Abstract
Emodepside is a resistance-breaking anthelmintic of a new chemical class, the cyclooctadepsipeptides. A major determinant of its anthelmintic effect is the calcium-activated potassium channel SLO-1. SLO-1 belongs to a family of channels that are highly conserved across the animal phyla and regulate neurosecretion, hormone release, muscle contraction, and neuronal network excitability. To investigate the selective toxicity of emodepside, we performed transgenic experiments in which the nematode SLO-1 channel was swapped for a mammalian ortholog, human KCNMA1. Expression of either the human channel or Caenorhabditis elegans slo-1 from the native slo-1 promoter in a C. elegans slo-1 functional null mutant rescued behavioral deficits that otherwise resulted from loss of slo-1 signaling. However, worms expressing the human channel were 10- to 100-fold less sensitive to emodepside than those expressing the nematode channel. Strains expressing the human KCNMA1 channel were preferentially sensitive to the mammalian channel agonists NS1619 and rottlerin. In the C. elegans pharyngeal nervous system, slo-1 is expressed in neurons, not muscle, and cell-specific rescue experiments have previously shown that emodepside inhibits serotonin-stimulated feeding by interfering with SLO-1 signaling in the nervous system. Here we show that ectopic overexpression of slo-1 in pharyngeal muscle confers sensitivity of the muscle to emodepside, consistent with a direct interaction of emodepside with the channel. Taken together, these data predict an emodepside-selective pharmacophore harbored by SLO-1. This has implications for the development of this drug/target interface for the treatment of helminth infections.
Introduction
Parasitic worms place a huge economic and health burden on society by causing disease in humans, livestock, and pets. They are controlled by anthelmintics, but these are losing their effectiveness as a result of the emergence of drug-resistant strains of worm (Gilleard, 2006). Emodepside is a new anthelmintic drug (Fig. 1) that paralyzes parasitic nematode worms, including those that have developed resistance to anthelmintics (von Samson-Himmelstjerna et al., 2005). It has a broad spectrum of action against gastrointestinal nematodes, including important parasites of livestock (Harder and von Samson-Himmelstjerna, 2002) and a filarial infection of humans, Onchocerca volvulus (Townson et al., 2005).
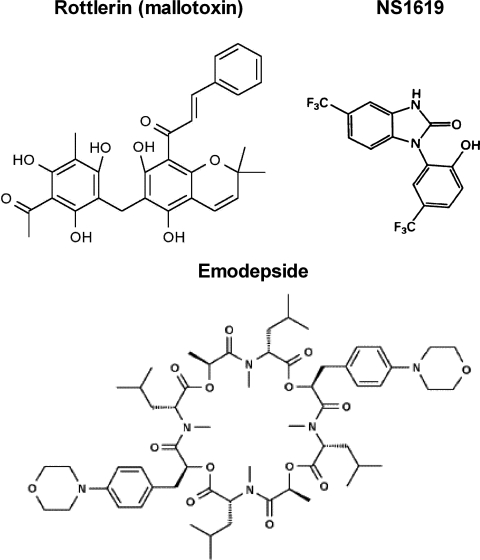
Fig. 1.
The structure of the calcium-activated potassium channel agonists compared with the novel cyclooctadepsipeptide anthelmintic emodepside (Harder et al., 2003). Rottlerin (mallotoxin; 1-[6-[(3-acetyl-2,4, 6-trihydroxy-5-methylphenyl)methyl]-5, 7-dihydroxy-2,2-dimethyl-2H-1-benzopyran-8-yl]-3-phenyl-2-propen-1-one) and NS1619 (1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one).
Studies to elucidate the mode of action of emodepside have been conducted on the parasitic nematode Ascaris suum and in the free-living model genetic nematode Caenorhabditis elegans. Taken together, these studies indicate that emodepside acts to inhibit neuromuscular transmission in nematodes and thus impairs the vital functions of motility, feeding, and reproduction (Willson et al., 2003, 2004; Bull et al., 2007). Mutagenesis screening for C. elegans resistant to the inhibitory effects of emodepside on locomotion identified SLO-1, a calcium- and voltage-activated potassium channel, as the major determinant of emodepside sensitivity (Guest et al., 2007; Holden-Dye et al., 2007). This is consistent with earlier in vitro electrophysiological experiments on A. suum muscle that demonstrated a calcium- and potassium-dependent hyperpolarization (Willson et al., 2003). Furthermore, in two independent genetic screens of 20,000 genomes for emodepside resistance, only loss-of-function or reduction-of-function alleles of slo-1 were recovered. Because slo-1 gain-of-function C. elegans mutants exhibit an inhibition of motility and egg-laying similar to that of emodepside-treated worms (Guest et al., 2007; Holden-Dye et al., 2007), a parsimonious explanation of emodepside's anthelmintic action is that it activates a SLO-1-dependent pathway to bring about neuromuscular inhibition and paralysis of the pathways that regulate feeding, locomotion, and egg-laying in the worm.
SLO-1 belongs to a family of calcium-activated potassium channels that are highly conserved throughout the animal phyla playing key physiological roles in the regulation of muscle and neuronal excitability, hormonal secretion, and neurotransmitter release (for review, see Salkoff et al., 2006). For example, in nematodes, SLO-1 regulates neurotransmitter release (Wang et al., 2001), whereas in humans, there is evidence linking mutations in calcium-activated potassium channels to seizures (Du et al., 2005). Nonetheless, emodepside is well tolerated by the mammalian hosts in which it has been tested to date (Harder et al., 2003), suggesting that it may achieve its selective toxicity through pharmacological differences between the channel in the nematode and its mammalian host.
In this study we have deployed a C. elegans slo-1-null mutant, js379, to either ectopically or heterologously express the wild-type C. elegans channel SLO-1 or a close mammalian ortholog, human kcnma1, in a genetic background devoid of native SLO-1 channel function. Ectopic overexpression of wild-type slo-1 in the pharyngeal muscle of C. elegans, a tissue that does not express the native channel (Wang et al., 2001; Chiang et al., 2006), conferred sensitivity to emodepside consistent with a role for SLO-1 as an emodepside receptor. Furthermore, although expression of human kcnma1 from the native slo-1 promoter provided full rescue of the distinct and quantifiable behavioral phenotypes of slo-1 js379 (strain NM1968; Wang et al., 2001), it did not confer sensitivity to emodepside. Instead, these strains exhibited responses to mammalian BK channel agonists (Fig. 1). Thus, we conclude that human KCNMA1 can functionally substitute for the nematode channel SLO-1 in vivo and that the two channels exhibit distinct pharmacological properties. With regard to the selective toxicity of emodepside and the potential of the new class of cyclooctodepsipeptides in tropical medicine for filariasis (Geary et al., 2010), the C. elegans channel is 10- to 100-fold more sensitive to emodepside than the human channel.
Materials and Methods
Culture.
C. elegans were grown on nematode growth medium plates (Brenner, 1974) seeded with Escherichia coli (OP50 strain) at 20°C. N2 (Bristol strain) C. elegans were employed as wild type. NM1968 is a strain carrying a predicted functional null mutation, js379, for slo-1 (Wang et al., 2001) and this was employed in these studies as a strain resistant to the effects of emodepside on locomotion (Guest et al., 2007). Transgenic C. elegans (described below) were always assayed in parallel with positive and negative controls for the emodepside sensitivity assays (i.e., on the same day with N2 and slo-1(js379) C. elegans, respectively).
Sequence Analysis.
SLO-1 is encoded by a single gene on chromosome V in C. elegans, and its human ortholog KCNMA1 is on chromosome X in the human genome. Multiple splice variants are generated by alternative splicing and post-transcriptional mechanisms (Salkoff et al., 2006). The primary sequence of C. elegans SLO-1 has greater than 50% amino acid identity with the mammalian channels (Butler et al., 1993; McCobb et al., 1995; Salkoff et al., 2006) and 66% identity with Drosophila melanogaster (Adelman et al., 1992). To identify and categorize mammalian orthologs of SLO-1a (NP_001024259), and specifically to test the relationship with KCNMA1 (NP_002238), we constructed a molecular phylogeny (see Supplemental Information S2 for details).
To further compare the sequence of SLO-1a (NP_001024259) and KCNMA1 (NP_002238), we performed Basic Local Alignment (NCBI BLAST) (Altschul et al., 1990) of translated protein sequences of the channels and their individual regions. We used Specialized BLAST (NCBI) program (Johnson et al., 2008) to align two protein sequences. Algorithm parameters were set to automatic, and the method used was “Compositional matrix adjust.”
Molecular Biology.
A number of different splice variants of slo-1 exist in C. elegans (http://www.wormbase.org); of these, slo-1a is the longest variant. pBK3.1 and pBK4.1, vectors for the neuronal and body-wall muscle expression of slo-1a, respectively, were initially provided by Lawrence Salkoff (Wang et al., 2001). The snb-1 promoter in pBK3.1 was replaced with the promoter for slo-1, which was amplified from C. elegans genomic DNA and cloned in front of slo-1a. (Primers to amplify the putative C. elegans slo-1 promoter region were designed based on the sequence of yeast artificial clone Y51A2D; GenBank accession no. AL021497.) The amplified putative promoter sequence was 3084 base pairs upstream of the start site. An analysis of the sequence indicated no recognizable promoter elements or transcription factor-binding sites. This construct gave apparently full rescue of the behavioral phenotype of the slo-1-null mutant js379. The open reading frame (ORF) of kcnma1 was amplified by polymerase chain reaction (PCR) using proof-reading polymerase PfuUltra (Invitrogen, Carslbad, CA) from pCMV6-XL4 vector (OriGene Technologies, Rockville, MD). 3′ and 5′ Primers contained BamHI and XbaI recognition sites, respectively. PCR products were separated on a 0.8% agarose gel, and the band of 3.5 kilobase pairs, corresponding to kcnma1 cDNA (ORF), was purified using a QIAGEN Gel Purification kit (QIAGEN, Valencia, CA). Kcnma1 ORF cDNA was then ligated into pCRII-Blunt-TOPO vector (TOPO cloning kit; Invitrogen) and further subligated into pBK3.1, pBK4.1, and cepslo1::pBK3.1 vectors. The ORF of kcnma1 and sites of ligation were sequenced in 3′ to 5′ direction using MWG value read sequencing service. ORF of kcnma1 was cut from pCRII-Blunt-TOPO vector using XbaI and BamHI enzymes. pBK3.1 and pBK4.1 vectors were digested with BamHI and XbaI. pslo1 promoter has two recognition sites for XbaI. pslo1::pBK3.1 vector was digested with BamHI, which was then inactivated by heating the digest mixture for 20 min at 65°C, followed by partial digest of the vector with XbaI. All plasmid DNA samples were verified for authenticity by sequencing the newly generated portions of cDNA (Eurofins MWG Operon, London, UK). A construct to drive expression of slo-1a in the pharyngeal muscle was created by ligating the slo-1a sequence from pBK3.1 downstream of the myo-2 promoter sequence in plasmid pPD30.69 (a gift from Andrew Fire, Stanford University, Stanford, CA; Okkema et al., 1993).
Transforming slo-1(js379) C. elegans with slo-1a and kcnma1 Genes.
slo-1(js379) C. elegans were injected with plasmids to drive expression of either slo-1a or kcnma1 from a pan-neuronal promoter (psnb-1) (Okkema et al., 1993; Nonet et al., 1998), a body-wall muscle promoter (pmyo-3) (Okkema et al., 1993), a pharyngeal muscle promoter (pmyo-2) (Okkema et al., 1993), or from the native slo-1 promoter (pslo-1). The plasmids were injected at 30 ng/μl. Transformed worms were identified by coinjecting pPD118.33 plasmid (50 ng/μl), which drives expression of green fluorescent protein from the pharyngeal muscle promoter, pmyo-2. The coinjected gfp transformation marker formed an extrachromosomal array with the plasmids carrying the calcium-activated potassium channel sequences, and thus worms with fluorescent green pharynxes could be identified as carrying the plasmid of interest. For all the experiments, at least two independently transformed lines of transgenic C. elegans expressing slo-1 or kcnma1 behind the specified promoter were assayed. Results between the independent lines for each construct were in good agreement, and the data presented are the pooled data from these independent lines. Expression of the transgenes was also confirmed by reverse transcription-PCR (Supplemental Information S1).
Locomotion Assays of C. elegans on Emodepside, 24-h Exposure.
NGM plates were modified with vehicle (0.5% ethanol) or emodepside as described previously (Bull et al., 2007). Emodepside modified plates contained drug in concentrations of 10 nM, 100 nM, 1 μM, and 10 μM. The maximum calculated concentration of ethanol was 86 mM, a concentration that does not exhibit inhibitory effects on the locomotion of C. elegans (Mitchell et al., 2007). Vehicle controls were performed for all experiments.
Experiments were performed on age synchronized worms. Larval stage 4 (L4) C. elegans were grown on NGM plates modified with emodepside or vehicle for 24 h before the assay. Each L4 + 1 day worm was moved to an NGM plate (without E. coli) for 30 s to remove any adhering bacteria, followed by the transfer to a fresh NGM plate, also without E. coli. After 1 min, locomotion of C. elegans was quantified by counting body bends the worm generated in 1 min. A body bend was specified as a movement of the worm in which the tip of the head or the tail of the animal makes one full sinusoidal wave. Only completed waves were counted as body bends. At 1 and 10 μM emodepside, some wild-type and transgenic C. elegans were moving forward by protruding their static anterior via frequent shallow waves of low amplitude generated by the rest of the body. In this case, one body bend was counted when the tip of the head moved forward the same distance as it does in one body bend in control animals.
Locomotion Assays of C. elegans on Emodepside- and NS1619-Containing Plates, 3-h Exposure.
NS1619 is a light-sensitive compound, and stability was therefore an issue for long-term exposure. Therefore, an alternative approach for drug treatment was adopted in which a 200-μl dose of vehicle or drug (emodepside or NS1619) was applied to the OP50 E. coli lawn on the NGM plate, and left to dry for 30 min. Parallel experiments were conducted using emodepside with the same protocol. L4 + 1 day C. elegans were exposed to the vehicle or drug in the food source for 3 h. Body bends were then counted as described above.
C. elegans Reversals Assay.
Wild-type C. elegans initiate foraging behavior in the absence of food, characterized by forward locomotion and spontaneous reversals (Chiba and Rankin, 1990). slo-1(js379) C. elegans have a higher frequency of reversals than wild-type (Wang et al., 2001; Guest et al., 2007). Age-synchronized (L4 plus 1 day) wild-type, slo-1(js379), and transgenic C. elegans described above were assayed on 9-cm NGM plates with no food. Each worm was first transferred to a no-food plate for approximately 30 s to remove any adhering bacteria and then moved to a second no-food plate. After 5 min of acclimatization on the no-food plate, the number of reversals was counted for 3 min. A “reversal” was identified as a movement of C. elegans in which its trajectory changes from forward to reverse and in which the tip of the tail traces at least one wave of sinusoidal shape. The tail does not always return to the same position in a wave. Ten worms were assayed per plate. Each transgenic strain was assayed in parallel with N2 and slo-1(js379) as internal controls.
C. elegans Pharyngeal Assays.
Well-fed age-synchronized (L4 plus 1 day old) C. elegans were transferred to a 3-cm Petri dish containing modified Dent's saline (10 mM d-glucose, 140 mM NaCl, 1 mM MgCl2, 3 mM CaCl2, 6 mM KCl, and 10 mM HEPES, pH 7.4) supplemented with 0.01% (w/v) bovine serum albumin. A transverse cut was made immediately posterior of the terminal bulb of the pharynx using a razor blade. The pharyngeal preparation was transferred to the recording chamber (volume, ∼1 ml). EPG recordings were made using methods described previously (Dillon et al., 2009). Recordings were made in the presence of perfusion at a constant rate of 5 ml/min. Data were acquired using Axoscope software (Molecular Devices, Sunnyvale, CA) and recorded with a sampling rate of 2 kHz. Recordings were made within 5 min of removal of the worm from the food plate and typically lasted no longer than 20 min. Each single pharyngeal feeding cycle, or “pump,” consisted of a contraction and relaxation of the radial pharyngeal muscle, which is recorded as an EPG waveform (Cook et al., 2006). EPG recordings were analyzed for pump frequency and for the pattern of pumping (i.e., whether or not pumping occurred at a constant rate or consisted of bursts of activity in which pumps occurred in groups). For the latter analysis, “pump groups” refers to the organization of individual pumps into clusters or groups. An individual pump was defined as belonging to a group if it occurred within 0.2 s from the previous pump. Basal pumping of N2 wild-type C. elegans typically includes pump groups of 1, 2, or 3. In contrast, slo-1(js379) mutants pump mainly in groups of higher than 3, displaying “bursting” pumping (Dillon et al., 2009). To evaluate rescue of this slo-1(js379) pharyngeal phenotype, transgenic C. elegans expressing either slo-1 or kcnma1 behind the native pslo-1 promoter were analyzed for the behavior of pumping in groups using AutoEPG software (Dillon et al., 2009). For this, pumping in groups was analyzed during the first 10 min of 20-min recordings.
For experiments involving application of emodepside, the following protocol was adopted to permit a quantification of the inhibitory effect of emodepside on pharyngeal pumping. It is based on establishing a reproducible stimulatory response to 5-HT (Rogers et al., 2001) against which emodepside inhibition can be measured. The first 2.5 min were recorded perfusing with Dent's saline followed by 1.5 min in 5-HT, 2 min in Dent's saline, followed by 1.5 min 5-HT, followed by 5 min in either Dent's saline (control) or emodepside, followed by 1.5 min 5-HT, and finally 5 min in Dent's saline (wash step). In the absence of any emodepside, the pharyngeal preparation exhibited a consistent increase in frequency of pumping in response to consecutive applications of 5-HT. The inhibitory effect of emodepside was quantified by determining the change in the 5-HT response after addition of emodepside as a percentage of the response obtained before addition of emodepside (control).
Drugs.
NS1619 (1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one) and rottlerin were obtained from Sigma-Aldrich UK (Dorset, UK). Emodepside was provided by Bayer (Monheim, Germany). Drugs were prepared in 100% ethanol at a concentration of 2 mM as a stock solution. Stock solutions of NS1619 were kept at −20°C for a maximum of 2 weeks. Stock solutions of emodepside and emodepside-containing plates were kept at 4°C for a maximum of 1 week. Final drug concentrations of 10 nM, 100 nM, 1 μM, and 10 μM in NGM or in a food source contained 0.5% ethanol; 0.5% vehicle, ethanol, was used as a control. For the electrophysiological experiments, emodepside was prepared fresh each day in 100% DMSO. Stock solution was further diluted in DMSO and Dent's saline to give a final concentration in the recording chamber of 100 nM or 1 μM emodepside and 0.01% DMSO. Serotonin creatinine sulfate complex (5-HT; Sigma UK) was freshly prepared each day in Dent's saline. The stock solution was further diluted in Dent's saline to give a final concentration of 300 nM.
Statistical Analysis.
Data are presented as the mean ± S.E.M. of n experiments. Inhibition curves were fitted to the modified logistic equation using Prism software (ver. 4.0; GraphPad Software, San Diego, CA) to determine IC50 values with 95% confidence limits. Statistical significance was determined using one-way ANOVA (significance level set at p < 0.05) followed by Bonferroni post-tests. For the electrophysiological experiments, statistical significance was determined using paired Student's t test or one-way ANOVA (statistical significance level was set at p < 0.05) as appropriate. The number of corresponding pumps and the number of individual worms used to perform the statistical analysis for each strain is stated in respective figure legends.
Results
Structural Comparison of SLO-1 and KCNMA1.
KCNMA1, the mammalian ortholog for SLO-1a (NP_001024259), was identified using Ensembl ortholog definitions (Flicek et al., 2011). This was confirmed by molecular phylogenetic analysis of SLO-1, KCNMA1, and the closest mammalian paralog identified by Ensembl, KCNU1 (Supplemental Information S2). Homo sapiens kcnma1 (Ensembl genome browser ENSG00000156113) is predicted to produce 26 transcripts, 20 of them coding for proteins. The protein product of Ensembl transcript KCNMA1-001 (peptide ENSP00000286627) corresponds to KCNMA1 variant 2/isoform b in the NCBI database (NP_002238). The transcript encoding this isoform (NM_002247) was available to purchase from OriGene Technologies.
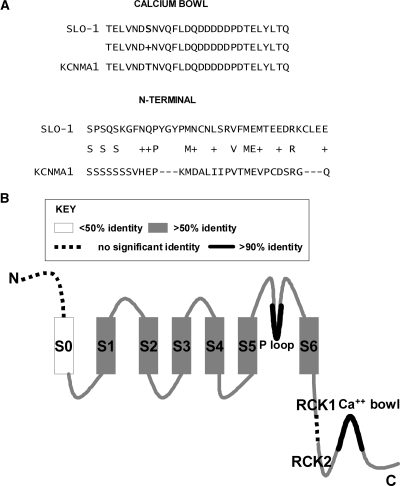
To further compare the sequence of SLO-1a (NP_001024259) and KCNMA1 (NP_002238), we performed basic local alignment (NCBI BLAST) (Johnson et al., 2008) of translated protein sequences of the channels and their individual regions. The alignment for SLO-1a and KCNMA1 shows 55% identity and 69% similarity between the two sequences (Supplemental Information S3). Further alignments were conducted for specific regions of the protein to discern regions of high conservation from those that are more divergent. The most conserved and divergent regions of the channel are the calcium bowl (96% identity, 100% similarity) and the N-terminal domain (no significant similarity), respectively (Fig. 2A). Other highly conserved regions are the transmembrane domains, with the exception of the first transmembrane region S0, the channel pore, and the regulatory domains, RCK1 and RCK2 (Fig. 2B). Between RCK1 and RCK2, there is an additional run of 49 residues in SLO-1.
Fig. 2.
The most divergent and most conserved regions of sequence between C. elegans SLO-1a and human KCNMA1 (isoform b). A, alignment of the calcium bowl (top) and N-terminal domain (bottom) using BLAST (NCBI). Identical amino acids are indicated by their letter symbols, and conservative substitutions are indicated by “+.” Gaps are indicted by “–” symbols and were introduced by the program to enable analysis of the most conserved parts of the sequences. Identity between amino acid sequences of the calcium bowl is 96% and similarity is 100%. There is no significant similarity in the N-terminal domain. B, a diagram of the channel to indicate the most conserved regions of sequence.
C. elegans slo-1 and Mammalian kcnma1 Rescue Behavioral Phenotypes in slo-1(js379).
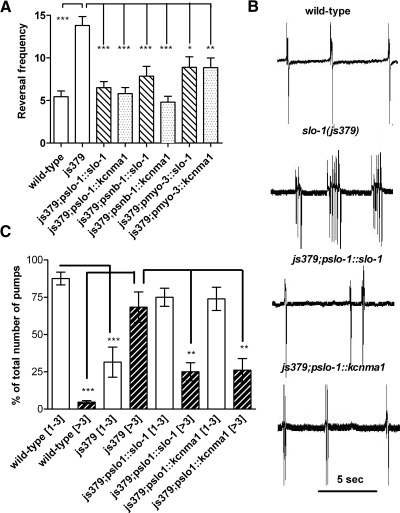
slo-1(js379) mutants move with similar speed and frequency of body bends compared with wild-type C. elegans, but their rate of reversals is significantly increased (Wang et al., 2001; Guest et al., 2007). To identify whether slo-1 and kcnma1 are functional orthologs, we expressed cDNAs of both genes in the slo-1(js379) mutant background from pan-neuronal (snb-1), body-wall muscle (myo-3), and the native slo-1 promoter and determined to what extent these rescued the reversal phenotype of slo-1(js379). Video analysis of slo-1(js379) C. elegans confirmed that these animals stop and reverse more often than wild-type (Fig. 3A; Supplemental Videos S1 and S2). Expression of either slo-1 or kcnma1 completely rescued the reversal phenotype of slo-1(js379) and the transgenic animals exhibited a pattern of reversals more similar to wild-type (Fig. 3A; Supplemental Videos S3 and S4). Furthermore, expression of slo-1 or kcnma1 in only neurons (psnb-1) was also sufficient to rescue this phenotype, in agreement with the neuronal basis of the aberrant pattern of locomotion in slo-1 mutants (Fig. 3A) (Wang et al., 2001). Expression of either slo-1 or kcnma1 in the body-wall muscle of slo-1(js379) also rescued the reversal phenotype (Fig. 3A).
Fig. 3.
A comparison of the rescue of C. elegans slo-1(js379) behavioral phenotypes by slo-1 and its human ortholog kcnma1. A, a comparison of reversal frequency for wild-type, slo-1(js379), and transgenic lines of slo-1(js379) expressing either slo-1 ▨ or kcnma1 ▩ behind the native promoter (pslo-1), a pan-neuronal promoter (psnb-1), or in body-wall muscle (pmyo-3). At least two stable lines for each js379;pslo-1::slo-1 and js379;pslo-1::kcnma1 transgenic strain were tested. All lines for the same strain showed comparable results, and the data are pooled. Data are the mean ± S.E.M. of n ≥20, ***, p < 0.001; **, p < 0.01; *, p < 0.05, one-way ANOVA with Bonferroni post hoc test. B, a comparison of the pattern of pharyngeal pumping in wild-type, slo-1(js379), and transgenic lines of slo-1(js379) expressing either slo-1 or kcnma1 behind the native promoter (pslo-1). Note the erratic pattern in slo-1(js379), which has a tendency to generate pharyngeal pumps in groups of three or more rather than as single evenly spaced pumps. This effect is ameliorated by expression of either slo-1 or kcnma1, bottom two traces. C, a summary of experiments conducted as shown in B. For each experiment, 10-min recording of basal pharyngeal pumping was acquired. This was subjected to analysis with AutoEPG (Dillon et al., 2009), which counted the number of pumps that occurred as single pumps and the number of pumps that occurred in groups of three or more. The data are expressed as the number of pumps that occurred in groups of one to three (open bars) or more than three (hatched bars) as a percentage of the total number of pumps. Data are the mean ± S.E.M. of n = 14 to 26. Note that the pattern of pumping in both the slo-1 and kcnma1 rescue lines is more like wild-type than slo-1(js379).
Mutations in slo-1 also confer a pharyngeal phenotype. Thus, although the pattern of pharyngeal pumping in wild-type worms consists of pumps that occur predominantly in groups of 1 to 3, in slo-1(js379), this pattern is disrupted, and pumps occur in larger groups (Dillon et al., 2009). This is consistent with the idea that SLO-1 has a role in regulating the excitability of neural networks and thus, in its absence, the stability of the network activity is disturbed, leading to bursts of activity. We confirmed this phenotype to reinforce the functional role of SLO-1 in the pharyngeal neural circuits and showed that worms expressing either slo-1 or kcnma1 from the native slo-1 promoter were rescued for this behavior (Fig. 3, B and C), thus indicating that either SLO-1 or KCNMA1 can regulate bursting activity in the pharyngeal circuit.
These studies on the functional rescue of two independent slo-1 behavioral phenotypes with either slo-1 or kcnma1 suggests that kcnma1, the mammalian ortholog of slo-1, can be functionally expressed in C. elegans and, indeed, can substitute for this channel to the extent that its expression can restore behavior to wild-type.
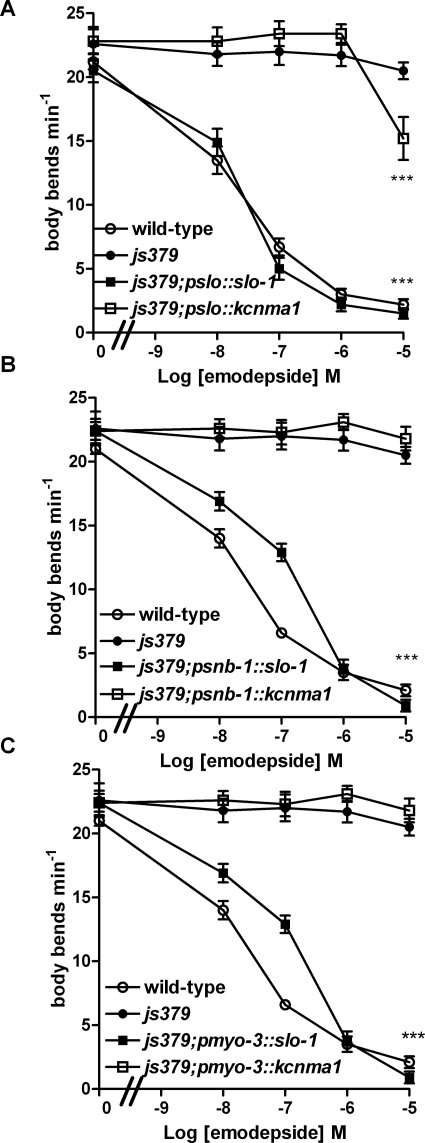
slo-1 but Not kcnma1 Confers Sensitivity to the Inhibitory Effects of Emodepside on Locomotion.
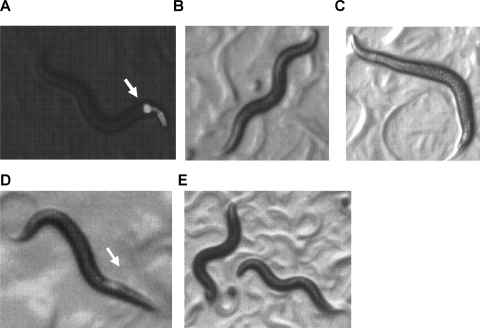
We compared the effect of emodepside on wild-type worm locomotion with its effect on slo-1(js379) C. elegans that had been transformed with either C. elegans slo-1 or its closest mammalian homolog, human kcnma1. We tested animals that expressed slo-1 or kcnma1 from three different promoters: the native slo-1 promoter (pslo-1) or tissue-specific promoters driving pan-neuronal expression (psnb-1) or body wall muscle expression (pmyo-3). These experiments were performed on worms that were synchronized for age (1-day-old adults) to avoid variability of emodepside effects on different developmental stages of C. elegans (Bull et al., 2007). Worms were identified as expressing the gene of interest by visualization of green fluorescent protein in the pharyngeal muscle as a selection marker (Fig. 4A).
Fig. 4.
Effects of emodepside on the body shape of wild-type and transgenic C. elegans. The comparisons shown are of wild-type, slo-1(js379), and transgenic lines of slo-1(js379) expressing either slo-1 or kcnma1 behind the native promoter (pslo-1) or a pan-neuronal promoter (psnb1). Transgenic worms were identified by the appearance of green fluorescence in the pharynx as a result of the expression of gfp driven from the pharyngeal promoter myo-2 as a positive selection marker. A, an example of an L4 + 1-day stage transgenic slo-1(js379) C. elegans. Green fluorescence in the area of pharyngeal muscle (white arrow) is driven by pmyo-2 promoter, which is used as a marker for transformation. B, wild-type L4 + 1-day-old worm on 0.5% ethanol vehicle control showing wild-type sinusoidal body posture. C, wild-type L4 + 1-day-old worm with 100 nM emodepside (24-h exposure) showing the typical flattened wave form, particularly in the anterior region and egg retention (enlarged posterior). D, L4 + 1-day-old js379;psnb-1::slo-1 on 100 nM emodepside (24-h exposure) showing a flattened body posture similar to wild-type in the anterior region (white arrow). E, L4 + 1-day-old js379;psnb-1::kcnma1 with 100 nM emodepside (24-h exposure) showing a normal sinusoidal body shape. (The body shape of js379;psnb-1::kcnma1 and js379;psnb-1::slo-1 in the absence of emodepside was indistinguishable from wild-type worms; not shown.)
The effect of emodepside on locomotion was assessed by observing the effect on body bends. Consistent with earlier reports for wild-type worms (Bull et al., 2007; Guest et al., 2007), 100 nM emodepside elicited a flaccid paralysis, particularly apparent in the anterior of the worm, and a decrease in amplitude of the sinusoidal body shape (Fig. 4, B and C). This effect of emodepside on body posture was not observed in slo-1(js379) mutants, consistent with earlier reports that this mutant exhibits high-level resistance to emodepside (Guest et al., 2007). However, reintroduction of a wild-type copy of slo-1 into the slo-1(js379) mutant restored the ability of emodepside to affect body posture, whereas expression of kcnma1 in the slo-1(js379) genetic background did not (Fig. 4, D and E).
To more quantitatively compare the effect of emodepside in the different transgenic lines, we analyzed concentration-dependent effects on locomotion by assaying the frequency of body bends. Wild-type worms were sensitive to emodepside, whereas slo-1(js379) were resistant [Fig. 5A; IC50 for wild-type worms was 16 nM (95% confidence interval, 11–24 nM), n = 10]. Expression of slo-1 from the native slo-1 promoter in the slo-1(js379) mutant background rescued the sensitivity to emodepside to the level of wild-type [IC50, 23 nM (95% confidence interval, 15–35 nM), n = 10; Fig. 5A]. In marked contrast, expression of kcnma1 from the native slo-1 promoter did not rescue sensitivity to emodepside at 10 nM, 100 nM, and 1 μM. Only at the highest concentration tested, 10 μM emodepside, was the mean number of body bends significantly reduced compared with control (p < 0.001; Fig. 5A). Closer inspection of the behavior of transgenic lines expressing KCNMA1 in the presence of this highest (10 μM) concentration of emodepside revealed an aberrant pattern of locomotion compared with vehicle controls. They stopped moving more often and exhibited repeated reverse movements. These periods of disorientated movement were not observed in the same transgenic animals on vehicle control and are thus best explained by an effect of emodepside at this high concentration rather than as an effect of overexpression of KCNMA1 channel in the transgenic strains. The different effects of emodepside on locomotion in worms expressing either SLO-1 or KCNMA1 can be viewed on Supplemental Videos S5, S6, S7, and S8. The repeated reversals observed for those worms expressing KCNMA1 in the presence of emodepside is reminiscent of the behavior of the slo-1 null mutant js379 and a possible explanation therefore is that at high concentrations, emodepside acts to inhibit signaling through the human calcium-activated potassium channel, KCNMA1.
Fig. 5.
A comparison of the effect of emodepside on locomotor frequency in worms expressing either slo-1 or its human ortholog kcnma1. These experiments measured the frequency of body bends of 1-day-old adult worms to provide a quantitative measure of the effect of 24-h exposure to emodepside on locomotor behavior. A, a comparison of the effect of emodepside on the frequency of body bends on wild-type, slo-1(js379), and js379 transgenic worms expressing slo-1 (js379;pslo-1::slo-1) or kcnma1 (js379;pslo-1::kcnma1) from the native promoter. n = 10 worms for each data point, mean ± S.E.M.. ***, p < 0.001 with respect to vehicle control, one-way ANOVA on last data points. B, a comparison of the effect of emodepside on the frequency of body bends on wild-type, slo-1(js379), and js379 transgenic worms expressing slo-1 (js379;psnb-1::slo-1) or kcnma1 (js379;psnb-1::kcnma1) from a pan-neuronal promoter. n = 10 worms for each data point, mean ± S.E.M. ***, p < 0.001 with respect to vehicle control. C, a comparison of the effect of emodepside on the frequency of body bends on wild-type, slo-1(js379), and js379 transgenic worms expressing slo-1 (js379;pmyo-3::slo-1) or kcnma1 (js379;pmyo-3::kcnma1) from the body-wall muscle promoter myo-3. n = 10 worms for each data point, mean ± S.E.M. ***, p < 0.001 with respect to vehicle control.
Further studies employing different promoters to drive expression of kcnma1 in the slo-1(js379) genetic background in either neurons or body-wall muscle provided additional evidence that expression of kcnma1 does not confer sensitivity to emodepside. In these strains, even the highest concentration of emodepside tested, 10 μM, had no effect on locomotion. This contrasts with the susceptibility of worms expressing kcnma1 from the native pslo-1 promoter to 10 μM emodepside, and this difference is consistent with the observation that the most effective promoter for providing rescue of the reversal locomotor phenotype is also the native pslo-1 promoter (Fig. 3A). In parallel, it was confirmed that expression of the native C. elegans SLO-1 channel from all the promoters tested resulted in sensitivity to emodepside in slo-1(js379) (Fig. 5, B and C).
slo-1 but Not kcnma1 Confers Sensitivity to the Inhibitory Effects of Emodepside on Feeding.
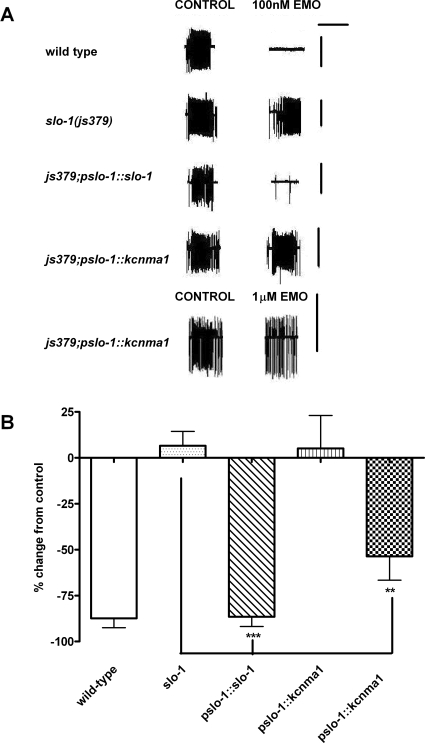
In the pharyngeal system of C. elegans, slo-1 is expressed in the neural circuits but not in the pharyngeal muscle (Wang et al., 2001; Chiang et al., 2006). It has been shown previously that emodepside inhibits the fast coordinated pumping activity of the pharynx that occurs in the presence of the stimulatory neurotransmitter 5-HT (Willson et al., 2004). This effect is mediated presynaptically (Willson et al., 2004) and is highly dependent on the presence of neuronally expressed slo-1 (Guest et al., 2007). In this study, we used the pharyngeal preparation to further test the sensitivity of strains expressing either SLO-1 or KCNMA1 to emodepside by employing the transgenics we had generated that expressed either slo-1 or kcnma1 in the slo-1(js379) mutant from the native slo-1 promoter.
In these assays, the pharyngeal preparation was exposed to three consecutive applications of 5-HT separated by a 2-min interval. In control experiments, each application of 5-HT elicited a robust excitatory response, as reported previously (Willson et al., 2004; Guest et al., 2007). To quantify the level of emodepside inhibition, we compared the pharyngeal pumping frequency observed in response to 300 nM 5-HT (a submaximal concentration) (Rogers et al., 2001) before and 5 min after addition of 100 nM emodepside (Fig. 6A). In wild-type controls, emodepside inhibited the response to 5-HT (Fig. 6A, top). slo-1(js379) were resistant to 100 nM emodepside (Fig. 6, A and B), whereas expression of slo-1 but not kcnma-1 in the js379 mutant from the native slo-1 promoter, pslo-1, restored emodepside sensitivity (Fig. 6, A and B). Only at the higher concentration of emodepside tested, 1 μM, was a partial, but nonetheless significant, inhibitory effect observed on pharyngeal pumping of the strains expressing kcnma1 (Fig. 6, A, bottom, and B).
Fig. 6.
A comparison of the effect of emodepside on the 5-HT-stimulated pharyngeal pumping rate of wild-type and transgenic worms expressing slo-1 or human kcnma1. A, extracellular recordings were made from the pharyngeal system to monitor the activity of the pharyngeal muscle in the presence and absence of emodepside. In these recordings, each upward and downward deflection provides a measure of a single pharyngeal feeding cycle or “pump.” On a slow time-base, a fast pumping rate appears as a continual block of activity, and individual pumps cannot be resolved. In the experiment, continuous recordings were made for 20 min in which the preparation was first perfused with saline, followed by three to four consecutive applications of 5-HT to stimulate pumping interspersed with wash periods in saline. In the absence of emodepside, the response to consecutive applications of 5-HT were similar (not shown). The traces show the response to 300 nM 5-HT before and 5 min after addition of emodepside. In the top four traces, 100 nM emodepside (emo) was applied; in the bottom trace, 1 μM emodepside was used. Scale bars indicate 2 min, 5 mV. Exposure of wild-type worms expressing the transformation marker only (pmyo-2::gfp) to emodepside resulted in a complete inhibition of pumping (top trace). B, the inhibitory effect of emodepside on the 5-HT response in the different strains was compared by expressing the pumping rate in the presence of emodepside as a percentage change compared with the control response (i.e., the pumping rate before the addition of emodepside); 1 μM emodepside was also tested in the strain expressing kcnma1 (checkered bar); (n ≥ 5, mean ± S.E.M.; ***, p < 0.001; **, p < 0.01; one-way ANOVA with Bonferroni post hoc test).
The data for the pharmacological actions of emodepside on the worms expressing either kcnma1 or slo-1 from the native pslo-1 promoter are summarized in Table 1 and provide evidence for a selective effect of emodepside on strains expressing slo-1.
TABLE 1.
A comparison of the ability of emodepside to inhibit pharyngeal pumping and locomotion in C. elegans expressing either slo-1 or kcnma1
Data are a summary of the effects of 100 nM and 10 μM emodepside from Figs. 5A and 6. The values are presented as percentage of control, where control is the average number of pumps (in the presence of 300 nM 5-HT) or body bends in worms on vehicle plates, assessed in parallel with the drug treatment group. Wild-type control are worms expressing the transformation marker myo-2::gfp.
| Percentage of Control |
|||
|---|---|---|---|
| Pharyngeal Pumps 100 nM emo | Body Bends |
||
| 100 nM emo | 10 μM emo | ||
| % | |||
| Wild-type | 8 ± 2 (n = 4)* | 32 ± 4 (n = 10)* | 10 ± 2 (n = 10)* |
| slo-1(js379) | 99 ± 9 (n = 7) | 101 ± 8 (n = 10) | 96 ± 9 (n = 10) |
| js379;pslo-1:: slo-1 | 10 ± 6 (n = 8)* | 26 ± 5 (n = 10)* | 8 ± 2 (n = 10)* |
| js379;pslo-1:: kcnma1 | 117 ± 15 (n = 6) | 104 ± 6 (n = 10) | 68 ± 8 (n = 10)* |
emo, emodepside.
Significantly different from control; one-way ANOVA with Bonferroni post hoc test.
A Pharmacological Characterization of Transgenic Lines Expressing slo-1 or kcnma1 Using Mammalian Calcium-Activated Potassium Channel Agonists.
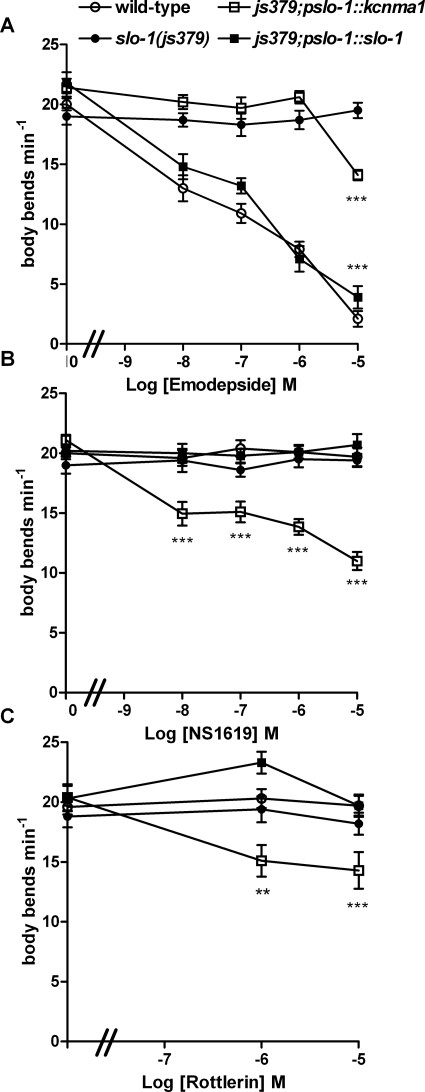
The experiments described in the previous two sections show that worms expressing kcnma1 are only weakly sensitive to emodepside despite the fact that expression of this channel from the native slo-1 promoter completely rescued the behavioral phenotypes of slo-1(js379). Therefore, we tested whether or not expression of kcnma1 in C. elegans conferred sensitivity to known agonists of this channel, as might be predicted from the observed functional rescue. We tested the effects of an activator of mammalian BK channels, NS1619 (Olesen et al., 1994), on locomotion of wild-type and slo-1(js379)-, js379;pslo-1::slo-1-, and js379;pslo-1::kcnma1-expressing strains of C. elegans. NS1619 is used widely for its ability to relax smooth muscle (Olesen et al., 1994) and inhibit neuronal activity (Lee et al., 1995) by a selective activation of calcium-activated BK channels. NS1619 was chosen for these experiments as it can act at mammalian channels in the absence of accessory β subunits (Zakharov et al., 2005). NS1619 has low stability; therefore, these experiments were conducted over a shorter time-frame of drug exposure, 3 rather than 24 h. Emodepside inhibited body bends of wild-type worms after this shorter incubation time but with a slightly higher IC50 (78 nM; 95% confidence interval, 47–131 nM; n = 10) compared with the 24-h emodepside treatment. These experiments using a shorter exposure to emodepside provided further evidence of the ability of only the high concentration to inhibit lines expressing kcnma1 (Fig. 7A). NS1619 at concentrations from 10 nM to 10 μM had no effect on locomotion of wild-type or js379;pslo-1::slo-1 C. elegans, suggesting that it does not interact with C. elegans slo-1 channels (Fig. 7B). In contrast, the locomotion of worms expressing kcnma1 (js379;pslo-1::kcnma1) was significantly impaired compared with vehicle control and wild type (Fig. 7B; Supplemental Videos S9, S10, S11, and S12). Worms did not appear paralyzed in the presence of NS1619 but rather seemed to repeatedly be “slipping” backward and immediately forward in the same spot on a plate without completing a complete sinusoidal body wave. This behavior was also noted in worms expressing kcnma1 (js379;pslo-1::kcnma1), in the presence of emodepside, but with NS1619 it was much more pronounced. These disruptions of normal coordinated movement became increasingly severe with increasing concentration of NS1619, which is reflected in a decreasing number of body bends (Fig. 7B). NS1619 did not cause a complete inhibition of body bends and elicited a maximal inhibition of 48% at the highest concentration tested.
Fig. 7.
A comparison of the effect of emodepside and the calcium-activated potassium channel agonists NS1619 and rottlerin on locomotion of C. elegans expressing SLO-1 or the human channel KCNMA1. For these experiments, 1-day-old adult worms were exposed to vehicle or drug at increasing concentrations for 3 h. A, the effect of a 3-h incubation with emodepside at the concentrations indicated on the frequency of body bends on wild-type, slo-1(js379), js379;pslo-1::slo-1, and js379;pslo-1:: kcnma1. n = 10 worms for each data point, mean ± S.E.M., ***, p < 0.001 compared with vehicle control for the same strain. B, the effect of a 3-h incubation with NS1619 at the concentrations indicated on the frequency of body bends on wild-type, slo-1(js379), js379;pslo-1::slo-1, and js379;pslo-1::kcnma1. n = 10 worms for each data point, except for js379;pslo-1::kcnma1, for which n = 20 worms for each data point, mean ± S.E.M., ***, p < 0.001 compared with vehicle control for the same strain. One-way ANOVA and Bonferroni post hoc test on last data points. C, the effect of a 3-h incubation with rottlerin at the concentrations indicated on the frequency of body bends on wild-type, slo-1(js379), js379;pslo-1::slo-1, and js379;pslo-1::kcnma1. n = 10 worms for each data point, **, p < 0.01; ***, p < 0.001 compared with vehicle control for the same strain.
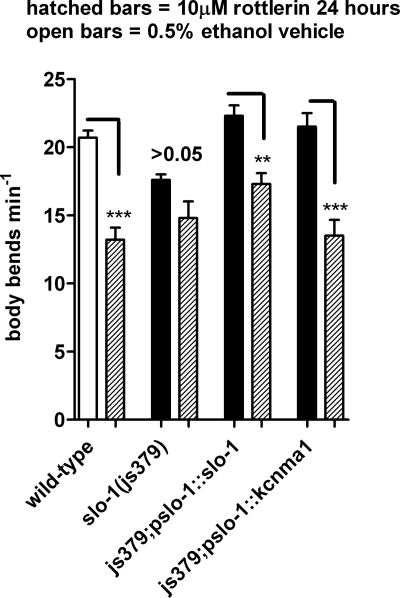
In a further series of experiments, we tested the effect of the BK channel agonist rottlerin (Zakharov et al., 2005) on the behavior of transgenic strains expressing either slo-1 or kcnma1. Rottlerin, also known as mallotoxin, is a lipid-soluble toxin isolated from Mallotus philippinensis (Wu et al., 2007), which potently activates BK channels (Zakharov et al., 2005; Wu et al., 2007). It activates heterologously expressed and mammalian muscle BK channels when applied to both extracellular (Zakharov et al., 2005) and intracellular parts of the membrane (Wu et al., 2007). Locomotion of C. elegans after both short-term (3-h) and long-term (24-h) exposure to rottlerin was assessed. Short-term (3-h) exposure to rottlerin did not inhibit locomotion of wild-type, slo-1(js379), or js379;pslo-1::slo-1 (Fig. 7C). However, movement was slowed in js379;pslo-1::kcnma1 C. elegans exposed to 1 and 10 μM rottlerin for 3 h (Fig. 7C). The worms expressing kcnma1 exposed to rottlerin also had periods during which they moved backward and forward on the same spot with some stalls, similar to their locomotion on NS1619, suggesting that rottlerin and NS1619 are affecting locomotion in a similar manner. Long-term (24-h) exposure to rottlerin affected locomotion of wild-type worms (Fig. 8). It is noteworthy that slo-1(js379) was resistant to the effect on locomotion of 24-h exposure to rottlerin, suggesting that the effect of rottlerin on wild-type worms may be mediated by SLO-1 (Fig. 8). This was confirmed by the observation that the inhibitory effect of rottlerin on locomotion was restored by expressing slo-1 from the native promoter (js379;pslo-1::slo-1 worms; Fig. 8). Furthermore, worms expressing kcnma1 were inhibited by 10 μM rottlerin in a fashion similar to those expressing slo-1. This suggests that rottlerin has less selectivity than NS1619 for the human over the C. elegans calcium-activated potassium channel.
Fig. 8.
A comparison of the effect of rottlerin (24-h exposure) on locomotion of C. elegans expressing SLO-1 or the human channel KCNMA1. For these experiments, 1-day-old adult worms were exposed to vehicle or 10 μM rottlerin for 24 h, and the effect on the frequency of body bends was assayed. Data are the mean ± S.E.M. of 10 determinations for each strain. **, p < 0.01; ***, p < 0.001; one-way ANOVA with Bonferroni post hoc test.
Ectopic Expression of slo-1 in C. elegans Pharyngeal Muscle.
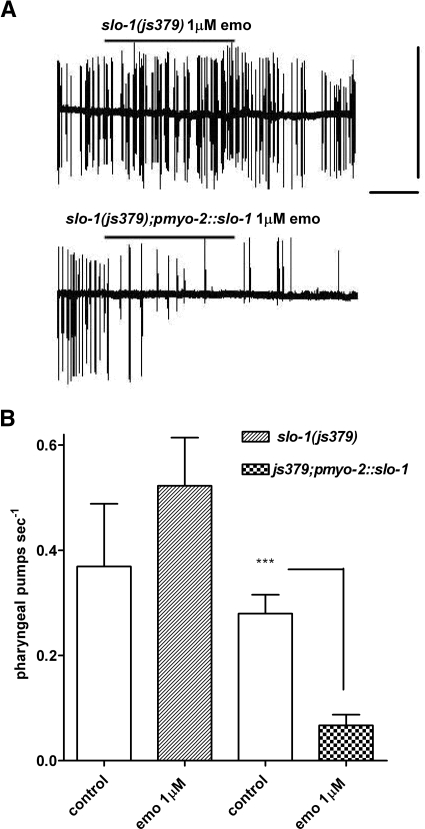
slo-1 is not expressed at detectable levels in pharyngeal muscle. In the pharyngeal system, it is selectively expressed in neurons (Wang et al., 2001; Chiang et al., 2006) to regulate the pattern of pharyngeal pumping activity (Dillon et al., 2009). Expression of wild-type slo-1a from the pan-neuronal promoter snb-1 in the slo-1 null mutant js379 has been shown to rescue the uncoordinated pattern of bursting pharyngeal pumping activity exhibited by the slo-1 mutant strain (Dillon et al., 2009), consistent with its role in the nervous system. In contrast, expression of slo-1a in the pharyngeal muscle of slo-1(js379) does not rescue this behavior (Dillon et al., 2009), consistent with the lack of native expression of, and thus physiological role for, the channel in this tissue (Dillon et al., 2009). Therefore, we exploited the pharyngeal muscle for ectopic expression of slo-1 to test its role in mediating the action of emodepside. In these studies, we determined the direct effect of emodepside on the pharynx in the absence of 5-HT stimulation. In wild-type worms, emodepside caused an almost complete inhibition of pumping (control, 0.49 ± 0.08 pumps/sec; with 1 μM emodepside, 0.04 ± 0.02 pumps/sec; n = 7; p < 0.001, paired Student's t test). Emodepside had no inhibitory effect on the pharyngeal muscle of the mutant slo-1(js379) but did inhibit the pumping activity of strains ectopically expressing slo-1a specifically in the pharyngeal muscle (Fig. 9, A and B). This effect of emodepside on strains expressing SLO-1 in the pharyngeal muscle was observed in the absence of rescue of the slo-1(js379) pharyngeal phenotype (i.e., the bursting activity), which has previously been shown to be derived from a neuronal effect of SLO-1 (Dillon et al., 2009). These data indicate an intimate link between emodepside response and the SLO-1 channel and are consistent with the proposal that the nematode channel harbors an emodepside-selective pharmacophore.
Fig. 9.
Ectopic expression of slo-1 in C. elegans pharyngeal muscle confers sensitivity to emodepside. A, example traces of extracellular (EPG) recordings of pharyngeal muscle. Each vertical deflection reports a single muscle contraction-relaxation cycle or “pump.” Scale bars, 9 mV, 4 min. The top trace is from a slo-1-null mutant and bottom trace is a slo-1-null mutant expressing wild-type slo-1 from a pharyngeal muscle promoter pmyo-2. The horizontal bar indicates the duration of application of emodepside. B, a summary of these experiments. Data are mean ± S.E.M., n ≥ 8; ***, p < 0.001; paired Student's t test.
Discussion
Emodepside has important resistance-breaking properties (Harder et al., 2003) that have stimulated interest in its molecular mechanisms of action. Two modes of action have been demonstrated: an emodepside receptor with homology to mammalian latrophilin G protein-coupled receptors has been expression-cloned from Haemonchus contortus (Saeger et al., 2001), and its C. elegans ortholog, LAT-1, confers sensitivity of the pharyngeal system to emodepside (Willson et al., 2004). However, C. elegans latrophilin mutants remain susceptible to the inhibitory effects of emodepside on locomotion (Guest et al., 2007), indicating that another effector must also be involved. Subsequently, a chemical mutagenesis screen identified SLO-1 as a key determinant for the drug's effects on nematode development and motility (Guest et al., 2007).
The kcnma1 channel gene is the mammalian ortholog of slo-1 and was therefore selected as a suitable candidate to test the selective toxicity of emodepside. To assess the effects of emodepside on SLO-1 and KCNMA1, both genes were expressed in a C. elegans mutant, slo-1(js379), that harbors a mutation in the channel (introducing a premature stop codon) and is therefore predicted to be a functional null mutant (Wang et al., 2001). The tractability of C. elegans for expression of transgenes from different tissue specific promoters was employed to drive expression from the native promoter, a pan-neuronal promoter and a promoter for expression in body-wall muscle. slo-1 has been shown to be widely expressed in C. elegans (Wang et al., 2001), in neurons, and in body-wall muscle. This accords with the observation that the slo-1 behavioral phenotypes of increased frequency of reversals (Wang et al., 2001; Guest et al., 2007) and erratic pharyngeal pumping (Dillon et al., 2009) were restored when a wild-type copy of slo-1 was expressed from either the native promoter or a pan-neuronal promoter. In addition, the reversal phenotype was significantly rescued by expression of slo-1 in body-wall muscle consistent with the observation that native slo-1 is also expressed in this tissue (Carre-Pierrat et al., 2006). These assays provided a platform for functional analysis of the human channel, kcnma1, expressed in C. elegans. Expression of this channel instead of slo-1 was observed to robustly rescue the slo-1-dependent locomotor and pharyngeal phenotypes. This suggests that KCNMA1 is able to substitute for SLO-1 in the neural circuits that regulate the pattern of locomotion and pharyngeal pumping and restore wild-type function in the slo-1-null mutant.
The heterologous expression of the mammalian channel protein KCNMA1 in C. elegans in this study provided a highly tractable in vivo model for the direct comparison of the susceptibility of the nematode versus the mammalian channel to emodepside. It has been established that the high level of resistance of the slo-1-null mutant js379 to the effect of emodepside on locomotion can be reversed by expression of a wild-type copy of slo-1 in either neurons or body wall muscle (Guest et al., 2007). For these studies, to more accurately reflect the endogenous expression of slo-1, we employed a transgenic strain expressing slo-1 from the native slo-1 promoter. In these lines, the susceptibility to emodepside was indistinguishable from that of wild-type. In parallel, experiments were performed on transgenic lines expressing human kcnma1, and in these lines, resistance to emodepside was not alleviated. This lack of susceptibility to emodepside is unlikely to be due to low-level functional expression of the human channel in the C. elegans biological background because these same lines exhibited a robust rescue of the behavioral phenotypes. Furthermore, reverse transcription-PCR indicated that the kcnma1 transgene was transcribed at levels similar to those of the slo-1 transgene (Supplemental Information 1). As noted above, kcnma is subject to alternative splicing, and we cannot rule out the possibility that one of the splice variants, other than the kcnma1 splice isoform tested here, may be more sensitive to emodepside. Nonetheless, taken at face value, these data indicate that the human channel, although functional in C. elegans, is not sensitive to submicromolar concentrations of emodepside. However, when strains expressing kcnma1 were tested in the presence of the highest concentration of emodepside, 10 μM, an inhibitory effect on locomotion was observed. This was unlikely to be due to a nonspecific effect of emodepside, because this inhibitory effect was not observed in the slo-1-null mutant. Thus, we conclude that emodepside can interact with the human channel KCNMA1 but with a much lower efficacy than its interaction with SLO-1. This conclusion is further supported by the observations made on the effects of emodepside on pharyngeal pumping. In these experiments, expression of slo-1, but not kcnma1, was required to confer susceptibility to low drug concentrations.
The generation of strains expressing functional human or nematode calcium-activated potassium channels enabled further comparative pharmacological analysis. Thus, in addition to providing evidence that the C. elegans but not the human channel is sensitive to nanomolar concentrations of emodepside, this study has also shown that the mammalian BK channel activator NS1619 (Lee et al., 1995) distinguishes between the two channels, in this case having higher efficacy for the human channel. NS1619 was without effect on wild-type worms, on slo-1(js379), or on any of the slo-1 rescue lines. However, NS1619 had marked effects on strains expressing kcnma1 at a concentration equivalent to those previously shown to activate the human channel (Zhang et al., 2003). This led to uncoordinated locomotion, which reduced the mean number of body bends in a dose-dependent manner. It is noteworthy that the behavior of these transgenic worms expressing the human channel on either 10 nM NS1619 or on a high (10 μM) concentration of emodepside was qualitatively similar in that they appeared to be repeatedly “slipping” backward and immediately forward in the same spot. These data are consistent with the suggestion that both these drugs interact with the KCNMA1 channel but that NS1619 has an efficacy 3 orders of magnitude greater than emodepside at the mammalian channel.
Another activator of mammalian BK channels is rottlerin, or mallotoxin (Zakharov et al., 2005; Wu et al., 2007). In contrast to NS1619, this drug inhibited locomotion in transgenic C. elegans expressing either slo-1 or kcnma1 and indeed was observed to inhibit locomotion of wild-type worms. This suggests that this drug is less selective for the C. elegans versus the human channel. The observation that slo-1(js379) mutants were resistant to rottlerin suggests that at least part of its effect is due to an action mediated by the calcium-activated potassium channels, either SLO-1 or KCNMA1.
Although NS1619 had a significant and characteristic effect on the locomotor behavior of worms expressing kcnma1, even at the highest concentration tested, it did not completely inhibit locomotion. This is in contrast to the effect of emodepside on wild-type worms in which a nearly complete inhibition of body bends was observed at micromolar concentrations. A similar observation was made with rottlerin, which also elicited a submaximal inhibition of locomotion. One possible explanation is that emodepside has a high efficacy at the SLO-1 channel that permits a greater effect on locomotion compared with the effects observed either with rottlerin acting through SLO-1 or KCNMA1 or with NS1619 acting through KCNMA1. Further experiments employing electrophysiological analysis are required to resolve this issue.
Finally, we took advantage of the lack of native slo-1 expression in the pharyngeal muscle to ectopically express the channel specifically in this tissue in an otherwise slo-1-null mutant genetic background. Emodepside had no effect on the mutant but inhibited muscle activity in strains expressing slo-1 only in the muscle. Taken together, the results are consistent with the proposal that SLO-1 harbors a selective pharmacophore for emodepside and provides a rationale for further structure-function analysis of this therapeutically important target. In this respect, it is noteworthy that recent progress has been made in the structural characterization of this family of channels (Wu et al., 2010).
The relatively high cost of emodepside is likely to preclude its widespread use in humans; however, our study provides a platform for further drug discovery based on SLO-1 as a target. The value of the experimental approach adopted here is reinforced by the observation that although human proteins have an established capability for expression in commonly used heterologous expression systems, the expression of invertebrate receptor proteins is much less robust. Thus the method we describe, using C. elegans as the expression assay, may provide a complementary approach to more conventional cell-based assays and overcome difficulties encountered in doing a comparative functional analysis across phyla.
Supplementary Material
Acknowledgments
We are grateful to Lawrence Salkoff for provision of the vectors for neuronal and body wall muscle expression of slo-1a. Some C. elegans strains used in this work were provided by the Caenorhabditis elegans Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the Biotechnology and Biological Research Council UK [Grant BB/F009208/1] and by a postgraduate studentship from Bayer Healthcare AG.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.071043.
- NGM
- nematode growth medium
- NCBI
- National Center for Biotechnology Information
- ORF
- open reading frame
- PCR
- polymerase chain reaction
- L4
- larval stage 4
- EPG
- electropharyngeogram
- 5-HT
- 5-hydroxytryptamine (serotonin)
- NS1619
- 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one
- DMSO
- dimethyl sulfoxide
- ANOVA
- analysis of variance
- BK
- large-conductance potassium channel.
Authorship Contributions
Participated in research design: Crisford, O'Connor, v Samson-Himmelstjerna, Walker, Harder, and Holden-Dye.
Conducted experiments: Crisford, Murray, Edwards, Kruger, and Welz.
Contributed new reagents or analytic tools: Harder.
Performed data analysis: Crisford and Holden-Dye.
Wrote or contributed to the writing of the manuscript: Crisford, O'Connor, Edwards, Walker, von Samson-Himmelstjerna, and Holden-Dye.
References
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. (1992) Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9:209–216 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990) Basic local alignment search tool. J Mol Biol 215:403–410 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull K, Cook A, Hopper NA, Harder A, Holden-Dye L, Walker RJ. (2007) Effects of the novel anthelmintic emodepside on the locomotion, egg-laying behaviour and development of Caenorhabditis elegans. Int J Parasitol 37:627–636 [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. (1993) mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261:221–224 [DOI] [PubMed] [Google Scholar]
- Carre-Pierrat M, Grisoni K, Gieseler K, Mariol MC, Martin E, Jospin M, Allard B, Ségalat L. (2006) The SLO-1 BK channel of Caenorhabditis elegans is critical for muscle function and is involved in dystrophin-dependent muscle dystrophy. J Mol Biol 358:387–395 [DOI] [PubMed] [Google Scholar]
- Chiang JT, Steciuk M, Shtonda B, Avery L. (2006) Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J Exp Biol 209:1859–1873 [DOI] [PubMed] [Google Scholar]
- Chiba CM, Rankin CH. (1990) A developmental analysis of spontaneous and reflexive reversals in the nematode. Caenorhabditis elegans. J Neurobiol 21:543–554 [DOI] [PubMed] [Google Scholar]
- Cook A, Franks CJ, Holden-Dye L. (2006) Electrophysiological recordings from the pharynx, in WormBook (C. elegans Research Community ed) pp 1–7, doi: 10.1895/wormbook.1.110.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon J, Andrianakis I, Bull K, Glautier S, O'Connor V, Holden-Dye L, James C. (2009) AutoEPG: software for the analysis of electrical activity in the microcircuit underpinning feeding behaviour of Caenorhabditis elegans. PLoS ONE 4:e8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, et al. (2005) Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37:733–738 [DOI] [PubMed] [Google Scholar]
- Flicek P, Amode MR, Barrell D, Beal K, Brent S, Chen Y, Clapham P, Coates G, Fairley S, Fitzgerald S, Gordon L, Hendrix M, Hourlier T, Johnson N, Kähäri A, Keefe D, Keenan S, Kinsella R, Kokocinski F, Kulesha E, Larsson P, Longden I, McLaren W, Overduin B, Pritchard B, Riat HS, Rios D, Ritchie GR, Ruffier M, Schuster M, Sobral D, Spudich G, Tang YA, Trevanion S, Vandrovcova J, Vilella AJ, White S, Wilder SP, Zadissa A, Zamora J, Aken BL, Birney E, Cunningham F, Dunham I, Durbin R, Fernández-Suarez XM, Herrero J, Hubbard TJ, Parker A, Proctor G, Vogel J, Searle SM., et al. (2011) Ensembl 2011. Nucleic Acids Res 39:D800–D806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, de Silva NR, Olliaro PL, Lazdins-Helds JK, Engels DA, et al. (2010) Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol 40:1–13 [DOI] [PubMed] [Google Scholar]
- Gilleard JS. (2006) Understanding anthelmintic resistance: the need for genomics and genetics. Int J Parasitol 36:1227–1239 [DOI] [PubMed] [Google Scholar]
- Guest M, Bull K, Walker RJ, Amliwala K, O'Connor V, Harder A, Holden-Dye L, Hopper NA. (2007) The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int J Parasitol 37:1577–1588 [DOI] [PubMed] [Google Scholar]
- Harder A, Schmitt-Wrede HP, Krücken J, Marinovski P, Wunderlich F, Willson J, Amliwala K, Holden-Dye L, Walker R. (2003) Cyclooctadepsipeptides-an anthelmintically active class of compounds exhibiting a novel mode of action. Int J Antimicrob Agents 22:318–331 [DOI] [PubMed] [Google Scholar]
- Harder A, von Samson-Himmelstjerna G. (2002) Cyclooctadepsipeptides—a new class of anthelmintically active compounds. Parasitol Res 88:481–488 [DOI] [PubMed] [Google Scholar]
- Holden-Dye L, O'Connor V, Hopper NA, Walker RJ, Harder A, Bull K, Guest M. (2007) SLO, SLO, quick, quick, slow: calcium-activated potassium channels as regulators of Caenorhabditis elegans behaviour and targets for anthelmintics. Invert Neurosci 7:199–208 [DOI] [PubMed] [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. (2008) NCBI BLAST: a better web interface. Nucleic Acids Res 36:W5–W9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Rowe IC, Ashford ML. (1995) NS 1619 activates BKCa channel activity in rat cortical neurones. Eur J Pharmacol 280:215–219 [DOI] [PubMed] [Google Scholar]
- McCobb DP, Fowler NL, Featherstone T, Lingle CJ, Saito M, Krause JE, Salkoff L. (1995) A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am J Physiol 269:H767–H777 [DOI] [PubMed] [Google Scholar]
- Mitchell PH, Bull K, Glautier S, Hopper NA, Holden-Dye L, O'Connor V. (2007) The concentration-dependent effects of ethanol on Caenorhabditis elegans behaviour. Pharmacogenomics J 7:411–417 [DOI] [PubMed] [Google Scholar]
- Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. (1998) Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci 18:70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A. (1993) Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135:385–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen SP, Munch E, Moldt P, Drejer J. (1994) Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol 251:53–59 [DOI] [PubMed] [Google Scholar]
- Rogers CM, Franks CJ, Walker RJ, Burke JF, Holden-Dye L. (2001) Regulation of the pharynx of Caenorhabditis elegans by 5-HT, octopamine, and FMRFamide-like neuropeptides. J Neurobiol 49:235–244 [DOI] [PubMed] [Google Scholar]
- Saeger B, Schmitt-Wrede HP, Dehnhardt M, Benten WP, Krücken J, Harder A, Von Samson-Himmelstjerna G, Wiegand H, Wunderlich F. (2001) Latrophilin-like receptor from the parasitic nematode Haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J 15:1332–1334 [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. (2006) High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7:921–931 [DOI] [PubMed] [Google Scholar]
- Townson S, Freeman A, Harris A, Harder A. (2005) Activity of the cyclooctadepsipeptide emodepside against Onchocerca gutterosa, Onchocerca lienalis and Brugia pahangi (Abstract). Am J Trop Med Hyg 73 (6 Suppl):93 [Google Scholar]
- von Samson-Himmelstjerna G, Harder A, Sangster NC, Coles GC. (2005) Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology 130:343–347 [DOI] [PubMed] [Google Scholar]
- Wang ZW, Saifee O, Nonet ML, Salkoff L. (2001) SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32:867–881 [DOI] [PubMed] [Google Scholar]
- Willson J, Amliwala K, Davis A, Cook A, Cuttle MF, Kriek N, Hopper NA, O'Connor V, Harder A, Walker RJ, Holden-Dye L. (2004) Latrotoxin receptor signaling engages the UNC-13-dependent vesicle-priming pathway in C. elegans. Curr Biol 14:1374–1379 [DOI] [PubMed] [Google Scholar]
- Willson J, Amliwala K, Harder A, Holden-Dye L, Walker RJ. (2003) The effect of the anthelmintic emodepside at the neuromuscular junction of the parasitic nematode Ascaris suum. Parasitology 126:79–86 [DOI] [PubMed] [Google Scholar]
- Wu SN, Wang YJ, Lin MW. (2007) Potent stimulation of large-conductance Ca2+-activated K+ channels by rottlerin, an inhibitor of protein kinase C-delta, in pituitary tumor (GH3) cells and in cortical neuronal (HCN-1A) cells. J Cell Physiol 210:655–666 [DOI] [PubMed] [Google Scholar]
- Wu Y, Yang Y, Ye S, Jiang Y. (2010) Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel. Nature 466:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov SI, Morrow JP, Liu G, Yang L, Marx SO. (2005) Activation of the BK (SLO1) potassium channel by mallotoxin. J Biol Chem 280:30882–30887 [DOI] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. (2003) Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience 122:1003–1011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.