Abstract
Acting as a negative gating modulator, (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphthylamine (NS8593) shifts the apparent Ca2+-dependence of the small-conductance Ca2+-activated K+ channels KCa2.1–2.3 to higher Ca2+ concentrations. Similar to the positive KCa channel-gating modulators 1-ethyl-2-benzimidazolinone (1-EBIO) and cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methylpyrimidin-4-yl]-amine (CyPPA), the binding site for NS8593 has been assumed to be located in the C-terminal region, in which these channels interact with their Ca2+ sensor calmodulin. However, by using a progressive chimeric approach, we were able to localize the site-of-action of NS8593 to the KCa2 pore. For example, when we transferred the C terminus from the NS8593-insensitive intermediate-conductance KCa3.1 channel to KCa2.3, the chimeric channel remained as sensitive to NS8593 as wild-type KCa2.3. In contrast, when we transferred the KCa2.3 pore to KCa3.1, the channel became sensitive to NS8593. Using site-directed mutagenesis, we subsequently identified two specific residues in the inner vestibule of KCa2.3 (Ser507 and Ala532) that determined the effect of NS8593. Mutation of these residues to the corresponding residues in KCa3.1 (Thr250 and Val275) made KCa2.3 insensitive to NS8593, whereas introduction of serine and alanine into KCa3.1 was sufficient to render this channel highly sensitive to NS8593. It is noteworthy that the same two residue positions have been found previously to mediate sensitivity of KCa3.1 to clotrimazole and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34). The location of Ser507 in the pore-loop near the selectivity filter and Ala532 in an adjacent position in S6 are within the region predicted to contain the KCa2 channel gate. Hence, we propose that NS8593-mediated gating modulation occurs via interaction with gating structures at a position deep within the inner pore vestibule.
Introduction
Within the superfamily of Ca2+-activated K+ channels (KCa) the group of small (KCa2.1–2.3) and intermediate-conductance (KCa3.1) channels are closely related in both structure and function. In contrast to the big conductance KCa1.1 channel, which is activated by both voltage and Ca2+, KCa2 and KCa3.1 channels are inward-rectifying, voltage-independent, and activated solely by intracellular Ca2+ (Stocker, 2004; Wulff et al., 2007). The opening of both KCa2 and KCa3.1 channels is initiated via Ca2+-binding to the N-lopes of calmodulin (CaM) constitutively attached to a calmodulin binding domain (CaMBD) located in the proximal intracellular C terminus (Xia et al., 1998; Fanger et al., 1999). The energy of the ensuing conformational change is transferred to the transmembrane (TM) regions to open the gate. Unlike Kv channels, which are gated by a rotational constriction of the intracellular aperture formed by the lower part of the four S6 TM helixes, the physical gates of KCa2 and KCa3.1 channels seem to be deeply buried in the inner pore vestibule, close to or even overlapping with the K+ selectivity filter (Bruening-Wright et al., 2002, 2007; Klein et al., 2007; Garneau et al., 2009).
Despite their structural and functional similarity, KCa2 and KCa3.1 channels have a very different pharmacology, the details of which are increasingly becoming better defined at the molecular level. Selective peptide inhibitors of KCa2 channels, such as apamin or scyllatoxin, and KCa3.1 channel-inhibiting peptides, such as charybdotoxin and maurotoxin, interact with extracellularly exposed amino acids in the outer pore vestibule (Ishii et al., 1997; Rauer et al., 2000; Castle et al., 2003) and are usually conceived to inhibit via a simple blocking mechanism (Lamy et al., 2010 describes an emergent different view on the apamin mode-of-action). The same applies to the positively charged small-molecule KCa2-selective blockers such as 6,10-diaza-3(1,3)8(1,4)-dibenzena-1,5(1,4)-diquinolinacyclodecaphane (UCL1684) (Campos Rosa et al., 2000), which were designed to mimic the charged part of the apamin molecule, as well as to bicuculline methiodide (Johnson and Seutin, 1997), a lower-affinity blocker.
In contrast, the inhibition by the established small-molecule blockers of KCa3.1, the triarylmethanes, exemplified by clotrimazole and the more selective 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) (Wulff et al., 2001), is mediated via two amino acids located in the lower part of the pore loop (Thr250) and in the S6 segment (Val275), respectively (Wulff et al., 2001). These amino acids form part of a ring of hydrophobic residues, which line and thus isolate the upper part of the watery inner pore vestibule. Their position close to the selectivity filter suggested a model in which TRAM-34 coordinates via its aryl groups to Thr250 and Val275 and interacts with the lower part of the selectivity filter via its pyrazole moiety, thereby blocking the KCa3.1 pore from the inside.
A new principle for selective KCa2 channel inhibition by small molecules has been described. Certain 2-(N-substituted)-aminobenzimidazoles, such as (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphthylamine (NS8593) (Fig. 1) and (1H-benzimidazol-2-yl)-(6,7-dichloro-1,2,3,4-tetrahydronaphthalen-1-yl)amine (NS11757), inhibit all three KCa2 channels via negative gating modulation rather than via a simple pore-blocking mechanism (Strøbaek et al., 2006; Sørensen et al., 2008). The hallmark of this mode-of-action is a right-shifted and less steep KCa2 channel Ca2+-response curve, leading to a strong Ca2+-dependence of inhibition. The physical binding site for the negative gating modulators is clearly distinct from the apamin binding site, because these compounds do not displace radiolabeled apamin. Furthermore, KCa2 channels that have been point-mutated to apamin insensitivity are still sensitive to NS8593 (Sørensen et al., 2008). Physiological gating modulation of KCa2 channels occurs via casein kinase 2-mediated threonine phosphorylation of CaM (Thr80) attached to the KCa2 channel (Bildl et al., 2004; Allen et al., 2007). This phosphorylation reduces the apparent Ca2+ affinity and the resulting right-shift and reduced steepness of the KCa2 channel Ca2+ response curve is essentially identical with the effect of NS8593. Hence, we originally hypothesized that negative gating modulation by NS8593 is a phosphorylation-independent, direct downstream effect on the physiological gating process. Furthermore, because the C-terminal CaM/CaMBD is the site-of-action for the classic positive KCa2 channel gating modulator 1-EBIO (Pedarzani et al., 2001), we assumed, because of the structural similarity between NS8593 and the positive gating modulators 1-EBIO and 6,7-dichloro-1H-indole-2,3-dione-3-oxime (NS309) (Fig. 1), that negative modulation by NS8593 was also mediated via interaction with the CaM/CaMBD. All three compounds contain a benzimidazole or related indole-dione scaffold, and it seemed reasonable to hypothesize that they might be binding to the same site with the larger and more lipophilic NS8593 reducing gating. In the present article, we adopted a KCa2/KCa3.1 channel chimeric/mutational approach to delineate the amino acids important for negative pharmacological KCa2 channel modulation. Surprisingly, NS8593 sensitivity was found to reside at residues Ser507 and Ala532 in KCa2.3, which are in equivalent positions to Thr250 and Val275 in KCa3.1, the amino acids conferring TRAM-34 sensitivity. We therefore conclude that NS8593 inhibition involves deep-pore amino acids and suggest that the compound's negative gating modulation may result from a direct interaction with the physical gate of KCa2 channels.
Fig. 1.
Chemical structures of the negative KCa2 channel modulator NS8593 and the positive modulators 1-EBIO and NS309.
Materials and Methods
Molecular Biology.
HEK293 cell lines stably expressing WT hKCa2.3 and hKCa3.1 channels were described previously (Hougaard et al., 2009). The N-terminal KCa3.1-KCa2.3 chimera was generated with overlapping polymerase chain reaction (Expand High Fidelity PCR System; Roche Diagnostics, Mannheim, Germany) using the oligonucleotides hIK-hSK3s GTCTCTGGCCGGCTGGGCActgatttttgggatgttt and hSK3-hIKas aaacatcccaaaaatcagTGCCCAGCCGGCCAGAGAC, a T7 primer, and an antisense primer in the 3′-polylinker end. The C-terminal KCa2.3-KCa3.1 chimera was generated by ligation of the following DNA fragment purchased from GenScript (Piscataway, NJ): ACCGGTATCATGGGTGCAGGCTGCACTGCCCTTGTGGTGGCCGTGGTGGCCCGAAAGCTGGAGTTTAACAAGGCAGAGAAGCACGTGCACAACTTCATGATGGATATCCAGTATACCAAAGAGATGAAGGAGTCCGCTGCCCGAGTGCTACAAGAAGCCTGGATGTTCTACAAACATACTCGCAGGAAGGAGTCTCATGCTGCCCGCAGGCATCAGCGCAAGCTGCTGGCCGCCATCAACGCGTTCCGCCAGGTGCGGCTGAAACACCGGAAGCTCCGGGAACAAGTGAACTCCATGGTGGACATCTCCAAGATGCACATGATCCTGTATGACCTGCAGCAGAATCTGAGCAGCTCACACCGGGCCCTGGAGAAACAGATTGACACGCTGGCGGGGAAGCTGGATGCCCTGACTGAGCTGCTTAGCACTGCCCTGGGGCCGAGGCAGCTTCCAGAACCCAGCCAGCAGTCCAAGTAGCTGGAGTGGCGGCCGC into hKCa2.3 with an AgeI site as described in Hougaard et al., (2009). The KCa3.1 mutants T250S and V275A and the KCa2.3 mutants S507T and S507T + A532V were described previously (Wulff et al., 2001). Additional point mutations were generated using WT KCa2.3 and KCa3.1 plasmids uracilated via Escherichia coli RZ1032 (Stratagene, La Jolla, CA) as templates in mutagenesis reactions. Four oligonucleotides (MWG-Biotech AG, Ebersberg, Germany) were used to introduce the mutations: hKCa2.3 S507T, CTCCATCACATTCCTTaCaATTGGTTATGGGGACA; hKCa2.3 A532V, TCACTGGCATCATGGGTGtAGGCTGtACaGCCCTTGTGGTGGCCG; hKCa3.1 T250S, GATCCCCATCACATTCCTGtCaATtGGCTATGGTGACGTG; and hKCa3.1 V275A, GCACTGGAGTCATGGGTGcaTGCTGCACAGCCCTGCT. The mutagenesis reactions were performed using T7 DNA polymerase and T4 DNA ligase (New England Biolabs, Ipswich, MA). E. coli XL1-Blue (Stratagene) was transformed with an aliquot of the reaction, and the resulting plasmid DNA was purified using standard methods. All constructs were verified by sequencing.
Electrophysiology.
All experiments were performed on transiently transfected HEK 293 cells in either the inside-out or the whole-cell configuration of the patch-clamp technique. Lipofectamine and standard transfection methods were used, and recordings were conducted 2 days after transfection. Cells for whole-cell experiments were detached by trypsinization and plated on coverslips (3.5 mm Ø) on the day of the experiments, whereas cells for inside-out recordings were plated 1 day before the experiments to attach them more firmly. For recordings, a coverslip was placed in a 15-μl recording chamber mounted on the cross-board of an inverted microscope (Olympus XI-70 equipped with fluorescence burner and filters; Olympus, Tokyo, Japan), and cell selection was guided by fluorescence from the cotransfected green fluorescent protein. The extracellular solutions contained 154 mM KCl, 10 mM HEPES, pH 7.4, 2 or 0.1 mM CaCl2, and 1 or 3 mM MgCl2 for inside-out/whole-cell experiments, respectively. Solutions on the intracellular side contained 154 mM KCl, 10 mM HEPES, pH 7.2, 10 mM EGTA or 1 mM EGTA plus 9 mM nitriloacetic acid, CaCl2, and MgCl2 to yield a calculated free Mg2+ concentration of 1 mM and calculated free Ca2+ concentrations of 0.01, 0.2, 0.3, 0.4, 0.5, 3, 10, and 30 μM. Solutions used for experiments with ATP were made with Na2+-ATP (Sigma-Aldrich, St. Louis, MO) and adjusted to yield 30 μM free Ca2+ and 1.6 mM Mg2+-ATP. Cells or membrane patches were perfused at 1 ml/min by gravity from a 10-position solution exchanger. Patch pipettes were pulled from borosilicate (Vitrex Medical A/S, Herlev, Denmark) or soda lime glass (micro-hematocrit tubes; Kimble Chase, Rochester, NY) and had resistances of 2 to 3 MΩ when submerged in the bath solution. Positioning of the patch electrode was controlled with a Patchman micromanipulator (Eppendorf North America, New York, NY). Any initial voltage difference between the patch electrode and the integrated and grounded bath electrode was eliminated before the pipette was attached to the cell. Experiments were controlled with a HEKA EPC-9 or EPC-10 amplifier and Pulse software (HEKA, Lambrecht/Pfalz, Germany). Cells were clamped to a holding potential of at 0 mV, and KCa currents were elicited by 200-ms voltage ramps from −80 to +80 mV applied every 5 s. Data analysis, fitting, and plotting were performed with IGOR-Pro (Wavemetrics, Lake Oswego, OR) or Origin 7 (OriginLab Corporation, Northampton, MA).
Chemicals and Reagents.
NS8593 and NS309 were synthesized at NeuroSearch A/S as described previously (Strøbaek et al., 2004, 2006). Bicuculline methobromide (BMB) and apamin were from Sigma-Aldrich, and charybdotoxin was from Bachem Biosciences (King of Prussia, PA). TRAM-34 was synthesized in the Wulff laboratory as described previously (Wulff et al., 2001). Standard laboratory chemicals were purchased from commercial dealers and were of the purest grade available.
Quantification and Statistics.
IC50 values were calculated as described previously (Strøbaek et al., 2006). Summary values are given as means ± S.D.
Results
The Inhibition of KCa2.3 by NS8593 Is Not Dependent on N- and C-Terminal Regions.
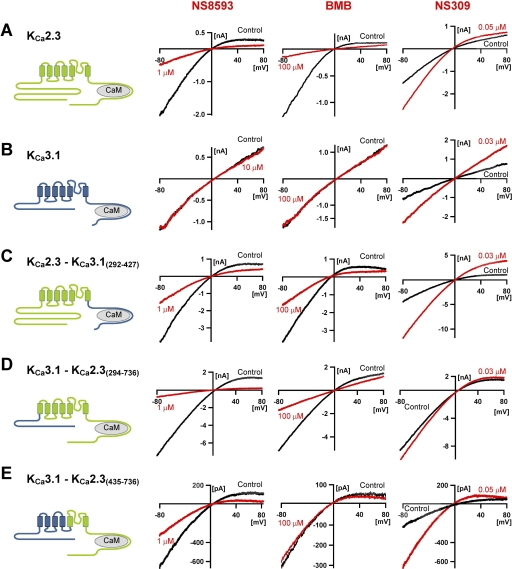
In whole-cell experiments, NS8593 inhibits KCa2.3 channels with a potency of 90 nM at a pipette [Ca2+] of 400 nM, whereas the related KCa3.1 channel is insensitive to this negative gating modifier (Strøbaek et al., 2006). To delineate KCa2 channel regions important for the rightward shift in the Ca2+ response curve induced by NS8593, we constructed a number of chimeras between the hKCa2.3 channel and the hKCa3.1 channel. The responses of these chimeras to NS8593, the KCa2 channel blocker BMB and NS309, a positive modulator of KCa2 and KCa3.1 channels, were tested in whole-cell patch-clamp experiments (Fig. 2 and Supplemental Table 1).
Fig. 2.
The inhibitory effect of NS8593 is not dependent on N- and C-terminal regions of KCa2.3 but on pore regions. Effect of NS8593, BMB, and NS309 on whole-cell currents from HEK293 cells expressing WT KCa2.3 (A), WT KCa3.1 (B), and the chimeric constructs KCa2.3-KCa3.1(292–427) (C), KCa3.1-KCa2.3(287–736) (D), and KCa3.1-KCa2.3(435–736) (E). Voltage ramps were applied every 5 s from a holding potential of 0 mV. Control traces (last sweep before compound application) are shown in black, and compound traces (last sweep before compound washout) are shown in red. Washout traces have been omitted for clarity. Symmetric K+ distributions with free [Ca2+] in the pipette solution buffered to 400 nM (see Materials and Methods for specifications).
Figure 2, A and B, show representative recordings from wild-type KCa2.3 and KCa3.1 currents elicited by 200-ms voltage ramps from −80 to +80 mV applied every 5 s with 400 nM free Ca2+ in the patch pipette. As published previously for recordings with symmetrical K+ concentrations in this expression system (Strøbaek et al., 2006; Hougaard et al., 2007), KCa2.3 exhibited a much more pronounced inward rectification than KCa3.1, which showed a nearly linear IV relationship. Both channels exhibited their characteristic pharmacology. KCa2.3 was potently inhibited by NS8593 with an IC50 of 104 nM (Tables 1 and 2) and almost fully blocked by 100 μM BMB (IC50 5 μM), which we routinely use to estimate leak when working with symmetrical K+ solutions. KCa3.1, in contrast, was completely insensitive to 10 μM NS8593 and 100 μM BMB but could be blocked completely by TRAM-34 (Tables 1 and 2). NS309 increased both KCa2.3 and KCa3.1 currents but was distinctly more effective on KCa3.1 in keeping with previous reports (Strøbaek et al., 2004).
TABLE 1.
Pharmacology of KCa2.3 and KCa3.1 chimeric channels
Numbers in a parentheses indicate the number of independent experiments. For information on the pharmacological properties of additional KCa2.3/KCa3.1 chimeras, see Supplemental Table 1.
| Chimeras | NS8593 IC50 | Current Inhibition by 100 μM BMB | Current Increase by 30 nM NS309 |
|---|---|---|---|
| μM | % | fold | |
| KCa2.3 WT | 0.104 ± 0.034 (13) | 83 ± 13 (10) | 2.1 ± 0.5 (6) |
| KCa3.1 WT | >10 (11) | 0 ± 0 (5) | 5.1 ± 2.1 (6) |
| KCa2.3-KCa3.1(292–427) | 0.117 ± 0.013 (3) | 57 ± 24 (3) | 2.9 ± 0.6 (3) |
| KCa3.1-KCa2.3(287–736) | 0.115 ± 0.040 (6) | 91 ± 3 (6) | 1.1 ± 0.5 (6) |
| KCa3.1-KCa2.3(435–736) | 0.947 ± 0.031 (4) | 0 ± 0 (3) | 2.3 ± 0.4 (3) |
TABLE 2.
Pharmacology of KCa2.3 and KCa3.1 point-mutated channels
Numbers in a parentheses indicate the number of independent experiments. For information on the pharmacological properties of additional KCa2.3/KCa3.1 chimeras, see Supplemental Table 1.
| Mutants | NS8593 IC50 | Current Inhibition by 100 μM BMB | TRAM-34 IC50 |
|---|---|---|---|
| μM | % | μM | |
| KCa2.3 WT | 0.104 ± 0.034 (13) | 83 ± 13 (10) | >20a |
| KCa2.3S507T | 2.5 ± 0.78 (9) | 42 ± 18 (5) | >>1 (3) |
| KCa2.3A532V | 3.1 ± 1.3 (4) | 67 ± 12 (2) | 2.7 (1) |
| KCa2.3S507T + A532V | >10 (3) | 65 ± 15 (3) | 0.064 ± 0.068 (2) |
| KCa3.1 WT | >10 (11) | 0 ± 0 (5) | 0.004 ± 0.002 (4) |
| KCa3.1T250S | 0.503 ± 0.209 (11) | 0 ± 0 (5) | 22 ± 14 (2) |
| KCa3.1V275A | 3.5 ± 1.6 (14) | 0 ± 0 (7) | 21 ± 4.3 (3) |
| KCa3.1T250S + V275A | 0.056 ± 0.024 (3) | 1 ± 1 (2) | >20a |
We next tested the effect of NS8593 on KCa2.3 chimeras that contained either the N- or the C-terminal regions of KCa3.1. Because both chimeras contained the pore region of KCa2.3, they exhibited the characteristic inward rectification of KCa2.3. Surprisingly, NS8593 inhibited both chimeras as potently as the WT KCa2.3 channel (Fig. 2, C and D), suggesting that the NS8593-induced negative gating modulation is not mediated via the N- or the C-terminal regions. Exchanging the C terminus between KCa2.3 and KCa3.1 also transferred the higher NS309 sensitivity of KCa3.1 to the resulting chimeric channel (Fig. 2C, right). This observation is reminiscent of the increase in 1-EBIO sensitivity that was reported for a KCa2.2 chimeric channel containing the C terminus of KCa3.1 (Pedarzani et al., 2001) and suggests that the binding site of the positive gating modulator NS309 is also located in this region.
Amino Acids in the Pore Region Confer Sensitivity to NS8593.
After having obtained the above results, we next focused our attention on the TM regions. Unfortunately, some KCa2.3/KCa3.1 chimeras in which various parts of TM5 and/or TM6 were swapped between the two channels did not seem to form functional channels or were expressed too poorly to allow for evaluation of modulator sensitivity (Supplemental Table 1). However, an important chimera that contained the transmembrane regions S1 to S4 from KCa3.1 and the pore and C terminus of KCa2.3 (Fig. 2E) expressed sufficiently well for pharmacological experiments. It is noteworthy that this chimera was as sensitive to NS8593 as the wild-type KCa2.3 channel (Fig. 2E, left) and was further found to be insensitive to the KCa2.3 pore blocker BMB. Both observations were surprising, the first suggesting that NS8593 might be exerting its effect by interacting with residues in the pore. As a consequence, we decided to follow-up this observation with point mutations in the inner pore region (see below). The latter observation could indicate that the presence of the S1 to S4 region from KCa3.1 in the chimera might have disturbed the architecture of the outer KCa2.3 vestibule, in which BMB, which exhibits voltage-dependent block (Grunnet et al., 2001) and displaces apamin (Finlayson et al., 2001), presumably binds. In this context, it should be mentioned that apamin was very recently reported to bind to the outer pore of KCa2 channels (Lamy et al., 2010), where it apparently acts more as an allosteric modulator of the selectivity filter than as a direct blocker such as tetraethyl ammonium or the larger scorpion toxins tamapin and scyllatoxin, which are able to span the KCa2 channel pore (Weatherall et al., 2010).
Two Amino Acids in the Inner Pore Region Are Required and Sufficient for Inhibition by NS8593.
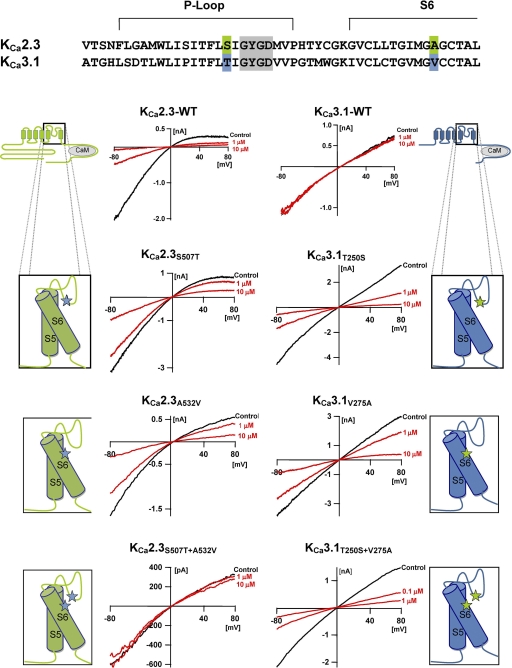
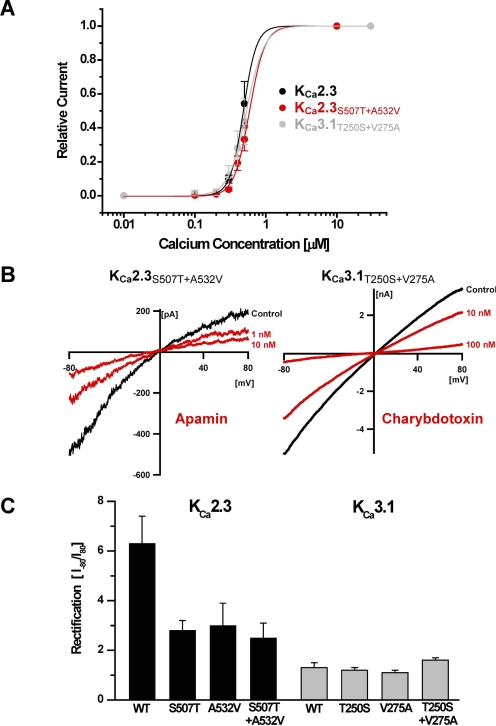
Two amino acids in the inner pore vestibule were reported previously to confer TRAM-34 and clotrimazole sensitivity to KCa3.1 (Wulff et al., 2001). These amino acids, Thr250 and Val275 in the KCa3.1 numbering, are located on either side of the selectivity filter, and we therefore speculated whether the effect of NS8593 might also be dependent on these amino acids. As shown in Tables 1 and 2 and Fig. 3, mutations of each of the corresponding residues in KCa2.3 (Ser507 and Ala532) reduced the potency of NS8593 roughly 20-fold, whereas introduction of the double mutation produced a KCa2.3 channel that was completely insensitive to NS8593. The importance of Ser507 and Ala532 was verified by showing that the reverse KCa3.1 mutants (T250S and V275A) became sensitive to NS8593 and that the KCa3.1 double mutant was at least as sensitive to NS8593 as the wild-type KCa2.3 channel (Fig. 3 and Tables 1 and 2). As a control of whether the point mutations disturbed the overall architecture and gating function of the channels we determined the calcium sensitivity and the pharmacology of the two double mutants. In inside-out patches, the KCa2.3S507T+A532V and the KCa3.1T250S+V275A mutants exhibited Ca2+ response curves that were very similar to the WT channels with EC50 values of 480 to 590 nM and Hill coefficients of 3.8 to 4.7 (Fig. 4A). In whole-cell recordings, both double mutants also showed the characteristic pharmacology of the WT channels. Although the KCa3.1T250S+V275A mutant was sensitive to charybdotoxin (IC50 17 ± 5.4 nM, n = 4) and insensitive to BMB and apamin, the KCa2.3S507T+A532V mutant was inhibited by apamin (IC50 1.4 ± 0.2 nM, n = 6) and BMB (Fig. 4B and Tables 1 and 2). These results suggest that the double point mutations did not significantly change the overall conformation of the KCa channels. However, as expected, the single and the double mutations changed TRAM-34 sensitivity (Tables 1 and 2), in keeping with our previous observations (Wulff et al., 2001).
Fig. 3.
Ser507 and Ala532 confer NS8593 sensitivity to KCa2.3, and their transfer into KCa3.1 renders KCa3.1 sensitive to NS8593. Effects of 1 and 10 μM NS8593 on KCa2.3 (left) and KCa3.1 (right) single and double mutants, in which inner pore residues were mutated to the equivalent amino acids of the other channel. A partial sequence alignment of hKCa3.1 and hKCa2.3 in the P-loop and the S6 region with the position of the residues highlighted is shown on top. Experimental details and color-coding are as stated in Fig. 1.
Fig. 4.
The KCa2.3S507T+A532V and the KCa3.1T250S+V275A double mutants exhibit similar biophysical and pharmacological properties as the WT channels. A, overlay of the calcium-response curves of KCa2.3 and the two double mutants measured from inside-out patches exposed to increasing Ca2+ concentrations. Data points are mean ± S.D. from seven or eight experiments per construct. The fit of the data to the Hill equation yielded the following results: KCa2.3 (EC50 480 ± 50 nM, nH = 4.8 ± 1.0); KCa2.3S507T+A532A (EC50 590 ± 40 nM, nH = 4.4 ± 0.5), and KCa3.1T250S+V275A (EC50 520 ± 70 nM, nH = 3.8 ± 0.9). B, whole-cell recording showing effects of the pore-blocking toxins apamin (left) and charybdotoxin (right) on the KCa2.3S507T+A532V and the KCa3.1T250S+V275A double mutants. Experimental details as stated in text to Fig. 1. (C) Rectification of the different KCa2.3 and KCa3.1 constructs determined by the ratio of the current amplitude at −80 and +80 mV. Values are mean ± S.D. (n = 2–15 per data point).
Another interesting feature of the inner pore mutations was that introduction of the respective KCa3.1 residues into KCa2.3 significantly reduced this channel's strong inward rectification. This is illustrated by comparing the ratio of the current amplitude at −80 and +80 mV between the different constructs (Fig. 4C). Although both KCa2.3 single mutants and the double mutant showed a pronounced reduction in rectification, the reverse substitutions in KCa3.1 did not increase the generally weak rectification of this channel. Part of the inward-rectification of KCa2 channels has been shown previously to be the result of voltage-dependent block by intracellular divalent cations, such as Ca2+ and Mg2+, at a site located below the selectivity filter involving Ser359 in rat KCa2.2 (Soh and Park, 2001, 2002), which corresponds to Ser507 in human KCa2.3. Mutation of this position to alanine or the larger threonine demonstrated that the hydroxyl group of this serine residue in KCa2.2 is critical for the binding of divalent cations to this site and thus for inward-rectification in their presence. Our results here confirm these observations in KCa2.3 and strongly underscore these amino acids as functionally important pore residues.
Gating Modulation of the KCa3.1T250S+V275A Mutant by NS8593 Is Much Less Ca2+- and NS309-Dependent than Gating Modulation of the WT KCa2.3 Channel.
Because the residues conferring NS8593 sensitivity to KCa2.3 and transferring it to KCa3.1 are located within in the region predicted to contain the gate of KCa2 and KCa3.1 channels, we wondered whether their mutations would also affect the mechanism of action of NS8593. To address this issue, we investigated two defining characteristics of negative gating modulation: 1) dependence on [Ca2+]i; and 2) reversibility in presence of the positive modulator NS309.
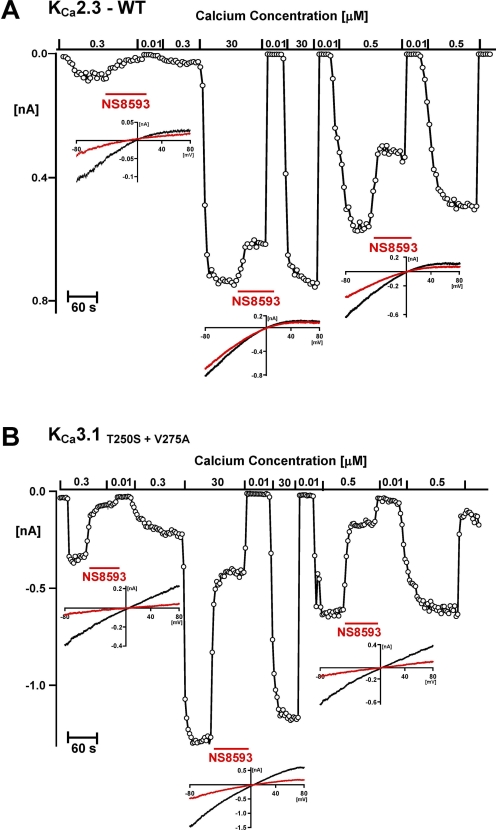
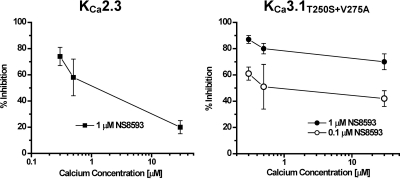
As described previously (Strøbaek et al., 2006), current reduction by the negative gating modulator NS8593 is strongly dependent on the [Ca2+]i, with the compound's potency decreasing with increasing [Ca2+]i. As shown in Fig. 5A, perfusion of 1 μM NS8593 onto KCa2.3 currents activated with 300 nM [Ca2+]i in inside-out patches blocked 74 ± 6% (n = 6) of the calcium-dependent inward current at −80 mV. However, when the same patch was subsequently exposed to 30 μM [Ca2+]i to maximally activate KCa2.3, 1 μM NS8593 only blocked 21 ± 5% (n = 6) of the current. After washout and another control for absence of contaminating leak current by a switch to 10 nM [Ca2+]i, KCa2.3 was then exposed to 0.5 μM [Ca2+]i, and 1 μM NS8593 was observed to exhibit an intermediate potency (58 ± 14% blockade, n = 5). In contrast, inhibition of the KCa3.1 double mutant (KCa3.1T250S+V275A) by NS8593 was much less calcium-dependent (Figs. 5B and 6). In a similar inside-out experiment, in which [Ca2+]i varied from 0.3 to 30 μM, NS8593 at 1 μM inhibited 87 ± 3% (n = 3) at 0.3 μM, 80 ± 4% (n = 3) at 0.5 μM, and 70 ± 6% (n = 5) of the current at 30 μM [Ca2+]i. However, the reduced calcium-dependence of the inhibitory effect of NS8593 on the KCa3.1 double mutant was not simply the result of the slightly higher affinity of NS8593 to the mutant channel because inhibition remained less calcium-sensitive even when the NS8593 concentration was reduced 10-fold from 1 μM to 100 nM (Fig. 6).
Fig. 5.
Inhibition of WT KCa2.3 (A) by NS8593 is more Ca2+-dependent than inhibition of the KCa3.1T250S+V275A mutant (B). Inside-out patches were exposed to a [Ca2+]i of 0.01, 0.3, 0.5, or 30 μM as indicated in the presence or absence of NS8593. The main figures show a continuous plot of the currents recorded at −75 mV. The inserts show control and NS8593 current traces. Experimental details and color-coding are as described in Fig. 1.
Fig. 6.
NS8593 inhibition of the KCa3.1T250S+V275A mutant shows reduced calcium-dependence at both 1 μM and 0.1 μM NS8593. Plot of the percentage of current inhibition by NS8593 versus [Ca2+]i concentration for WT KCa2.3 (right) and the KCa3.1T250S+V275A mutant (left). Values are mean ± S.D. (n = 3–15).
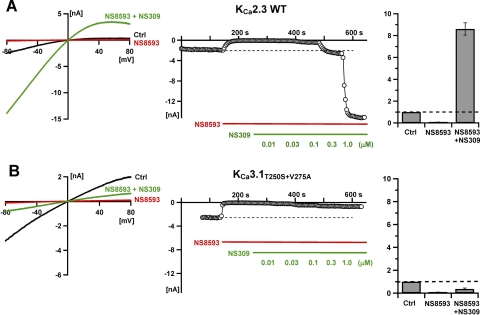
The NS8593 inhibition of KCa channels at low [Ca2+]i is essentially abolished upon the addition of the positive modulator NS309 (Strøbaek et al., 2006; Ji et al., 2009). Figure 7 compares the ability of NS309 to reverse the inhibitory effect of NS8593 on KCa2.3 (Fig. 7A) and the KCa3.1T250S+V275A mutant (Fig. 7B). In both cases, upon stabilization of the whole-cell current (black traces, left) 1 μM NS8593 was superfused causing nearly 100% inhibition (red traces, middle, after 150 s). Increasing concentrations of NS309 were then cosuperfused with NS8593 leading to a concentration-dependent reversal and “overshoot” of the KCa2.3 current, whereas the KCa3.1T250S+V275A current remained largely inhibited even at an NS309 concentration of 1 μM (green traces, middle, after 250 s). This series of experiments has been quantified (Fig.7, right), in which the fold decrease/increase relative to the control current is plotted for the NS8593 (1 μM) equilibrium inhibition and for the combined NS8593 + NS309 (1 μM) effect. The overall conclusion from these experiments is that NS8593-mediated inhibition of the KCa3.1T250S+V275A construct is not reversible with a positive modulator and in this respect has lost its negative modulation character and rather resembles a blocker.
Fig. 7.
Whole-cell experiments (symmetric K+, free pipette [Ca2+] buffered to 400 nM) showing that NS8593 inhibition of KCa2.3 is abolished by the positive modulator NS309 (A), whereas inhibition of KCa3.1T250S+V275A is not (B). Right, ramp traces obtained before (black) and after (red) superfusion with 1 μM NS8593 and after cosuperfusion with 1 μM NS309 (green). Middle, time courses (tick separation, 100 s) of the currents recorded at −80 mV. Right, diagram showing quantification of these effects relative to the control current (before NS8593 addition) defined as 1. The concentration for the coapplication with NS309 is 1 μM.
Finally, in a last attempt to demonstrate negative gating modulation characteristics on the KCa3.1T250S+V275A construct, we performed a series of inside-out experiments with combined high [Ca2+]i (30 μM) and Mg2+-ATP (1.6 mM) or NS309 (1 μM). The rationale for using Mg2+-ATP quote back to the observation that the Ca2+-dependent open channel probability Po(max) value of KCa3.1 is significantly lower than the corresponding value for KCa2 in some studies (Gerlach et al., 2001; Jones et al., 2007), and that this value can be increased significantly in the presence of Mg2+-ATP. In our hands, Mg2+-ATP and NS309 both induced slight increases (28%, n = 2, and 23%, n = 3, respectively), in the maximal current level of KCa3.1T250S+V275A (results not shown), and the ensuing addition of NS8593 (0.3 μM) caused current reductions that were not significantly different from the effects without Mg2+-ATP or NS309 (53 ± 8%, n = 5 versus 52 ± 7%, n = 3, and 51 ± 7%, n = 4, respectively). Likewise, the higher NS8593 concentration of 1 μM blocked the KCa3.1T250S+V275A mutant channel by 70% in both the absence (n = 5) and the presence of 1.6 mM ATP (n = 2).
Taken together, these data suggest that although the KCa3.1T250S+V275A mutation perfectly transfers the interaction site for NS8593 in terms of potency, the characteristic coupling to the gate is less pronounced in this construct, as shown by diminished Ca2+- and NS309-dependence of inhibition and by the preservation of its inhibitory potency in the presence of Mg2+-ATP.
Discussion
In comparison with the classic KCa2 channel blocker apamin, NS8593 acts by a different mechanism and via a different site because KCa2.3 channels mutated in the apamin binding site remain sensitive to its actions (Sørensen al., 2008). NS8593 produces a rightward-shift in the KCa2 channel Ca2+ activation curve and has therefore been termed a negative gating modulator rather than a pore blocker (Strøbaek et al., 2006; Sørensen et al., 2008). This article attempts to define the site-of-action for NS8593.
The selectivity of NS8593 for KCa2 channels over the structurally related KCa3.1 channel allowed us to use a chimeric/mutagenesis approach to identify regions and specific amino acids important for the NS8593 effect. In short, we have shown that KCa2.3 loses sensitivity toward NS8593 inhibition by the point mutations S507T and A532V and, on the other hand, that KCa3.1 gains sensitivity to NS8593 by the equivalently positioned mutations T250S and V275A. These results have at least two noteworthy implications. First, according to the generally accepted gross architecture of 6-TM K+-channels, both of these amino acids are located in the inner pore vestibule (inner pore helix of S5 and S6, respectively) close to the inside of the selectivity filter, in which they define open channel properties such as inward rectification/divalent cation block. Hence, because of the coupling between NS8593 inhibition and Ca2+-dependent gating (Strøbaek et al., 2006), we assume that Ser507 and Ala532 are positioned close to the physical gate of the channel. Second, these positions are the same two positions that define KCa3.1 sensitivity (and KCa2 insensitivity) toward triarylmethanes such as TRAM-34 and clotrimazole (Wulff et al., 2001) and to arachidonic acid (Hamilton et al., 2003). This coincidence of equivalent sites for selective inhibitors of the two channels underscores the close structural resemblance between the KCa2 and KCa3 families. However, our data also point toward significant functional differences between the two channel families. Although NS8593 inhibition via this region in KCa2.3 exhibits strong negative gating modulation (abolishable by Ca2+ and coapplication of positive modulators), Ca2+-dependence and NS309-mediated reversion are barely detectable for its inhibition of the KCa3.1T250S+V275A construct. The present results seem not to support the interpretation that the increased potency and abolished gating dependence is due solely to a low Po(max) value (favoring a possible closed state binding of NS8593), because combined experimental conditions tending toward a high Po (high [Ca2+]i + NS309 or Mg2+-ATP), do not shift the potency significantly. It is noteworthy that the same is the case for the TRAM-34/clotrimazole inhibition of KCa3.1, in which we have previously found that clotrimazole and TRAM-34 inhibited NS309 or naphtho[1,2-d]thiazol-2-ylamine (SKA-31) activated KCa3.1 channels with essentially the same potency as nonactivated channels (Strøbaek et al., 2004; Sankaranarayanan et al., 2009). Together, this series of results may warrant the consideration of whether these residue positions are simply less intimately coupled to the gating process in KCa3.1 than the equivalent positions in KCa2.3.
Equivalent deep-pore amino acid positions allowing 3 orders of magnitude of inhibitory selectivity between KCa2 and KCa3 families provoke the question of whether interaction with these positions might potentially also cause selectivity between the KCa2.1, KCa2.2, and KCa2.3 subtypes, a matter of considerable pharmacological and potential clinical relevance: KCa2.1 and KCa2.2 are mostly expressed in the cortical/limbic structures of the brain, whereas KCa2.3 is preferentially expressed in the basal ganglia and in other subcortical regions (Sailer et al., 2004). KCa inhibitors have been considered for improvement of cognitive performance (primarily KCa2.2) (Hammond et al., 2006), whereas there is evidence for the use of KCa2.3 inhibitors for mood disorders such as depression (Jacobsen et al., 2008). Unfortunately, both Ser507 and Ala532 are conserved among the KCa2 subtypes (Ser330 and Ala355 in KCa2.1; Ser359 and Ala384 in KCa2.2), which implies that selective inhibition of the KCa2 channel members may be difficult to achieve. In support of this interpretation, a detailed structure-activity analysis on NS8593 analogs revealed no subtype-selectivity between KCa2.2 and KCa2.3, despite the achievement of considerably increased potency for negative gating modulation (Sørensen et al., 2008). In a study of non–apamin-displacing KCa2 inhibitors of a different chemotype [4-(aminomethylaryl)pyrazolopyrimidine], subtype selectivity was also not observed (Gentles et al., 2008), but whether these compounds are interacting with the same site as NS8593 is currently not known. However, it might of course be possible to achieve some functional selectivity among the KCa2 subtypes based on the different Ca2+ concentrations in the microscopic environment surrounding the channel's calmodulin and its phosphorylation state (Bildl et al., 2004; Allen et al., 2007) in different types of neurons and brain regions.
An Emerging Picture of KCa2 Channel-Gating Pharmacology.
Because the predominant effects of the prototypical positive and negative gating modulators are left-shifting/right-shifting of the [Ca2+]i-response curve, respectively, their phenomenological mode-of-actions were originally attributed to selective increases/decreases in the “apparent Ca2+ affinity” of KCa2 channels, not excluding a priori an interference with the genuine CaM binding affinity for Ca2+. The demonstration that the C terminus is the site of 1-EBIO-mediated positive modulation of KCa2.1 (Pedarzani et al., 2001), a finding that has been confirmed by the KCa2.3/KCa2.2-selective cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methylpyrimidin-4-yl]-amine (KCa2.3>KCa2.2≫KCa2.1 = KCa3.1) (Hougaard et al., 2008), further strengthened the view of positive modulation occurring by a comparatively simple “local” C-terminal mechanism. We initially imagined a similar C-terminally “delimited” action for negative gating modulation, in particular with reference to the quite similar negative gating effect of casein kinase 2-mediated phosphorylation of Thr80 in the attached CaM (Bildl et al., 2004; Allen et al., 2007).
However, a number of findings significantly complicate this simple, unifying picture. First, localization of the site-of-action for NS8593 to deep-pore amino acids immediately excludes the possibility that positive and negative modulators simply share the same binding site and just differ in their (positive or negative) coupling to the gating process in analogy to the action of benzodiazepines on the GABAA receptor (Sieghart, 1994). In addition, recent findings show that NS309, which is a classic positive modulator like 1-EBIO, strengthens the link between CaM-Ca2+ and channel opening rather than increasing Ca2+ binding to CaM per se (Li et al., 2009). Furthermore, we have described recently a KCa2.1-selective activator (KCa2.1>KCa2.2 = KCa2.3≫KCa3.1) 4-(2-methoxy-phenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X), which exhibits a complex, partial agonist-like mode-of-action (Hougaard et al., 2009) that is also independent of the CaMBD/C-terminal region but is dependent on selective interaction with Ser293 (Leu476 in KCa2.3) in the S5 segment. In line with the present analysis of the interaction site for negative gating modulation by NS8593, the GW542573X results were interpreted as evidence for KCa2.1 activation via “deep-pore” gating structures. At the present time we therefore favor the hypothesis, that the diverse positive/negative modulator pharmacology directly reflects the complexity and extended participation of even remotely positioned parts of KCa2 (and accessory proteins) in the gating process. Cysteine scanning experiments have clearly shown that the gate of both KCa2 and KCa3 channels is positioned very close to or even encompasses the quite outwardly displaced K+ selectivity filter (Bruening-Wright et al., 2002, 2007; Klein et al., 2007; Garneau et al., 2009), a finding that has to be reconciled with the primary Ca2+-binding event occurring on CaM at the cytoplasmic C terminus. We think of KCa2 (and KCa3) channel gating as a series of events comprising Ca2+-binding, CaM/CaMBD/C-terminal conformational change, leading to a transduction via S6 (possibly involving S5 stabilization) to deep-pore gating structures and eventual opening of the channel. The emerging complexity of the gating modulators, in terms of site-of-actions, selectivity, and mode-of-actions, most likely reflects the existence of several points for pharmacological intervention along the chain of molecular events leading from Ca2+ binding to the eventual channel opening. Both negative and positive allosteric-like gating modulation can be achieved, as well as partial agonism-like activation. However, no simple unifying relation seems to exist between the position of interaction sites and mode-of-actions. Despite the present results obtained with NS8593, we do therefore not exclude that future negative gating modulators of KCa2 channels might act on different sites than Ser507 and Ala532. Indeed, different sites of modulation coupled to different selectivities are not unprecedented for K+ channel gating modulators. Although the “classic” Kv7.2-Kv7.5 channel activator retigabine interacts with a hydrophobic pocket formed upon channel opening between the cytoplasmic parts of S5 and S6 (Wuttke et al., 2005), the more Kv7.2/7.3-selective activator N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243) was found recently to act through a voltage-sensor domain site located in transmembrane segments S1–S3 (Padilla et al., 2009). In analogy, alternative gating inhibitor sites on KCa2 channels may exist, possibly providing a better opportunity for the achievement of subtype-selectivity.
Supplementary Material
Acknowledgments
We gratefully acknowledge the excellent technical skills of Lene Gylle Larsen in making the chimeric constructs and those of Anne Stryhn Meincke in performing patch-clamp experiments.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant UL1-RR024146]; a Clinical and Translational Sciences Center Highly Innovative Award; and a gift from NeuroSearch A/S.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.069807.
- KCa
- Ca2+-activated K+ channel
- CaM
- calmodulin
- BMB
- bicuculline methobromid
- CaMBD
- calmodulin binding domain
- 1-EBIO
- 1-ethyl-2-benzimidazolinone
- GW542573X
- 4-(2-methoxy-phenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester
- TM
- transmembrane
- HEK
- human embryonic kidney
- KCa1.1
- big conductance Ca2+-activated K+ channel
- KCa2
- small conductance Ca2+-activated K+ channel
- KCa3.1
- intermediate conductance Ca2+-activated K+ channel
- NS309
- 6,7-dichloro-1H-indole-2,3-dione 3-oxime
- NS8593
- (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine
- TRAM-34
- 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- WT
- wild type
- SKA-31
- naphtho[1,2-d]thiazol-2-ylamine
- UCL1684
- 6,10-diaza-3(1,3)8(1,4)-dibenzena-1,5(1,4)-diquinolinacyclodecaphane
- NS11757
- (1H-benzimidazol-2-yl)-(6,7-dichloro-1,2,3,4-tetrahydronaphthalen-1-yl)amine
- ICA-27243
- N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide.
Authorship Contributions
Participated in research design: Jenkins, Strøbæk, Christophersen, and Wulff.
Conducted experiments: Jenkins, Strøbæk, and Hougaard.
Contributed new reagents or analytic tools: Jensen, Hummel (molecular biology), and Sørensen (chemistry).
Performed data analysis: Jenkins, Strøbæk, and Wulff.
Wrote or contributed to the writing of the manuscript: Jenkins, Strøbæk, Christophersen, and Wulff.
References
- Allen D, Fakler B, Maylie J, Adelman JP. (2007) Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci 27:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, et al. (2004) Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating. Neuron 43:847–858 [DOI] [PubMed] [Google Scholar]
- Bruening-Wright A, Lee WS, Adelman JP, Maylie J. (2007) Evidence for a deep pore activation gate in small conductance Ca2+-activated K+ channels. J Gen Physiol 130:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening-Wright A, Schumacher MA, Adelman JP, Maylie J. (2002) Localization of the activation gate for small conductance Ca2+-activated K+ channels. J Neurosci 22:6499–6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos Rosa J, Galanakis D, Piergentili A, Bhandari K, Ganellin CR, Dunn PM, Jenkinson DH. (2000) Synthesis, molecular modeling, and pharmacological testing of bis-quinolinium cyclophanes: potent, non-peptidic blockers of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem 43:420–431 [DOI] [PubMed] [Google Scholar]
- Castle NA, London DO, Creech C, Fajloun Z, Stocker JW, Sabatier JM. (2003) Maurotoxin: a potent inhibitor of the intermediate conductance Ca2+-activated potassium channel. Mol Pharmacol 63:409–418 [DOI] [PubMed] [Google Scholar]
- Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, Beckingham K, Chandy KG, Cahalan MD, Aiyar J. (1999) Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem 274:5746–5754 [DOI] [PubMed] [Google Scholar]
- Finlayson K, McLuckie J, Hern J, Aramori I, Olverman HJ, Kelly JS. (2001) Characterisation of [125I]-apamin binding sites in rat brain membranes with HE293 cells transfected with SK channel subtypes. Neuropharmacology 41:341–350 [DOI] [PubMed] [Google Scholar]
- Garneau L, Klein H, Banderali U, Longpré-Lauzon A, Parent L, Sauvé R. (2009) Hydrophobic interactions as key determinants to the KCa3.1 channel closed configuration. An analysis of KCa3.1 mutants constitutively active in zero Ca2+. J Biol Chem 284:389–403 [DOI] [PubMed] [Google Scholar]
- Gentles RG, Hu S, Huang Y, Grant-Young K, Poss MA, Andres C, Fiedler T, Knox R, Lodge N, Weaver CD, et al. (2008) Preliminary SAR studies on non-apamin-displacing 4-(aminomethylaryl)pyrrazolopyrimidine K(Ca) channel blockers. Bioorg Med Chem Lett 18:5694–5697 [DOI] [PubMed] [Google Scholar]
- Gerlach AC, Syme CA, Giltinan L, Adelman JP, Devors DC. (2001) ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a C-terminal domain. J Biol Chem 276:10963–10970 [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Angelo K, Frøkjaer-Jensen C, Klaerke DA, Olesen SP, Jensen BS. (2001) Pharmacological modulation of SK3 channels. Neuropharmacology 40:879–887 [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Syme CA, Devor DC. (2003) Molecular localization of the inhibitory arachidonic acid binding site to the pore of hIK1. J Biol Chem 278:16690–16697 [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. (2006) Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci 26:1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C, Eriksen BL, Jørgensen S, Johansen TH, Dyhring T, Madsen LS, Strøbaek D, Christophersen P. (2007) Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol 151:655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C, Hummel R, Johansen T, Eriksen BL, Strøbæk D, Christophersen P. (2008) Positive modulation by the SK2/SK3-selective compound, CyPPA, is mediated via the C-terminal tail (Abstract). Biophys J 94:2199 [Google Scholar]
- Hougaard C, Jensen ML, Dale TJ, Miller DD, Davies DJ, Eriksen BL, Strøbaek D, Trezise DJ, Christophersen P. (2009) Selective activation of the SK1 subtype of human small-conductance Ca2+-activated K+ channels by 4-(2-methoxyphenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) is dependent on serine 293 in the S5 segment. Mol Pharmacol 76:569–578 [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. (1997) A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94:11651–11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Weikop P, Hansen HH, Mikkelsen JD, Redrobe JP, Holst D, Bond CT, Adelman JP, Christophersen P, Mirza NR. (2008) SK3 K+ channel-deficient mice have enhanced dopamine and serotonin release and altered emotional behaviors. Genes Brain Behav 7:836–848 [DOI] [PubMed] [Google Scholar]
- Ji H, Hougaard C, Herrik KF, Strøbaek D, Christophersen P, Shepard PD. (2009) Tuning the excitability of midbrain dopamine neurons by modulating the Ca2+ sensitivity of SK channels. Eur J Neurosci 29:1883–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. (1997) Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett 231:13–16 [DOI] [PubMed] [Google Scholar]
- Jones HM, Bailey MA, Baty CJ, Macgregor GG, Syme CA, Hamilton KL, Devor DC. (2007) An NH2-terminal multi-basic RKR motif is required for the ATP-dependent regulation of hIK1. Channels 1:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H, Garneau L, Banderali U, Simoes M, Parent L, Sauvé R. (2007) Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis. J Gen Physiol 129:299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy C, Goodchild SJ, Weatherall KL, Jane DE, Liégeois JF, Seutin V, Marrion NV. (2010) Allosteric block of KCa2 channels by apamin. J Biol Chem 285:27067–27077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Halling DB, Hall AW, Aldrich RW. (2009) EF hands at the N-lobe of calmodulin are required for both SK channel gating and stable SK-calmodulin interaction. J Gen Physiol 134:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla K, Wickenden AD, Gerlach AC, McCormack K. (2009) The KCNQ2/3 selective channel opener ICA-27243 binds to a novel voltage-sensor domain site. Neurosci Lett 465:138–142 [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. (2001) Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 276:9762–9769 [DOI] [PubMed] [Google Scholar]
- Rauer H, Lanigan MD, Pennington MW, Aiyar J, Ghanshani S, Cahalan MD, Norton RS, Chandy KG. (2000) Structure-guided transformation of charybdotoxin yields an analog that selectively targets Ca2+-activated over voltage-gated K+ channels. J Biol Chem 275:1201–1208 [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. (2004) Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci 26:458–469 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Köhler R, Wulff H. (2009) Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 75:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. (1994) Pharmacology of benzodiazepine receptors: an update. J Psychiatry Neurosci 19:24–29 [PMC free article] [PubMed] [Google Scholar]
- Soh H, Park CS. (2001) Inwardly rectifying current-voltage relationship of small-conductance Ca2+-activated K+ channels rendered by intracellular divalent cation blockade. Biophys J 80:2207–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh H, Park CS. (2002) Localization of divalent cation-binding site in the pore of a small conductance Ca2+-activated K+ channel and its role in determining current-voltage relationship. Biophys J 83:2528–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen US, Strøbaek D, Christophersen P, Hougaard C, Jensen ML, Nielsen EØ, Peters D, Teuber L. (2008) Synthesis and structure-activity relationship studies of 2-(N-substituted)-aminobenzimidazoles as potent negative gating modulators of small conductance Ca2+-activated K+ channels. J Med Chem 51:7625–7634 [DOI] [PubMed] [Google Scholar]
- Stocker M. (2004) Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5:758–770 [DOI] [PubMed] [Google Scholar]
- Strøbaek D, Hougaard C, Johansen TH, Sørensen US, Nielsen EØ, Nielsen KS, Taylor RD, Pedarzani P, Christophersen P. (2006) Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol 70:1771–1782 [DOI] [PubMed] [Google Scholar]
- Strøbaek D, Teuber L, Jørgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B. (2004) Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim Biophys Acta 1665:1–5 [DOI] [PubMed] [Google Scholar]
- Weatherall KL, Goodchild SJ, Jane DE, Marrion NV. (2010) Small conductance calcium-activated potassium channels: from structure to function. Prog Neurobiol 91:242–255 [DOI] [PubMed] [Google Scholar]
- Wulff H, Gutman GA, Cahalan MD, Chandy KG. (2001) Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem 276:32040–32045 [DOI] [PubMed] [Google Scholar]
- Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. (2007) Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem 14:1437–1457 [DOI] [PubMed] [Google Scholar]
- Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. (2005) The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol 67:1009–1017 [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, et al. (1998) Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395:503–507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.