Abstract
The aim of this study was to generate new insight into chemical regulation of transient receptor potential (TRP) channels with relevance to glucose homeostasis and the metabolic syndrome. Human TRP melastatin 2 (TRPM2), TRPM3, and TRP canonical 5 (TRPC5) were conditionally overexpressed in human embryonic kidney 293 cells and studied by using calcium-measurement and patch-clamp techniques. Rosiglitazone and other peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists were investigated. TRPM2 was unaffected by rosiglitazone at concentrations up to 10 μM but was inhibited completely at higher concentrations (IC50, ∼22.5 μM). TRPM3 was more potently inhibited, with effects occurring in a biphasic concentration-dependent manner such that there was approximately 20% inhibition at low concentrations (0.1–1 μM) and full inhibition at higher concentrations (IC50, 5–10 μM). PPAR-γ antagonism by 2-chloro-5-nitrobenzanilide (GW9662) did not prevent inhibition of TRPM3 by rosiglitazone. TRPC5 was strongly stimulated by rosiglitazone at concentrations of ≥10 μM (EC50, ∼30 μM). Effects on TRPM3 and TRPC5 occurred rapidly and reversibly. Troglitazone and pioglitazone inhibited TRPM3 (IC50, 12 μM) but lacked effect on TRPC5, suggesting no relevance of PPAR-γ or the thiazolidinedione moiety to rosiglitazone stimulation of TRPC5. A rosiglitazone-related but nonthiazolidinedione PPAR-γ agonist, N-(2-benzoylphenyl)-O-[2-(methyl-2-pyridinylamino)ethyl]-l-tyrosine (GW1929), was a weak stimulator of TRPM3 and TRPC5. The natural PPAR-γ agonist 15-deoxy prostaglandin J2, had no effect on TRPM3 or TRPC5. The data suggest that rosiglitazone contains chemical moieties that rapidly, strongly, and differentially modulate TRP channels independently of PPAR-γ, potentially contributing to biological consequences of the agent and providing the basis for novel TRP channel pharmacology.
Introduction
Transient receptor potential (TRP) proteins are ion pore-forming subunits of cationic channels that often confer Ca2+ and Na+ entry on cells (Venkatachalam and Montell, 2007; Damann et al., 2008). In mammals, the proteins are encoded by 28 genes and are divided into six subfamilies based on amino acid sequence similarities; the two largest subfamilies are the canonical (TRPC) and melastatin (TRPM) channels. The TRP channels appear to serve primarily chemical-sensing functions, many of them showing complex polymodal activation by multiple chemicals. In several cases, specific TRP channels have been linked to sensing of food chemicals such as menthol and have been shown to be determinants of sensory perception (Damann et al., 2008). However, TRP channels are often widely expressed across the body and many different functions have been suggested. Consequently there is broad relevance to mammalian biology.
Specific relevance of TRP channels to glucose homeostasis and the metabolic syndrome is starting to emerge. Disruption of Trpm2 or Trpm5 genes in mice was found to impair insulin secretion (Colsoul et al., 2010; Uchida et al., 2011), and stimulation of insulin secretion by pregnenolone sulfate was transduced by TRPM3 in isolated mouse pancreatic β-cells (Wagner et al., 2008). Up-regulated expression of TRPC channels, such as TRPC5, is a characteristic of the metabolic syndrome (Edwards et al., 2010; Hu et al., 2009; Wuensch et al., 2010), and TRPC channels have been implicated in vascular remodeling and hypertension (Yu et al., 2004; Xu et al., 2006; Al-Shawaf et al., 2010; Chen et al., 2010), which are features of the metabolic syndrome. Furthermore, proteomic analysis of hyperglycemia-induced endothelial injury identified TRPC5 as one of five proteins that was most up-regulated (Nath et al., 2009). TRPC channels are not known to affect insulin secretion, but disruption of Trpc3 gene in mice was observed to suppress pancreatitis (Kim et al., 2009).
Hydrogen peroxide is considered the primary activator of TRPM2 channels, probably acting via elevation of intracellular ADP ribose (Jiang et al., 2010). The strongest known activator of TRPM3 channels is pregnenolone sulfate (Wagner et al., 2008; Majeed et al., 2010). However, the concentrations of pregnenolone sulfate required for activation are supraphysiological (Wagner et al., 2008; Naylor et al., 2010). Other strong activators of TRPM3 are not known but they may not be required because constitutive cholesterol-regulated activity is possible (Naylor et al., 2010). TRPC5 channels are more complicated because they often arise through heteromultimerization with other TRPC channels. Activators include oxidized phospholipids and metal ions such as gadolinium and lead (Plant and Schaefer, 2005; Al-Shawaf et al., 2010; Sukumar and Beech, 2010). TRPC5 is also activated, apparently directly, by lysophospholipids such as lysophosphatidylcholine, which is a primary component of oxidized low-density lipoprotein complexes (Flemming et al., 2006). As such, TRPC5 is a putative lipid ionotropic receptor (Beech et al., 2009).
Peroxisome-proliferator-activated receptor-γ (PPAR-γ) is one of a family of lipid-regulated transcription factors (Lehmann et al., 1995). It has attracted much attention as a regulator of metabolic status and target for the thiazolidinedione drugs that are licensed for use in the treatment of type-2 diabetes and include rosiglitazone and pioglitazone (Quinn et al., 2008). Like TRP channels, PPAR-γ has promiscuous sensitivity to a range of ligands (Kliewer et al., 1997). Biologically relevant PPAR-γ agonists include polyunsaturated fatty acids (e.g., linolenic acid and linoleic acid), 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), and oxidized phospholipids (Kliewer et al., 1995; Xu et al., 1999; Davies et al., 2001).
Because TRP channels are considered promiscuous chemical sensors, it is important to acquire extensive knowledge of their chemical-sensing profiles. In chemical screens of TRPC5 and TRPM3 channel activities designed to discover novel modulators, we identified rosiglitazone as an activator of TRPC5 and an inhibitor of TRPM3. Here we report on our investigation of these hits.
Materials and Methods
Cell Culture.
Human TRPM2, TRPM3, and TRPC5 were expressed as described previously (McHugh et al., 2003; Zeng et al., 2004; Majeed et al., 2010). TRP channel cDNA, stably incorporated into human embryonic kidney 293 cells, was under the control of a tetracycline-inducible promoter such that addition of 1 μg/ml tetracycline (Tet+) induced expression of channels. Cells not treated with tetracycline (Tet−) were used as control. Cells were maintained in Dulbecco's modified Eagle's medium-F12 + GlutaMAX (Invitrogen, Paisley, UK) supplemented with 10% fetal calf serum, 100 units/ml penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO), and the selection antibiotics (10 μg/ml blasticidin and 400 μg/ml phleomycin (Zeocin); Invitrogen) at 37°C in a 5% CO2 incubator.
Ca2+ Measurement.
Twenty-four hours before intracellular Ca2+ measurements, cells were plated at 80 to 90% confluence in clear-bottomed poly-d-lysine–coated 96-well plates (Corning Life Sciences, Lowell, MA). Immediately before recordings, cells were incubated for 1 h at 37°C in SBS containing 2 μM fluo-4 acetoxymethyl ester (with 0.01% Pluronic acid and 2.5 mM probenecid) and then washed with SBS three times before adding the recording buffer (SBS with appropriate solvent). SBS in de-ionized water contained 130 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 8 mM glucose, and 10 mM HEPES. Osmolality was adjusted to 290 mOsm/kg by using d-mannitol, and pH was titrated to 7.4 by using 4 M NaOH. Measurements were made on a 96-well fluorescence plate reader (FlexStation II384; Molecular Devices, Sunnyvale, CA). Fluo-4 was excited at 485 nm, and emitted light was collected at 525 nm. Wells of the 96-well plate were studied in a column format and loaded alternately for test and control conditions. All experiments were at 23 ± 2°C. Intracellular calcium concentration ([Ca2+]i) is shown as fluo-4 fluorescence emission intensity (F). Where the basal [Ca2+]i was not altered by the drug treatment, changes in absolute fluorescence (ΔF) are given for sake of clarity. An alternative method is to divide F values by their initial value (F/F0), but this method prevents detection of drug-induced shifts in basal channel activity and so we did not use it; in cases in which there was no change in baseline, it was demonstrated that the ΔF and F/F0 methods gave similar results (Fig. 1b; see also Supplemental Fig. I).
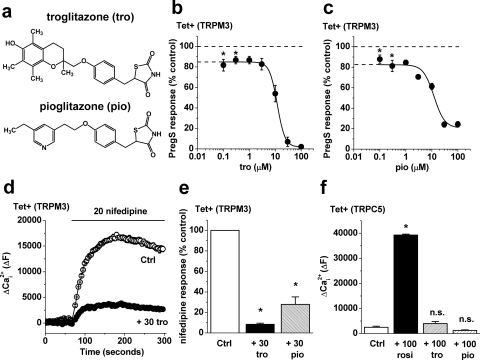
Fig. 1.
Fast and reversible inhibition of TRPM3 activity by rosiglitazone. a, two-dimensional structure of rosiglitazone (rosi) with the thiazolidinedione (TZD) moiety highlighted. b–e, data were generated by Ca2+ measurement (b) or whole-cell patch-clamp (c–e) in cells overexpressing TRPM3 (Tet+) unless specified by Tet− (in b). b, example parallel comparisons of 5 μM PregS responses in the presence of 10 μM rosi or vehicle applied for 15-min before PregS application and maintained throughout the recordings (N = 4). c, time-series plot showing the effect of bath-applied 5 μM PregS and then the addition of 10 μM rosi. Shown are outward and inward currents sampled during the voltage ramp. d, typical I-V relationships for the PregS-induced currents immediately before (PregS) and after the response to 10 μM rosi (+10 rosi). e, for experiments of the type shown in c, the mean residual PregS-evoked current after application of rosi normalized to the current amplitude immediately before application of rosi (n = 9).
Electrophysiology.
Recordings were made using the whole-cell configuration of the patch-clamp technique. Borosilicate glass capillaries (o.d., 1 mm; i.d., 0.58 mm; Harvard Apparatus, Holliston, MA) were used as the basis for patch pipettes. Pipettes were pulled using a PP-830 vertical two-stage pipette-puller (Narishige, Tokyo, Japan). Pipette resistances after fire-polishing and filling with pipette solution were 3 to 5 MΩ. Pipettes were mounted either on a CV203BU or CV201A head-stage (Molecular Devices) connected to a three-way coarse manipulator and micromanipulator (Newport 300P; Newport Corporation, Irvine, CA; or Piezo PCS500; Burleigh, ON, Canada). Electrodes comprised silver wires coated with chloride ions. Electrical signals were amplified and recorded using an Axopatch 200B or 200A amplifier and pCLAMP software (Molecular Devices). Data were filtered at 1 kHz and sampled digitally at 2 kHz via a Digidata 1322A analog-to-digital converter (Molecular Devices). Series resistances were <10 MΩ. The voltage protocol consisted of a step from a holding potential of 0 mV to −100 mV, followed by a 0.1-s ramp to +100 mV, before returning to 0 mV (repeated every 10 s). Analysis was performed off-line using Clampfit 8.2 or 10.2 (Molecular Devices) and Origin 7.5 software (OriginLab Corp., Northampton, MA). The TRPC5 electrophysiology data presented in Fig. 3d were generated using the Patchliner planar patch-clamp system (Nanion, München, Germany) in whole-cell mode (Milligan et al., 2009). Before recordings, cells were detached from culture flasks using 0.05% trypsin/EDTA and resuspended at a density of 106 to 5 × 107 cells/ml. For TRPM3 the extracellular solution comprised 130 mM NaCl, 5 mM KCl, 10 mM CsCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 8 mM glucose, and 10 mM HEPES, with pH titrated to 7.4 using 4 M NaOH. The osmolality of this solution was 295 mOsm/kg. The patch-pipette solution comprised 80 mM cesium aspartate, 45 mM CsCl, 10 mM HEPES, 10 mM BAPTA sodium, and 4 mM Na2ATP; osmolality was adjusted to 290 mOsm/kg by using d-mannitol, and the pH was titrated to 7.2 by using 4 M CsOH. For TRPC5,. the extracellular solution comprised 135 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 8 mM glucose, and 10 mM HEPES in deionized water. Osmolality was adjusted to 290 mOsm/kg by using d-mannitol (except for Fig. 6), and pH was titrated to 7.4 by using 4 M NaOH. The patch-pipette solution comprised 135 mM CsCl, 2 mM MgCl2, 1 mM EGTA, 10 mM HEPES, 5 mM Na2ATP, and 0.1 mM Na2GTP. The pH was titrated to 7.2 by using 4 M CsOH. Patch pipette solutions were filtered using a 0.2-μm membrane filter (Minisart; Sartorius Stedim Biotech, Goettingen, Germany), divided into aliquots of ∼50 μl, and stored at −20°C.
Fig. 3.
Stimulation of TRPC5 activity by rosiglitazone. Data were from cells overexpressing TRPC5 (Tet+) unless specified by Tet−. a, typical Ca2+ measurement experiment showing the effect of 100 μM rosiglitazone (rosi) in TRPC5-expressing cells (N = 4 each). b, example whole-cell patch-clamp experiment for currents at +80 and −80 mV, showing the effect of bath-applied 100 μM rosi. c, I-V relationship for the rosi-induced current of b. d, concentration-dependent stimulation of TRPC5 determined after 500-s (white circles) and 15-min (black circles) applications of rosi in Ca2+ measurement experiments (n/N = 3/18 each). The curve is a fitted Hill equation to the 15-min data (EC50, 31.1 μM; slope, 1.65). Also included are whole-cell patch-clamp data for responses to rosi at −80 mV (white triangles) and +80 mV (white squares) (n = 5).
Fig. 6.
Insensitivity of TRPM3 and TRPC5 to 15-deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2). a, two-dimensional structure of 15d-PGJ2. Data were generated in cells overexpressing TRPM3 (b–d) or TRPC5 (e). b and c, example time-series for a whole-cell voltage-clamp experiment showing the effect of bath-applied 10 μM 15d-PGJ2 on currents elicited by 5 μM PregS. c, I-V relationship for PregS-evoked currents before (PregS) and after application of 15d-PGJ2 (+10 15d-PGJ2). d, mean data for the experiments exemplified in b and analyzed 5 and 10 min after 15d-PGJ2 application (n = 7). e, Ca2+ measurement data comparing the amplitude of the signals elicited by 10 μM 15d-PGJ2 or 100 μM Gd3+ in cells overexpressing TRPC5 (Tet+) and control (Tet−) cells (n/N = 3/24 for each point).
Chemicals.
All chemicals were purchased from Sigma-Aldrich and dissolved at the required concentration in 100% dimethyl sulfoxide unless otherwise specified. Rosiglitazone, troglitazone, pioglitazone, and ciglitazone were purchased from Enzo Life Sciences, Inc., (Farmingdale, NY). 15d-PGJ2 (Enzo Life Sciences, Inc.) was supplied as a 3 mM stock in methyl acetate. N-(2-Benzoylphenyl)-O-[2-(methyl-2-pyridinylamino)ethyl]-l-tyrosine (GW1929) and 2-chloro-5-nitrobenzanilide (GW9662) were prepared as 50 mM stocks in 100% dimethyl sulfoxide. All chemicals were stored at −20°C. Gadolinium was prepared as 20 or 100 mM stock solutions in de-ionized water.
Data Analysis.
Averaged data are expressed as mean ± S.E.M. Control and test data were produced in pairs and compared by using an independent Student's t test (intracellular Ca2+ measurement) or paired Student's t test (electrophysiology performed on the same cell). Probability (P) of less than 0.05 was considered statistically significant. All intracellular Ca2+ measurement data are presented as n/N, where n is the number of independent experiments (i.e., on different 96-well plates) and N is the number of wells used in the 96-well plates. For patch-clamp recordings, n is the number of single cells from which measurements were made. Origin 7.5 software (OriginLab, Northampton, MA) was used for data analysis and presentation.
Results
Potent Inhibition of TRPM3 but not TRPM2 by Rosiglitazone.
Rosiglitazone (Fig. 1a) inhibited TRPM3-dependent Ca2+-influx evoked by the TRPM3 stimulator pregnenolone sulfate (PregS) (Fig. 1b). Likewise, TRPM3-dependent ionic current was inhibited (Fig. 1, c–e). The onset and washout of the effect occurred relatively rapidly within 100 to 200 s, a time course similar to that of PregS (Fig. 1c). Inhibition of ionic current was the same at negative and positive voltages, suggesting voltage-independent action (Fig. 1, d and e).
Concentration-response curves were constructed against TRPM3 stimulated by the structurally independent TRPM3 modulator nifedipine (Fig. 2, a and b) or PregS (Fig. 2c). In both cases we observed that low concentrations of rosiglitazone (0.1–1 μM) caused approximately 20% inhibition of TRPM3 (Fig. 2, a–c). In Fig. 2b, the effect is seen as the difference between the dashed line at 100% (i.e., no effect) and the data points at approximately 80% (i.e., ∼20% inhibition). Concentration-response curves were not constructed for this effect because the signals were small, making it difficult to precisely determine IC50 values. Higher concentrations of rosiglitazone caused stronger and complete TRPM3 inhibition with IC50 values of 9.5 and 4.6 μM against nifedipine- and PregS-evoked activity (Fig. 2, b and c). In contrast, rosiglitazone had no effect on TRPM2 activity at concentrations up to 10 μM (Fig. 2c). Higher concentrations of rosiglitazone blocked TRPM2, leading to a steep concentration-response curve and approximate IC50 of 22.5 μM (Fig. 2c). These data suggest that rosiglitazone is a blocker of TRPM2 and TRPM3 channels with greatest potency and a biphasic concentration-response relationship at TRPM3.
Fig. 2.
Concentration-dependence of TRPM3 inhibition by rosiglitazone. Data were generated by Ca2+ measurement. a, example direct comparison of the effects of different concentrations of rosiglitazone (rosi) on TRPM3 activity evoked by 20 μM nifedipine. Rosi was applied 15 min before nifedipine and was continuously present thereafter. There was no response to nifedipine in the absence of TRPM3 expression (data not shown). b, mean normalized data for experiments of the type exemplified in a. Signals were measured 90 s (black circles) and 240 s (white circles) after nifedipine application (n/N = 3/12). The Hill equation fitted to the 90-s data points had an IC50 of 9.52 μM and slope of 1.34. c, mean concentration-response data for rosi inhibition of TRPM3 activated by 5 μM PregS (black circles; IC50, 4.6 μM; slope, 1.1) or TRPM2 activated by 1 mM H2O2 (white squares; IC50, 22.5 μM; slope, 3.4) (n/N = 3/12 for each data point). b and c, statistical comparisons (*, P < 0.05) were made against paired solvent controls (i.e., 100%, no effect) and measured 90 s after application of the channel stimulator.
Stimulation of TRPC5 by Rosiglitazone.
Measurement of [Ca2+]i as an indicator of TRPC5 activity revealed a strong stimulatory effect of direct application of rosiglitazone (Fig. 3a). Rosiglitazone had no effect on cells that were not induced to express TRPC5 (Fig. 3a) or when extracellular Ca2+ was omitted in experiments on TRPC5-expressing cells (n/N = 4/28, data not shown). Rosiglitazone also evoked ionic current in TRPC5-expressing cells, with a tendency for current at positive voltages to be evoked most rapidly (Fig. 3b). The onset and washout of effects occurred within 100 to 200 s (Fig. 3b). The evoked currents were not from background (endogenous) ion channels because they did not occur in control (noninduced) cells (data not shown), and the evoked currents had the distinctive current-voltage (I-V) relationship expected for TRPC5 channels, which showed inward rectification at negative voltages and outward rectification at positive voltages with a plateau around 0 mV, conferring an approximate inverted S-shape (Fig. 3c). Three different experimental protocols were used to determine the concentration-dependence of the effect of rosiglitazone on TRPC5 activity. Each yielded a similar result, giving an EC50 of ∼30 μM (Fig. 3d). The data suggest that rosiglitazone is a strong stimulator of TRPC5 channels. In contrast, direct application of rosiglitazone did not stimulate TRPM2 or TRPM3 channels (n/N = 3/12 each, data not shown).
Effects of Troglitazone and Pioglitazone on TRPM3 but Not TRPC5.
To provide insight into the chemical features of rosiglitazone that are required for TRP channel modulation, we tested troglitazone and pioglitazone, which, like rosiglitazone, contain the thiazolidinedione moiety (Fig. 4a; see also Fig. 1a). Both agents inhibited TRPM3, showing effects that were similar to those caused by rosiglitazone (Fig. 4, b and c). Also as with rosiglitazone, there was inhibition of nifedipine- as well as PregS-evoked TRPM3 activity (Fig. 4, d and e). In marked contrast, troglitazone and pioglitazone completely failed to evoke TRPC5 activity (Fig. 4f). Likewise, ciglitazone failed to stimulate TRPC5 (n/N = 3/12, data not shown). The data suggest that the thiazolidinedione moiety is important for TRPM3 modulation but insufficient for TRPC5 modulation.
Fig. 4.
Differential requirement for the thiazolidinedione moiety. Data were generated by Ca2+ measurement in cells over-expressing TRPM3 (b–e) or TRPC5 (f). a, two-dimensional structures of troglitazone (tro) and pioglitazone (pio). b and c, mean data for effects of tro (b) and pio (c) on TRPM3 stimulated by 5 μM PregS. Statistical comparisons were made with paired solvent controls. The fitted Hill equations gave IC50 values of 11.97 μM (slope, 2.91) and 12.10 μM (slope, 2.11) for tro and pio, respectively (n/N = 3/12 for each point). d, example experiment showing the effect of 15-min treatment with 30 μM tro on TRPM3 stimulated by 20 μM nifedipine. e, mean normalized data for experiments with tro (as exemplified in d) and 30 μM pio (n/N = 3/24 for each point). f, mean Ca2+ responses evoked by 100 μM rosi, 100 μM tro, 100 μM pio, or the vehicle control (dimethylsulfoxide) in cells overexpressing TRPC5 (n/N = 3/12 each).
PPAR-γ Antagonism Did Not Affect TRPM3 Inhibition by Rosiglitazone.
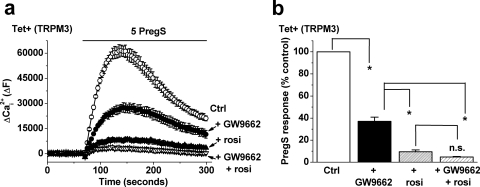
The observation that rosiglitazone, troglitazone, and pioglitazone all inhibited TRPM3 could be explained by effects through PPAR-γ, even though the effects were quite rapid and thus presumably not mediated via transcriptional control. To investigate the role of PPAR-γ, cells were pretreated for 24 h in GW9662, an irreversible PPAR-γ antagonist (Leesnitzer et al., 2002). Pretreatment with GW9662 partially inhibited the PregS response (Fig. 5, a and b). However, the inhibitory effect of rosiglitazone was retained, unaltered in amplitude compared with that in control cells (Fig. 5, a and b). The data suggested that the effect of rosiglitazone was not mediated by PPAR-γ.
Fig. 5.
Insensitivity of the rosiglitazone effect on TRPM3 to PPAR-γ antagonism. Data were generated by Ca2+ measurement in cells overexpressing TRPM3 (Tet+). a, cells were pretreated with 50 μM GW9662 (or vehicle) for 24 h before experiments and the GW9662 was maintained during the recordings. Pretreatment with 10 μM rosiglitazone (rosi) was as described for Fig. 1b, and PregS was applied at 5 μM. b, mean data for experiments exemplified in a, showing the effect of GW9662 alone (+ GW9662), rosi alone (+ rosi), or GW9662 with rosi (+ GW9662 + rosi) on the PregS-induced response (n/N = 3/12 for each condition).
Lack of Effect of an Endogenous PPAR-γ Agonist.
To investigate further whether the action of rosiglitazone on TRP channels occurred via PPAR-γ, we tested 15d-PGJ2, an endogenous PPAR-γ agonist that is chemically unrelated to rosiglitazone (Fig. 6a; see also Fig. 1a). 15d-PGJ2 had no effect on TRPM3 (Fig. 6, b–d). Likewise, there was also no stimulation of TRPC5 activity (Fig. 6e) compared with the effect of gadolinium (Gd3+), which is a direct TRPC5 stimulator and was used as a positive control.
Effect of an Alternative Pharmacological PPAR-γ Agonist, GW1929.
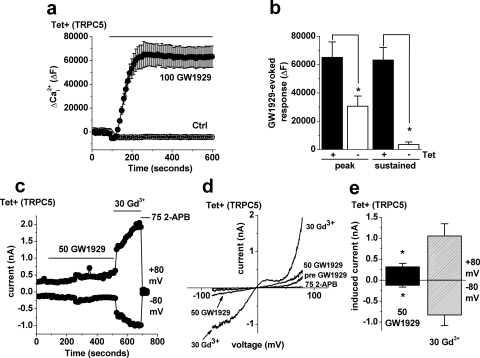
To further investigate the relationship to PPAR-γ, we used GW1929, a PPAR-γ agonist that lacks the thiazolidinedione moiety but resembles rosiglitazone in other chemical features (Fig. 7a; see also Fig. 1a). Unlike rosiglitazone, GW1929 failed to inhibit TRPM3-dependent Ca2+ entry (Fig. 7, b and c). However, it had a small but significant stimulatory effect on basal TRPM3 activity (Fig. 7, b and d). The effect of GW1929 on TRPC5 was also investigated. Ca2+ entry was evoked by GW1929 in TRPC5-expressing cells (Fig. 8, a and b). However, unlike rosiglitazone, it also evoked Ca2+ signals in noninduced cells (Fig. 8b). Although TRPC5-independent effects were more transient and smaller than those in TRPC5 cells, the presence of TRPC5-independent effects complicated interpretation of the data because TRPC5 activity can be potentiated by elevated intracellular Ca2+ levels (Hui et al., 2006; Blair et al., 2009). In whole-cell patch-clamp recordings, GW1929 elicited currents, but they were small in amplitude relative to currents evoked by the standard TRPC5 stimulator, gadolinium (Fig. 8, c–e). In most recordings, GW1929-evoked currents did not exhibit a distinct TRPC5 I-V relationship but in one recording, we observed clear activation of a TRPC5 I-V relationship (Supplemental Fig. 2). The data suggest that GW1929 is a weak stimulator of TRPM3 and TRPC5 but that its effect is complicated by other actions on intracellular Ca2+ signaling.
Fig. 7.
Effect of a nonthiazolidinedione PPAR-γ agonist (GW1929) on TRPM3. Data were generated by Ca2+ measurement in cells overexpressing TRPM3 (Tet+) or control (Tet−) cells. a, two-dimensional structure of GW1929. b, example parallel comparisons of 5 μM PregS responses in the presence of 50 μM GW1929 or vehicle (Ctrl) applied for 15 min before PregS application and maintained throughout the recordings (N = 8). Note the small increase in basal Ca2+ (indicated by arrows) in the presence of GW1929. c and d, mean data for experiments exemplified in (b), showing the effect of pretreatment with GW1929 on the PregS response (c) and GW1929-evoked increase in basal Ca2+ in Tet+ (TRPM3) but not Tet− cells (d) (n/N = 3/24 each).
Fig. 8.
Effect of GW1929 on TRPC5. a–e, data were generated by Ca2+ measurement (a and b) or whole-cell patch-clamp recording (c–e). a, example experiment showing the effect of application of 100 μM GW1929 or vehicle (Ctrl) on cells overexpressing TRPC5. b, mean data for the peak and sustained components of the GW1929-evoked Ca2+ response in cells overexpressing TRPC5 (Tet+) or control (Tet−) cells (n/N = 3/12 each). c, example time-series plot from a whole-cell voltage-clamp experiment showing the effect of bath-applied 50 μM GW1929, 30 μM Gd3+ and then 75 μM 2-aminoethoxydiphenylborate (2-APB). d, example I-V relationship from the experiment in c. e, mean currents induced by 50 μM GW1929 or 30 μM Gd3+ in cells overexpressing TRPC5 (Tet+) (n = 5). Statistical analysis was of the response to GW1929 relative to its vehicle (pre-GW1929) control.
Discussion
The study has shown that rosiglitazone is an inhibitor of TRPM2 and TRPM3 channels and an activator of TRPC5 channels with a sensitivity order of TRPM3 > TRPC5 = TRPM2. The study focused on TRPM3 and TRPC5, showing that for both channels, the natural PPAR-γ agonist 15d-PGJ2 had no effect, which suggests that rosiglitazone did not act via PPAR-γ. Further suggestions of independence from PPAR-γ came from the observations that PPAR-γ antagonism by GW9662 failed to affect the inhibition of TRPM3 by rosiglitazone, the PPAR-γ agonist GW1929 failed to inhibit TRPM3, and TRPC5 stimulation by rosiglitazone was not mimicked by troglitazone, pioglitazone, or ciglitazone and was only very weakly mimicked by GW1929. In addition to PPAR-γ independence, the relatively fast-occurring effects of rosiglitazone were suggestive of nongenomic mechanisms of action. These relatively direct effects were not chemically identical for TRPM3 and TRPC5, with the thiazolidinedione moiety showing importance for TRPM3 inhibition but not TRPC5 stimulation, for example. Therefore, the data suggest distinct but related drug binding sites for modulation of TRPM3 and TRPC5 channels.
Thiazolidinediones have not previously been shown to affect TRP channels, but effects on other types of ion channel have been described. Troglitazone inhibited voltage-dependent Ca2+ and K+ currents in vascular smooth muscle cells with IC50 values of 2 and 18 μM, contrasting with rosiglitazone and pioglitazone, which had weak effects (Eto et al., 2001). Likewise, troglitazone and rosiglitazone inhibited ATP-sensitive K+ channels in vascular smooth muscle cells with IC50 values of 1 and 20 μM, respectively (Mishra and Aaronson, 1999), and overexpressed KV1.3 channels with IC50 values of 4 and 19 μM, respectively (Ahn et al., 2007). Therefore, there has been a theme of relatively potent blockade by troglitazone and weaker effects of rosiglitazone, which is different from what we observed with TRPM3 and TRPC5 channels; i.e., troglitazone inhibited TRPM3 with an IC50 of 12 μM (IC50 values were <0.1 and 5–10 μM for rosiglitazone) and lacked effect on TRPC5 at 100 μM. GW1929, the nonthiazolidinedione compound, has previously been reported to inhibit voltage-dependent Ca2+ current with an IC50 of 5 μM (Heppner et al., 2005). These effects on ion channels have occurred relatively rapidly and so would also seem likely to have been independent of transcriptional control and regulation of transcription by PPAR-γ.
The inhibitory effects of thiazolidinediones on TRPM3 may be relevant to their clinical usage because plasma concentrations of rosiglitazone after administration of a single oral dose of 8 mg reach 2 to 3 μM (Thompson-Culkin et al., 2002; Chu et al., 2007; Aramwit et al., 2008), which is in the threshold concentration range for affecting TRP channels. Based on current, limited, knowledge of TRPM3 functions, it would be anticipated that consequences of blocking TRPM3 would be reduced insulin secretion from the pancreas, increased interleukin secretion from vascular smooth muscle cells, and increased hyaluronan secretion from synovial fibroblasts of patients with rheumatoid arthritis (Wagner et al., 2008; Ciurtin et al., 2010; Naylor et al., 2010). All of these effects would presumably be unwanted, consistent with the emerging concept of TRPM3 as a beneficial ion channel that is stimulated by “fountain of youth” steroids. Stimulation of TRPC5 by rosiglitazone may also have adverse consequences because TRPC5-containing channels have been linked to unwanted vascular remodeling (Al-Shawaf et al., 2010).
In summary, the findings of this study have revealed previously unrecognized chemical modulation of TRP channels that may provide foundations for novel and subtype-selective TRP channel modulators because differential effects were observed on three types of TRP channel, distinct chemical structure-activity relationships were identified, and the potency of rosiglitazone was greater than that of troglitazone, which suggests the capacity for selectivity relative to other channel types. Nevertheless, there should be caution because the polarity of the effects is potentially unwanted; that is, the prediction is that it would be therapeutically advantageous to have TRPM3 activators rather than inhibitors, and TRPC5 inhibitors rather than stimulators.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by Wellcome Trust [Grant 083857]; the British Heart Foundation [Grant 26679]; a University of Leeds studentship (to Y.M.); an Egyptian Ministry of Higher Education scholarship (to Y.B.); and a Biotechnology and Biological Sciences Research Council -AstraZeneca PhD Studentship (to L.A.W.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.069922.
- TRP
- transient receptor potential
- TRPC
- transient receptor potential canonical
- TRPM
- transient receptor potential melastatin
- PPAR
- peroxisome-proliferator-activated receptor
- 15d-PGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- SBS
- standard bath solution
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- GW1929
- N-(2-benzoylphenyl)-O-[2-(methyl-2-pyridinylamino)ethyl]-l-tyrosine
- GW9662
- 2-chloro-5-nitrobenzanilide
- PregS
- pregnenolone sulfate
- I-V
- current-voltage.
Authorship Contributions
Participated in research design: Majeed, Bahnasi, and Beech.
Conducted experiments: Majeed, Bahnasi, Seymour, Wilson, Milligan, Sukumar, and Naylor.
Contributed new reagents or analytical tools: Agarwal.
Performed data analysis: Majeed, Bahnasi, Seymour, Wilson, and Milligan.
Wrote or contributed to the writing of the manuscript: Majeed and Beech.
References
- Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Kim SY, et al. (2007) Open channel block of Kv1.3 by rosiglitazone and troglitazone: Kv1.3 as the pharmacological target for rosiglitazone. Naunyn Schmiedebergs Arch Pharmacol 374:305–309 [DOI] [PubMed] [Google Scholar]
- Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O'Regan D, Porter KE, Li J, Beech DJ. (2010) Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol 30:1453–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramwit P, Supasyndh O, Sriboonruang T. (2008) Pharmacokinetics of single-dose rosiglitazone in chronic ambulatory peritoneal dialysis patients. J Clin Pharm Ther 33:685–690 [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. (2009) TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium 45:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Kaczmarek JS, Clapham DE. (2009) Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 133:525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang D, Ma S, He H, Luo Z, Feng X, Cao T, Ma L, Yan Z, Liu D, et al. (2010) Increased rhythmicity in hypertensive arterial smooth muscle is linked to transient receptor potential canonical channels. J Cell Mol Med 14:2483–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KM, Hu OY, Pao LH, Hsiong CH. (2007) Pharmacokinetics of oral rosiglitazone in Taiwanese and post hoc comparisons with Caucasian, Japanese, Korean, and mainland Chinese subjects. J Pharm Pharm Sci 10:411–419 [DOI] [PubMed] [Google Scholar]
- Ciurtin C, Majeed Y, Naylor J, Sukumar P, English AA, Emery P, Beech DJ. (2010) TRPM3 channel stimulated by pregnenolone sulphate in synovial fibroblasts and negatively coupled to hyaluronan. BMC Musculoskelet Disord 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, et al. (2010) Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci USA 107:5208–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damann N, Voets T, Nilius B. (2008) TRPs in our senses. Curr Biol 18:R880–R889 [DOI] [PubMed] [Google Scholar]
- Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, Hilaire AS, Prescott SM, Zimmerman GA, et al. (2001) Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem 276:16015–16023 [DOI] [PubMed] [Google Scholar]
- Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. (2010) Exercise training decreases store-operated Ca2+entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res 85:631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M. (2001) Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol 423:1–7 [DOI] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ. (2006) Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem 281:4977–4982 [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Eckman DM, Gomez MF, Petkov GV, Nelson MT. (2005) Novel PPARgamma agonists GI 262570, GW 7845, GW 1929, and pioglitazone decrease calcium channel function and myogenic tone in rat mesenteric arteries. Pharmacology 73:15–22 [DOI] [PubMed] [Google Scholar]
- Hu G, Oboukhova EA, Kumar S, Sturek M, Obukhov AG. (2009) Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol Endocrinol 23:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui H, McHugh D, Hannan M, Zeng F, Xu SZ, Khan SU, Levenson R, Beech DJ, Weiss JL. (2006) Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol 572:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Yang W, Zou J, Beech DJ. (2010) TRPM2 channel properties, functions and therapeutic potentials. Expert Opin Ther Targets 14:973–988 [DOI] [PubMed] [Google Scholar]
- Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. (2009) Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology 137:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. (1995) A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 83:813–819 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, et al. (1997) Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 94:4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, et al. (2002) Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41:6640–6650 [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270:12953–12956 [DOI] [PubMed] [Google Scholar]
- Majeed Y, Agarwal AK, Naylor J, Seymour VA, Jiang S, Muraki K, Fishwick CW, Beech DJ. (2010) Cis-isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br J Pharmacol 161:430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. (2003) Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278:11002–11006 [DOI] [PubMed] [Google Scholar]
- Milligan CJ, Li J, Sukumar P, Majeed Y, Dallas ML, English A, Emery P, Porter KE, Smith AM, McFadzean I, et al. (2009) Robotic multiwell planar patch-clamp for native and primary mammalian cells. Nat Protoc 4:244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Aaronson PI. (1999) Differential block by troglitazone and rosiglitazone of glibenclamide-sensitive K+ current in rat aorta myocytes. Eur J Pharmacol 386:121–125 [DOI] [PubMed] [Google Scholar]
- Nath AK, Krauthammer M, Li P, Davidov E, Butler LC, Copel J, Katajamaa M, Oresic M, Buhimschi I, Buhimschi C, et al. (2009) Proteomic-based detection of a protein cluster dysregulated during cardiovascular development identifies biomarkers of congenital heart defects. PLoS One 4:e4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, et al. (2010) Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res 106:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TD, Schaefer M. (2005) Receptor-operated cation channels formed by TRPC4 and TRPC5. Naunyn Schmiedebergs Arch Pharmacol 371:266–276 [DOI] [PubMed] [Google Scholar]
- Quinn CE, Hamilton PK, Lockhart CJ, McVeigh GE. (2008) Thiazolidinediones: effects on insulin resistance and the cardiovascular system. Br J Pharmacol 153:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar P, Beech DJ. (2010) Stimulation of TRPC5 cationic channels by low micromolar concentrations of lead ions (Pb2+). Biochem Biophys Res Commun 393:50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Culkin K, Zussman B, Miller AK, Freed MI. (2002) Pharmacokinetics of rosiglitazone in patients with end-stage renal disease. J Int Med Res 30:391–399 [DOI] [PubMed] [Google Scholar]
- Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, Yada T, Minokoshi Y, Tominaga M. (2011) Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 60:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. (2007) TRP channels. Annu Rev Biochem 76:387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, et al. (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 10:1421–1430 [DOI] [PubMed] [Google Scholar]
- Wuensch T, Thilo F, Krueger K, Scholze A, Ristow M, Tepel M. (2010) High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes 59:844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, et al. (1999) Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 3:397–403 [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, et al. (2006) A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. (2004) Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101:13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ. (2004) Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol 559:739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.