3 cells expressing MrgX1. BAM-22P, a ligand that activates MrgX1, possesses negatively charged amino acids in close proximity to the hydrophobic Trp residues (Glu-Trp-Trp-Met-Asp; Table 1). Our finding that BAM-22P caused degranulation in RBL-2H3 cells expressing MrgX1, but PMX-53 did not, suggests that there are important differences in binding properties of these receptors.
An interesting and consistent finding of the present study was that although C5a caused transient Ca2+ mobilization, agonists for MrgX1 and MrgX2 induced more sustained responses in human mast cells and transfected RBL-2H3 cells. The reason for this difference is unclear but could reflect differences in the phosphorylation and desensitization of these receptors. CD88 undergoes phosphorylation in response to agonist stimulation resulting in their desensitization (Christophe et al., 2000; Braun et al., 2003). Furthermore, phosphorylation-deficient mutants of CD88 are resistant to desensitization (Pollok-Kopp et al., 2007). Solinski et al. (2010) showed that MrgX1 is one of the few GPCRs that does not associate with β-arrestin and is resistant to agonist-induced receptor internalization. Thus, the possibility that MrgX2 receptor is also resistant to agonist-induced phosphorylation, β-arrestin recruitment, and desensitization remains to be determined.
Our attempt to identify the mechanisms by which C5a causes mast cell degranulation led us to the unexpected finding that CD88 antagonist PMX-53 induces degranulation in human mast cells via MrgX2. Outside the dorsal root ganglia, MrgX2 is expressed only in human mast cells (Tatemoto et al., 2006). Furthermore, we have shown a striking selectivity of PMX-53 for promoting degranulation in human but not rodent mast cells. It is noteworthy that although PMX-53 has been shown to be effective in modulating a number of experimental inflammatory diseases in animal models, one study reported that orally administered PMX-53, despite reaching serum levels high enough for C5aR-blocking activity, did not reduce synovial inflammation in humans with rheumatoid arthritis (Vergunst et al., 2007). It is possible, therefore, that its lack of effectiveness in humans could reflect opposing effects on blocking CD88 and activating MrgX2 in human mast cells. Given that mast cells play important roles in a number inflammatory diseases, caution should be exercised in using peptides containing amphiphilic residues for therapeutic purposes.
MrgX2 is a low-affinity and low-specificity GPCR that can be activated by short amphiphilic peptides. It is currently unknown whether this receptor is activated by endogenous peptides produced in the context of innate immunity and inflammation. However, human defensins, which are amphiphilic peptides, induce mast cell degranulation at concentrations similar to those observed for PMX-53 (Chen et al., 2007; Niyonsaba et al., 2010). This raises the interesting possibility that defensins produced by epithelial cells during microbial infection may activate human mast cells via MrgX2 to promote innate immunity. It is possible, therefore, that the dual effect of PMX-53 could be pharmacologically relevant in promoting both anti-inflammatory activity and innate immunity. Thus, at low concentrations, PMX-53 could block inflammation by acting as a CD88 antagonist, but at higher concentrations, it can promote innate immunity by mimicking the actions of defensins on mast cell activation. These exciting possibilities will be the subject of our future investigations.
Acknowledgments
We are grateful to Dr. Joseph Butterfield (Mayo Clinic, Rochester, MN) for supplying the HMC-1 cells. We also thank Drs. Arnold Kirshenbaum and Dean Metcalfe (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) for providing LAD2 mast cells and the FACS core facilities of the Schools of Medicine and Dental Medicine, University of Pennsylvania, for acquisition, analysis, and cell sorting. We are grateful to Dr. Jörg Köhl (University Hospital of Schleswig-Holstein, Campus Lübeck, Lübeck, Germany) for providing RBL-2H3 cells expressing CD88.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL085774] and National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant AI068730].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.071472.
- GPCR
- G protein-coupled receptor
- Mrg
- Mas-related gene
- CBMC
- cord blood-derived mast cell
- PMX-53
- AcPhe(ornithine-Pro-cyclohexylamine-Trp-Arg)
- HA
- hemagglutinin
- CST
- cortistatin-14
- BAM-22P
- bovine adrenal medulla docosapeptide
- rhSCF
- recombinant human stem-cell factor
- IL
- interleukin
- BMMC
- bone marrow-derived mast cells
- RT-PCR
- reverse transcription-polymerase chain reaction
- DNP
- dinitrophenol
- BSA
- bovine serum albumin
- PMX-53S
- scrambled linear PMX-53 peptide
- PMX-53C
- scrambled control PMX-53 peptide
- GPA-2
- G protein antagonist 2 (pGlu-Gln-dTrp-Phe-dTrp-dTrp-Met-NH2)
- PTx
- pertussis toxin
- ANOVA
- analysis of variance
- Indo-1AM
- 2[4-(bis(carboxymethyl)amino)-3-[2-[2-(bis(carboxymethyl)amino)-5-methylphenoxy]ethoxy]phenyl]-1H-indole-6-carboxylic acid, acetoxymethyl ester
- C5aP
- C5a peptide.
Authorship Contributions
Participated in research design: Subramanian, Kashem, and Ali.
Conducted experiments: Subramanian, Kashem, and Collington.
Contributed new reagents or analytical tools: Qu and Lambris.
Performed data analysis: Subramanian, Kashem and Collington.
Wrote or contributed to the writing of the manuscript: Subramanian, Collington, and Ali.
References
- Ali H, Ahamed J, Hernandez-Munain C, Baron JL, Krangel MS, Patel DD. (2000) Chemokine production by G protein-coupled receptor activation in a human mast cell line: roles of extracellular signal-regulated kinase and NFAT. J Immunol 165:7215–7223 [DOI] [PubMed] [Google Scholar]
- Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. (1993) Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem 268:24247–24254 [PubMed] [Google Scholar]
- Ali H, Richardson RM, Tomhave ED, DuBose RA, Haribabu B, Snyderman R. (1994) Regulation of stably transfected platelet activating factor receptor in RBL-2H3 cells. Role of multiple G proteins and receptor phosphorylation. J Biol Chem 269:24557–24563 [PubMed] [Google Scholar]
- Arumugam TV, Shiels IA, Woodruff TM, Reid RC, Fairlie DP, Taylor SM. (2002) Protective effect of a new C5a receptor antagonist against ischemia-reperfusion injury in the rat small intestine. J Surg Res 103:260–267 [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Woodruff TM, Stocks SZ, Proctor LM, Pollitt S, Shiels IA, Reid RC, Fairlie DP, Taylor SM. (2004) Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol 40:934–941 [DOI] [PubMed] [Google Scholar]
- Braun L, Christophe T, Boulay F. (2003) Phosphorylation of key serine residues is required for internalization of the complement 5a (C5a) anaphylatoxin receptor via a beta-arrestin, dynamin, and clathrin-dependent pathway. J Biol Chem 278:4277–4285 [DOI] [PubMed] [Google Scholar]
- Burstein ES, Ott TR, Feddock M, Ma JN, Fuhs S, Wong S, Schiffer HH, Brann MR, Nash NR. (2006) Characterization of the Mas-related gene family: structural and functional conservation of human and rhesus MrgX receptors. Br J Pharmacol 147:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, Saito H, Nagaoka I, Ikeda S, Okumura K, et al. (2007) Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol 37:434–444 [DOI] [PubMed] [Google Scholar]
- Christophe T, Rabiet MJ, Tardif M, Milcent MD, Boulay F. (2000) Human complement 5a (C5a) anaphylatoxin receptor (CD88) phosphorylation sites and their specific role in receptor phosphorylation and attenuation of G protein-mediated responses. Desensitization of C5a receptor controls superoxide production but not receptor sequestration in HL-60 cells. J Biol Chem 275:1656–1664 [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. (2001) A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106:619–632 [DOI] [PubMed] [Google Scholar]
- el-Lati SG, Dahinden CA, Church MK. (1994) Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol 102:803–806 [DOI] [PubMed] [Google Scholar]
- Ferry X, Brehin S, Kamel R, Landry Y. (2002) G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides 23:1507–1515 [DOI] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. (1999) Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem 42:1965–1974 [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. (2005) Role of c5a in inflammatory responses. Annu Rev Immunol 23:821–852 [DOI] [PubMed] [Google Scholar]
- Hartmann K, Henz BM, Krüger-Krasagakes S, Köhl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. (1997) C3a and C5a stimulate chemotaxis of human mast cells. Blood 89:2863–2870 [PubMed] [Google Scholar]
- Haynes DR, Harkin DG, Bignold LP, Hutchens MJ, Taylor SM, Fairlie DP. (2000) Inhibition of C5a-induced neutrophil chemotaxis and macrophage cytokine production in vitro by a new C5a receptor antagonist. Biochem Pharmacol 60:729–733 [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. (2003) Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res 27:677–682 [DOI] [PubMed] [Google Scholar]
- Köhl J. (2006) Drug evaluation: the C5a receptor antagonist PMX-53. Curr Opin Mol Ther 8:529–538 [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, et al. (2002) Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci 5:201–209 [DOI] [PubMed] [Google Scholar]
- March DR, Proctor LM, Stoermer MJ, Sbaglia R, Abbenante G, Reid RC, Woodruff TM, Wadi K, Paczkowski N, Tyndall JD, et al. (2004) Potent cyclic antagonists of the complement C5a receptor on human polymorphonuclear leukocytes. Relationships between structures and activity. Mol Pharmacol 65:868–879 [DOI] [PubMed] [Google Scholar]
- Mousli M, Hugli TE, Landry Y, Bronner C. (1994) Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology 27:1–11 [DOI] [PubMed] [Google Scholar]
- Mukai H, Munekata E, Higashijima T. (1992) G protein antagonists. A novel hydrophobic peptide competes with receptor for G protein binding. J Biol Chem 267:16237–16243 [PubMed] [Google Scholar]
- Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. (1996) C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol 157:1693–1698 [PubMed] [Google Scholar]
- Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, Kajiwara N, Saito H, Nagaoka I, Ogawa H, et al. (2010) Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol 184:3526–3534 [DOI] [PubMed] [Google Scholar]
- Nothacker HP, Wang Z, Zeng H, Mahata SK, O'Connor DT, Civelli O. (2005) Proadrenomedullin N-terminal peptide and cortistatin activation of MrgX2 receptor is based on a common structural motif. Eur J Pharmacol 519:191–193 [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. (2005) Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol 115:1162–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok-Kopp B, Hüttenrauch F, Rethorn S, Oppermann M. (2007) Dynamics of protein kinase C-mediated phosphorylation of the complement C5a receptor on serine 334. J Biol Chem 282:4345–4353 [DOI] [PubMed] [Google Scholar]
- Qu H, Ricklin D, Lambris JD. (2009) Recent developments in low molecular weight complement inhibitors. Mol Immunol 47:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM. (2010) Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol Chapter 7:Unit 7.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robas N, Mead E, Fidock M. (2003) MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem 278:44400–44404 [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Boekhoff I, Bouvier M, Gudermann T, Breit A. (2010) Sensory neuron-specific MAS-related gene-X1 receptors resist agonist-promoted endocytosis. Mol Pharmacol 78:249–259 [DOI] [PubMed] [Google Scholar]
- Strachan AJ, Shiels IA, Reid RC, Fairlie DP, Taylor SM. (2001) Inhibition of immune-complex mediated dermal inflammation in rats following either oral or topical administration of a small molecule C5a receptor antagonist. Br J Pharmacol 134:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, et al. (2006) Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349:1322–1328 [DOI] [PubMed] [Google Scholar]
- Tomhave ED, Richardson RM, Didsbury JR, Menard L, Snyderman R, Ali H. (1994) Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J Immunol 153:3267–3275 [PubMed] [Google Scholar]
- Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. (2005) Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol 42:581–587 [DOI] [PubMed] [Google Scholar]
- Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJ, Sanders ME, Reedquist KA, Tak PP. (2007) Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 46:1773–1778 [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM. (2004) Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res 116:81–90 [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Crane JW, Proctor LM, Buller KM, Shek AB, de Vos K, Pollitt S, Williams HM, Shiels IA, Monk PN, et al. (2006) Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J 20:1407–1417 [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Strachan AJ, Dryburgh N, Shiels IA, Reid RC, Fairlie DP, Taylor SM. (2002) Antiarthritic activity of an orally active C5a receptor antagonist against antigen-induced monarticular arthritis in the rat. Arthritis Rheum 46:2476–2485 [DOI] [PubMed] [Google Scholar]
- Woodruff TM, Strachan AJ, Sanderson SD, Monk PN, Wong AK, Fairlie DP, Taylor SM. (2001) Species dependence for binding of small molecule agonist and antagonists to the C5a receptor on polymorphonuclear leukocytes. Inflammation 25:171–177 [DOI] [PubMed] [Google Scholar]