Abstract
Mouse embryonic spinal cord neurons in culture exhibit spontaneous calcium oscillations from day in vitro (DIV) 6 through DIV 10. Such spontaneous activity in developing spinal cord contributes to maturation of synapses and development of pattern-generating circuits. Here we demonstrate that these calcium oscillations are regulated by κ opioid receptors (KORs). The κ opioid agonist dynorphin (Dyn)-A (1–13) suppressed calcium oscillations in a concentration-dependent manner, and both the nonselective opioid antagonist naloxone and the κ-selective blocker norbinaltorphimine eliminated this effect. The KOR-selective agonist (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide (U69593) mimicked the effect of Dyn-A (1–13) on calcium oscillations. A κ-specific peptide antagonist, zyklophin, was also able to prevent the suppression of calcium oscillations caused by Dyn-A (1–13). These spontaneous calcium oscillations were blocked by 1 μM tetrodotoxin, indicating that they are action potential-dependent. Although the L-type voltage-gated calcium channel blocker nifedipine did not suppress calcium oscillations, the N-type calcium channel blocker ω-conotoxin inhibited this spontaneous response. Blockers of ionotropic glutamate receptors, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline and dizocilpine maleate (MK-801), also suppressed calcium oscillations, revealing a dependence on glutamate-mediated signaling. Finally, we have demonstrated expression of KORs in glutamatergic spinal neurons and localization in a presynaptic compartment, consistent with previous reports of KOR-mediated inhibition of glutamate release. The KOR-mediated inhibition of spontaneous calcium oscillations may therefore be a consequence of presynaptic inhibition of glutamate release.
Introduction
Periodic spontaneous network activity is a characteristic feature of the developing nervous system (Blankenship and Feller, 2010). In the traditional model of brain development, it has been proposed that this form of spontaneous neural activity facilitates the refinement of wiring of the nervous system laid out by predetermined genetic programs. Spontaneous events have been observed in a wide range of developing brain structures in vivo, including the cerebral cortex, spinal cord, and retina (Feller, 1999; O'Donovan, 1999). Spontaneous activity in the developing spinal cord contributes to motor neuron path-finding, maturation of synapses, and development of pattern-generating circuits (Blankenship and Feller, 2010). Spontaneous calcium transients have been implicated in regulating plasticity in developing neurons (Spitzer et al., 1995). Although there are basic similarities in the mechanisms responsible for spontaneous network activity in different brain regions, the signaling processes underlying these phenomena may vary with brain structure and developmental stage. Studies in isolated mouse spinal cord-limb preparations suggest that the depolarizing actions of GABA and acetylcholine are responsible for spontaneous network activity at early stages of development, whereas at later stages, they become more dependent on glutamatergic neurotransmission (Hanson and Landmesser, 2003).
Opioid receptor activation in the central nervous system (CNS) has been shown to result in inhibition of neuronal firing and reduction of neurotransmitter release. κ Opioid receptors (KORs) are widely expressed in the CNS and are specifically activated by endogenous opioids derived from prodynorphin (Chavkin et al., 1982). KORs and dynorphin mRNA initially appear during early embryonic development in both mouse and human CNS (Brana et al., 1995; Zhu et al., 1998). Dynorphin has been shown to inhibit excitatory transmission in the CNS either when exogenously applied (Castillo et al., 1996; Simmons and Chavkin, 1996; Iremonger and Bains, 2009) or endogenously released (Wagner et al., 1993; Iremonger and Bains, 2009). Although the molecular mechanism for dynorphin inhibition of excitatory transmission remains to be fully understood, an increasing body of evidence suggests that G-protein-coupled receptor stimulated release of Gβγ subunits directly inhibits the synaptic release machinery (Delaney et al., 2007; Iremonger and Bains, 2009).
Despite numerous reports of dynorphin-mediated depression of synaptic transmission, little is known regarding its role and relevance in CNS development. We found that calcium oscillations driven by excitatory neurotransmission appear at DIV 6 to 8 in spinal cord neurons. We also observed that these spontaneous calcium oscillations in spinal neurons were suppressed through a KOR-dependent mechanism. We further demonstrated that spontaneous calcium oscillations are dependent on glutamatergic neurotransmission and that the majority of the KORs are localized to glutamatergic spinal cord neurons. Although μ and δ opioid receptor-mediated regulation of calcium dynamics has been described, this study represents the first report of KOR regulation of spontaneous calcium oscillations.
Materials and Methods
Materials.
Trypsin, penicillin, streptomycin, horse serum, and soybean trypsin inhibitor were obtained from Atlanta Biologicals (Norcross, GA). Leibovitz medium, neurobasal medium, B27, and anti-synaptophysin were purchased from Invitrogen (Carlsbad, CA). DNase, poly-l-lysine, nor-BNI, DAMGO, DPDPE, naloxone, cytosine arabinoside, and Fluoromount aqueous mounting medium were purchased from Sigma (St. Louis, MO). Pluronic acid F127 and fluo-3 AM were purchased from Invitrogen. VGLUT2 antibody and Cy3-conjugated secondary antibody were purchased from Millipore Bioscience Research Reagents (Temecula, CA). PSD-95 antibody was obtained from Thermo Fisher Scientific (Waltham, MA). The antibody to KOR1 was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). ω-Conotoxin, tetrodotoxin, and nifedipine were purchased from Tocris Bioscience (Ellisville, MO). NBQX, dizocilpine maleate (MK-801), and d-(2R)-Amino-5-phosphonovaleric acid were purchased from Ascent Scientific (Princeton, NJ). Dyn-A (1–13) was purchased from American Peptide (Sunnyvale, CA). Dyn-A (1–17), Dyn-A (2–17), and zyklophin were synthesized as described previously (Patkar et al., 2005). (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide (U69593) was purchased from Sigma-Aldrich (St. Louis, MO).
Neuronal Culture.
Primary cultures of embryonic spinal cord neurons were harvested from embryos of Swiss-Webster mice on embryonic day 14. In brief, pregnant mice were euthanized with CO2, and vertebral columns were extracted from mouse embryos and dissected using sterile fine-tipped forceps to isolate spinal cords. Embryonic cords (10–12) were collected, meninges and dorsal root ganglia were removed from each cord, and cords were minced to 1-mm3 slices. Tissue was again macerated using a fire-polished plugged pipette and digested with 0.025% trypsin for 25 min at 37°C. The cells were then dissociated by two successive trituration and sedimentation steps in soybean trypsin inhibitor and DNase containing isolation buffer, centrifuged, and resuspended in neurobasal medium with 2% B-27, 1% penicillin-streptomycin, and 0.5% l-glutamine, pH 7.4. The cells were strained using a 70-μm cell strainer (Falcon; BD Biosciences Discovery Labware, Bedford, MA) to remove any undigested coarse particles. Cells were then counted using a hemocytometer counting chamber and plated onto poly-l-lysine–coated 96-well (9-mm) clear-bottomed black culture plates at a density of 0.8 × 106 cells/ml in Leibovitz (L-15) medium supplemented with glucose, 2% B-27, 2% horse serum, 0.5% l-glutamine, 1% penicillin-streptomycin, and 7.5% sodium bicarbonate. Culture plates were incubated at 37°C in a 5% CO2 and 95% humidity atmosphere. Cytosine arabinoside (10 μM) was added to the culture medium on day 2 after plating to prevent proliferation of non-neuronal cells. The culture medium was changed twice weekly and replaced with serum-free neurobasal medium. Cultures from DIV 6 to 8 were used for calcium oscillation experiments. For Western blotting, cells were grown in a 12-well plate at 1.2 × 106 cells/ml, and cell lysates were collected from cultures from DIV 1 to 9. For immunocytochemistry experiments, cells were grown on poly-l-lysine–coated square coverslips (22 mm2) inserted in to 6-well plates at 106 cells/ml. All animal use protocols were approved by the Institutional Animal Care and Use Committee of Creighton University.
Intracellular Ca2+ Monitoring.
Embryonic spinal cord neurons grown in 96-well plates (1.2 × 105 cells per well) were used for intracellular Ca2+ concentration ([Ca2+]i) measurements. The growth medium was removed and replaced with dye-loading medium (100 μl/well) containing 4 μM fluo-3 AM and 0.04% Pluronic acid F127 in Locke's buffer (154 mM NaCl, 5.6 mM KCl, 1.0 mM MgCl2, 2.3 mM CaCl2, 8.6 mM HEPES, 5.6 mM glucose, and 0.1 mM glycine, pH 7.4). After 1 h of incubation in dye-loading medium, the neurons were washed four times in fresh Locke's buffer (200 μl/well, 22°C) using an automated microplate washer (BioTek Instruments, Winooski, VT) and then transferred to a FLEXStation II (Molecular Devices, Sunnyvale, CA) benchtop scanning fluorometer chamber. The final volume of Locke's buffer in each well was 150 μl. Fluorescence measurements were carried out at 37°C. The neurons were excited at 488 nm and Ca2+-bound fluo-3 emission was recorded at 538 nm at 1.2-s intervals. After recording basal calcium oscillations (300–500 s), a 4× concentration of test compounds was added to the cells at a rate of 26 μl/s, yielding a final volume of 200 μl/well; the fluorescence was monitored for an additional period of 300–500 s.
Immunoblotting.
Immunoblot analysis was performed in cells plated in 12-well plates (1.2 × 106 cells per well) and grown for 1 to 9 days after plating (DIV 1–9). After washing with ice-cold Locke's buffer, cells were harvested in ice-cold lysis buffer containing 50 mM Tris, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Tergitol-type NP-40, 0.1% SDS, 2 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and 25 mM β-glycerophosphate. Phenylmethylsulfonyl fluoride (1 mM) and 1× protease inhibitor cocktail were then added, and the lysate was incubated for 15 min at 4°C. Cell lysates were then sonicated and centrifuged at 12,000g for 15 min at 4°C. The supernatant was assayed by the Bradford method to determine protein content. Equal amounts of protein were mixed with the Laemmli sample buffer and boiled for 5 min. The samples were loaded onto a 12% SDS-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane by electroblotting. The membranes were blocked in TBST (20 mM Tris, 150 mM NaCl, and 0.1% Tween 20) with 5% bovine serum albumin for 1 h at room temperature. After blocking, membranes were incubated overnight at 4°C in primary antibody diluted in TBST containing 5% bovine serum albumin. The blots were washed and incubated with the secondary antibody conjugated with horseradish peroxidase for 2 h, washed three times in TBST, and exposed with ECL Plus (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) for 4 min. Blots were exposed to Hyperfilm (GE Healthcare) and developed. Immunoblot densitometry data were obtained using MCID Basic 7.0 Software (Imaging Research, Inc. Cambridge, UK).
Subcellular Fractionation.
Subcellular fractionation was performed as described previously with minor modifications (Toda et al., 2006). Lysates were prepared from DIV 8 spinal neuronal culture in ice-cold buffer containing 0.32 M sucrose, 10 mM HEPES, and 1 mM EDTA, pH 7.4. Homogenates were cleared three times at 1000g for 10 min to remove nuclear fraction. The resulting supernatants were concentrated twice at 10,000g for 20 min to obtain crude synaptosome fraction, and subsequently were lysed hypo-osmotically in 1% Triton X-100-containing buffer with gentle rotation for 1 h at 4°C. This lysate was centrifuged at 16,000g for 30 min to obtain the PSD-95 enriched fraction (LP1). The resulting supernatant was then centrifuged at 200,000g for 90 min to obtain the crude synaptic vesicle-enriched fraction (LP2). The enrichment of LP1 and LP2 fractions were verified by immunoblotting, respectively, for PSD-95 and synaptophysin.
Immunocytochemistry (Double Immunofluorescence).
We performed a double immunofluorescence technique using antibodies to PSD-95 and synaptophysin to document the role of synapse formation in the development of spontaneous calcium oscillations. We also performed double immunofluorescence with antibodies to the vesicular glutamate transporter-2 (VGlut2) and KOR to demonstrate the localization of KOR in glutamatergic neurons. Cells (DIV 7) grown on 22-mm2 coverslips inserted in six-well plates were used. The cells were washed with phosphate-buffered saline (PBS) (37°C, pH 7.4) and fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. After fixing, cells were washed, blocked, and permeabilized by incubation for 30 min with PBS containing 6% goat serum and 0.15% Triton X-100. Permeabilized cells were blocked in 3% serum and incubated with primary antibodies (anti-synaptophysin and anti-PSD-95 or anti-VGlut2 and anti-KOR) in 0.15% Triton X-100 overnight at 4°C. The cells were washed, blocked, and incubated with Cy-3 and fluorescein isothiocyanate-conjugated secondary antibodies in 0.15% Triton X-100 for 2 h at room temperature in the dark. Cells were washed in PBS twice, and coverslips were mounted on glass slides using Fluoromount. Confocal images were captured using a spinning-disk confocal microscope (IX 71; Olympus, Tokyo, Japan) and a digital camera (ORCA-ER; Hamamatsu Corporation, Bridgewater, NJ).
Results
Ontogeny of Spinal Neuron Calcium Oscillations.
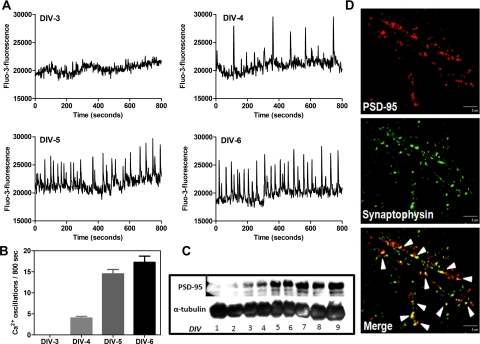
Fluo-3 AM–loaded spinal neuron cultures were scanned in a FLEX Station II from DIV-1 onward to study the ontogeny of spontaneous calcium oscillations. Spontaneous calcium oscillations were not detectable in spinal neurons up to DIV-3. Spinal cord cultures exhibited spontaneous calcium oscillations as early as DIV-4, and these calcium transients became more frequent and robust by DIV-6 (Fig. 1, A and B). The calcium oscillations persisted through DIV-11. As previously demonstrated for cerebral cortical neurons (Dravid and Murray, 2004), the Ca2+ oscillations were highly synchronized in populations of neurons in culture, and their mean frequency was 1.88 ± 0.01/min. The amplitude of the oscillations was somewhat variable among different cultures with basal amplitudes ranging between 3000 and 8000 fluorescence units. The synchronized nature of Ca2+ oscillations suggested that network activity played a key role in the generation of the transients. To examine the relationship between spontaneous calcium oscillations and synapse formation, we investigated the ontogeny of PSD-95 expression in spinal neuron cultures. We also examined the formation of synapses in spinal neurons using synaptophysin as a presynaptic marker and PSD-95 as a postsynaptic marker. PSD-95 is a multidomain postsynaptic density protein that clusters glutamate receptors and associated signaling complexes; hence, PSD-95 content is a primary determinant of the size and strength of synapses. Western blotting experiments with cell lysates collected from DIV 1 to 9 spinal cord neurons showed that the ontogeny for PSD-95 expression paralleled that of spontaneous calcium oscillations (Fig. 1C). PSD-95 was not detectable on DIV 1 or 2; it appeared on DIV 3, and its expression continued to increase from DIV 4 to 9 (Fig. 1C). In addition to the expression of PSD-95, using a double immunofluorescence technique, we documented the development of synapse formation in spinal neuron cultures. Synapses were detected as yellow puncta indicating the colocalization of PSD-95 and synaptophysin in DIV 8 spinal neurons (Fig. 1D). These data are consistent with the hypothesis that spontaneous calcium oscillations require the development of neuronal connectivity and formation of functional synapses. Given that the spontaneous calcium oscillations became robust by DIV 6, DIV 6 through DIV 8 spinal cultures were employed for further studies.
Fig. 1.
Ontogeny of spinal neuron calcium oscillations. A, spontaneous calcium oscillations emerged on DIV 4 and became robust by DIV 6. Traces shown are from a representative experiment repeated six times. B, summary histogram representing the ontogeny of spinal neuron calcium oscillations. C, ontogeny of spinal neuron calcium oscillations paralleled the ontogeny of PSD-95 expression. Data shown are from a representative experiment repeated three times. D, confocal images show immunolabeling for PSD-95 (red), synaptophysin (green), and the regions of colocalization of PSD-95 and synaptophysin as puncta (yellow) to indicate synapses (scale bar, 10 μm) in DIV-8 spinal neurons.
DynA (1–13)-Induced Suppression of Spontaneous Calcium Oscillations in Spinal Neurons Is Opioid Receptor-Mediated.
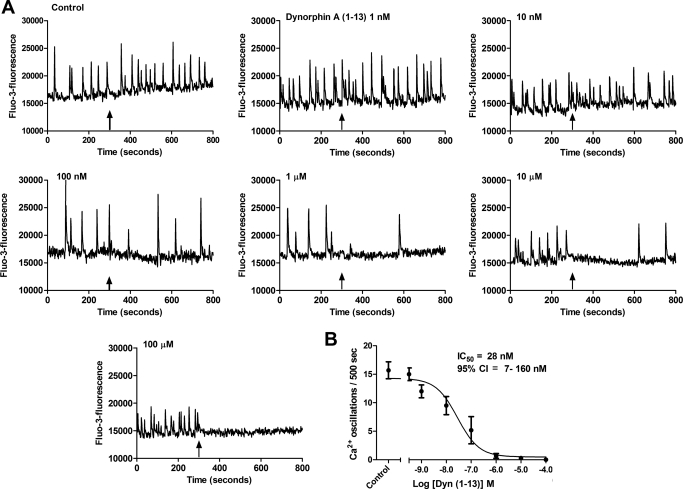
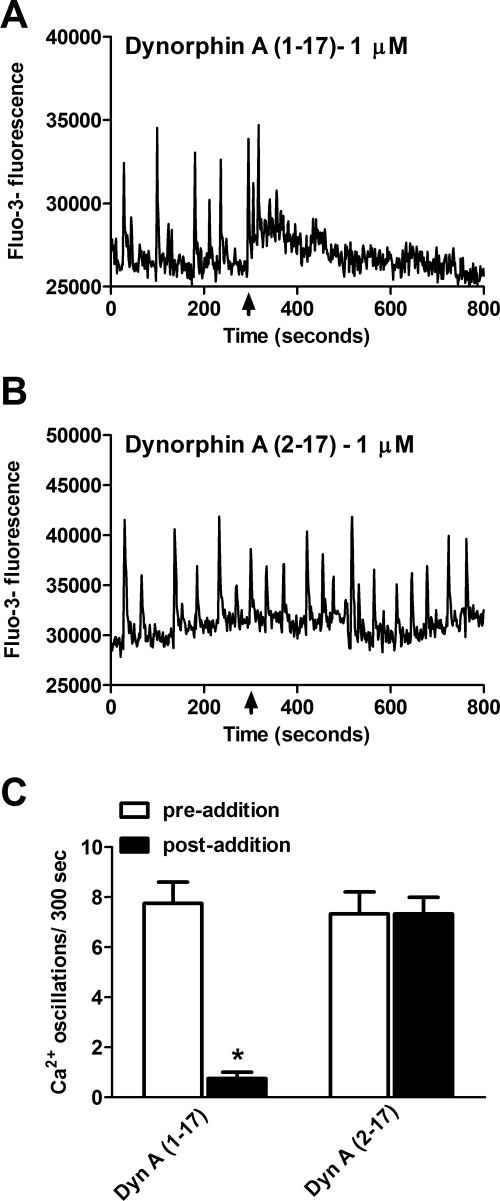
Dyn-A (1–13) is a fragment of the full-length endogenous peptide Dyn-A (1–17) that acts as a κ-selective ligand. Spinal cord neurons were treated with either Locke's buffer (control) or varying concentrations (1 nM–100 μM) of Dyn-A (1–13) to examine its effects on spontaneous calcium oscillations. Dyn-A (1–13) produced a concentration-dependent suppression of spontaneous calcium oscillations in spinal neurons (Fig. 2A). Nonlinear regression analysis of Dyn-A (1–13) concentration-response data yielded an IC50 of 28 nM (7–160 nM, 95% CI) (Fig. 2B). To further evaluate the pharmacologic signature of the receptor mediating dynorphin-induced suppression of calcium oscillations, we compared the effects Dyn-A (1–17) and Dyn-A (2–17) on calcium oscillations (Fig. 3). Inasmuch as the first amino acid (tyrosine) is essential for imparting opioid receptor activity (Chavkin and Goldstein, 1981), the truncated dynorphin analog Dyn-A (2–17) is inactive at opioid receptors. A 1 μM concentration of Dyn-A (1–17) produced an almost complete suppression of calcium oscillations, whereas 1 μM Dyn-A (2–17) was without effect (Fig. 3). These data are therefore consistent with an opioid receptor mediation of the Dyn-A (1–13)-induced suppression of calcium oscillations in spinal neurons.
Fig. 2.
Dynorphin A (1–13) suppresses spinal neuron calcium oscillations. A, Dyn-A (1–13) suppressed spontaneous calcium oscillations in a concentration-dependent manner. Arrow indicates the time of addition of drug. B, nonlinear regression analysis of the concentration-response data for Dyn-A (1–13) suppression of calcium oscillations yielded an IC50 of 28 nM (95% CI, 7–160 nM). Each point represents the mean ± S.E.M. (n = 8) of oscillations after addition of Dyn-A (1–13). Experiment was repeated eight times in independent cultures.
Fig. 3.
Dyn-A (1–13) induced suppression of calcium oscillations is opioid receptor-mediated. A and B, 1 μM Dyn-A (1–17) produced a suppression of calcium oscillations, whereas 1 μM Dyn-A (2–17) did not affect calcium oscillations. C, summary histogram of data represent the mean ± S.E.M. (n = 4) of calcium oscillations (for 300 s) before and after compound addition. *, p < 0.01, paired t test between the average number of oscillations before and after the addition of Dyn-A (1–17) 1 μM and Dyn-A (2–17) 1 μM.
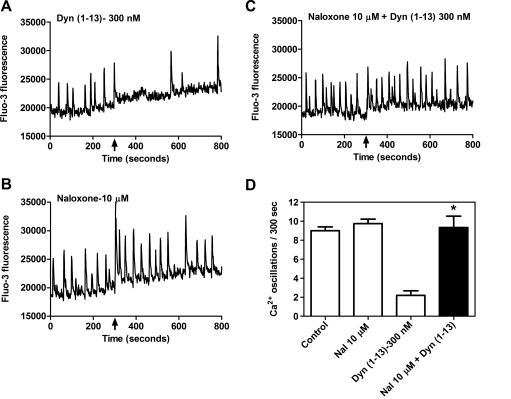
To further establish the identity of the opioid receptor involved in the effect of Dyn-A (1–13) on spontaneous calcium oscillations, we tested the nonselective opioid antagonist naloxone (10 μM) against Dyn-A (1–13) (300 nM) challenge (Fig. 4). Naloxone by itself had no effect on spontaneous calcium oscillations (Fig. 4B); however, this pretreatment completely abolished the ability of Dyn-A (1–13) to suppress calcium oscillations (Fig. 4C). The calcium oscillation frequency in the neurons pretreated with naloxone alone did not differ significantly from the control level (Fig. 4D). The naloxone antagonism of Dyn-A (1–13)-mediated suppression of calcium oscillations provided additional evidence for opioid receptor involvement in this effect.
Fig. 4.
Dyn-A (1–13)-induced suppression of calcium oscillations is opioid receptor-mediated. A, 300 nM Dyn-A (1–13) produced a suppression of spinal neuron calcium oscillations. B, the nonselective opioid antagonist naloxone (10 μM) alone had no effect on spontaneous calcium oscillations. C, pretreatment with naloxone (10 μM) eliminated Dyn-A (1–13)-induced suppression of calcium oscillations. D, summary histogram of data represent the mean ± S.E.M. (n = 5) of calcium oscillations (for 300 s) after compound addition. *, p < 0.01, unpaired t test between 300 nM Dyn-A (1–13) and Dyn-A (1–13) + naloxone (10 μM).
Dyn-A (1–13)-Induced Suppression of Spinal Neuron Calcium Oscillations Is Mediated by κ-Opioid Receptors.
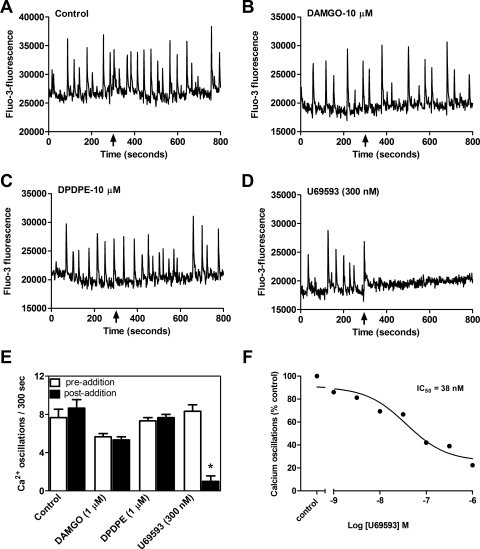
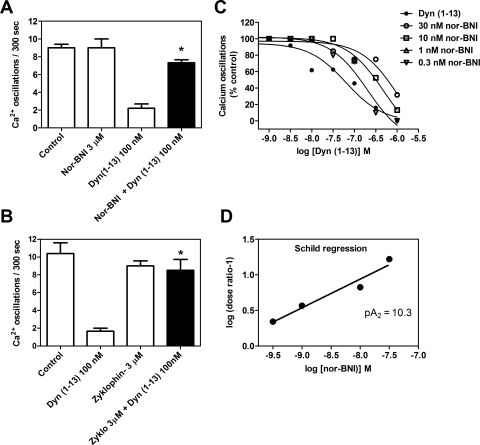
Next, we assessed the opioid receptor subtype involved in Dyn-A (1–13)-induced suppression of spinal neuron calcium oscillations. Inasmuch as Dyn-A (1–13) displays substantial affinity for μ and δ opioid receptors (Arttamangkul et al., 1995), we tested selective ligands for μ (DAMGO), δ (DPDPE), and κ (U69593) receptors (Fig. 5, B–D). At a concentration of 10 μM DAMGO and DPDPE were without effect (Fig. 5, B and C). In contrast, U69593 (300 nM) was able to completely suppress spinal neuron calcium oscillations (Fig. 5D). An evaluation of the concentration dependence of U69593 suppression of calcium transients revealed an IC50 value of 38 nM (10–135 nM; 95% CI) (Fig. 5F). These findings ruled out the possibility that the observed suppression of calcium oscillations produced by Dyn-A (1–13) involved either μ or δ opioid receptors. To confirm the κ opioid receptor involvement in the actions of Dyn-A (1–13), we used nor-BNI, a specific small-molecule antagonist of κ opioid receptor (Fig. 6, A, C, and D). Initially, nor-BNI was tested at a 3 μM concentration for its ability to block the suppression of calcium oscillations induced by 300 nM Dyn-A (1–13). Pretreatment with nor-BNI alone did not influence spinal neuron calcium oscillations; however, this concentration of nor-BNI abrogated the inhibitory response to Dyn-A (1–13). Next, we generated a Schild plot derived from Dyn-A (1–13) concentration-response experiments performed in the absence and presence of a series of nor-BNI concentrations. The resultant Schild regression depicted in Fig. 6D revealed a nor-BNI pA2 value of 10.3 ± 0.12, which is in close agreement with that of a previous study (Birch et al., 1987).
Fig. 5.
κ, But not μ or δ, opioid agonists inhibit spinal neuron spontaneous calcium oscillations. A, control. DAMGO (μ agonist; B) and DPDPE (δ agonist; C) did not affect spontaneous calcium oscillations. D, U69593 (κ agonist) induced suppression of calcium oscillations. E, summary histogram of the data to show that activation of KOR mediates suppression of spinal neuron calcium oscillations. Data represent the mean ± S.E.M. (n = 5) of calcium oscillations before (empty bars) and after compound addition (filled bars). Only the KOR agonist, U69593, suppressed calcium oscillations. *, p < 0.05, paired t test between pre- and postaddition oscillations for U69593. DAMGO and DPDPE did not affect calcium oscillations. F, nonlinear regression analysis of the concentration-response data for U69593-induced suppression of calcium oscillations yielded an IC50 of 38 nM.
Fig. 6.
Summary of κ receptor antagonist block of Dyn-A (1–13) inhibition of calcium oscillations (A and B) and Schild analysis of nor-BNI antagonism (C and D). Data in A and B are the mean ± S.E.M. of calcium oscillations remaining after addition of respective compounds. Pretreatment with nor-BNI or zyklophin eliminated dynorphin-induced suppression of calcium oscillations [n = 5 for nor-BNI experiment; n = 6 for zyklophin experiment; *, p < 0.01, unpaired t test between Dyn-A (1–13) and nor-BNI + Dyn-A (1–13) 300 nM; Dyn-A (1–13) and zyklophin + Dyn-A (1–13) 100 nM]. C, dose-response curves for Dyn-A (1–13) in the absence and presence of fixed concentrations of nor-BNI. D, Schild regression for nor-BNI antagonism of Dyn-A (1–13). Each data point represents the mean of three different experiments.
In addition to the small-molecule antagonist nor-BNI, we also tested a selective peptide antagonist of κ opioid receptors, zyklophin (Aldrich and McLaughlin, 2009), which is [N-benzyl-Tyr1,cyclo(d-Asp5,Dap8)] Dyn A-(1–11) amide (Fig. 6B). A 3 μM concentration of zyklophin completely eliminated the ability of Dyn-A (1–13) (100 nM) to suppress calcium oscillations. Together, these observations underscore the involvement of κ opioid receptors in the regulation of spontaneous calcium oscillations in spinal neurons.
Spontaneous Spinal Neuron Calcium Oscillations Are Dependent on Propagation of Action Potentials and N-Type Calcium Channels.
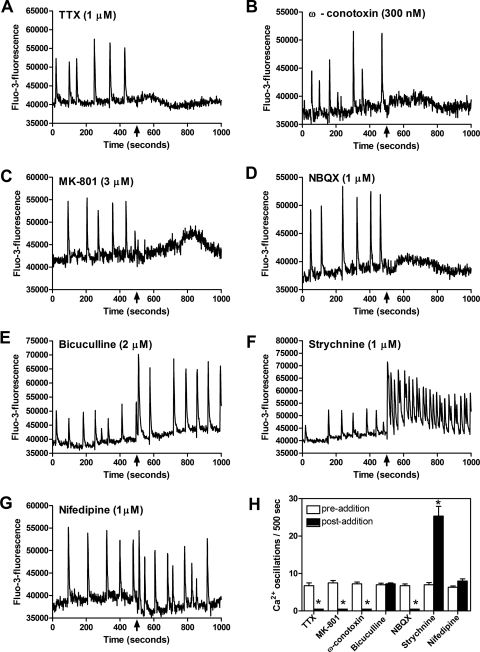
To determine whether spontaneous spinal neuron calcium oscillations were action potential dependent, we used tetrodotoxin (TTX; 1 μM), a voltage-gated sodium channel blocker. In agreement with an earlier report using cerebrocortical neurons (Dravid and Murray, 2004), TTX caused a complete elimination of the spontaneous calcium oscillations, indicating that they are dependent on action potentials generated by activation of voltage-gated sodium channels (Fig. 7A). Action potential triggered neurotransmitter release involves both synchronous (phasic) and asynchronous components at select nerve terminals. Asynchronous release can complement phasic release and may contribute to postsynaptic responses under certain conditions (Hefft and Jonas, 2005; Iremonger and Bains, 2007). Asynchronous release from presynaptic nerve endings relies heavily on Ca2+ influx through N-type Ca2+ channels. The N-type Ca2+ channel antagonist ω-conotoxin at 300 nM inhibited spontaneous calcium oscillations (Fig. 7B), whereas neither the L-type voltage-gated calcium channel blocker nifedipine (1 μM; Fig. 7G) nor the P/Q-type calcium channel blocker ω-agatoxin (100 nM; data not shown) affected spontaneous calcium transients. The suppression of calcium oscillations by ω-conotoxin was concentration-dependent with an IC50 of 120 nM (7 nM–2 μM, 95% CI) (data not shown). These data indicate that spontaneous calcium oscillations in spinal neurons are dependent on action potential propagation and Ca2+ influx through N-type calcium channels.
Fig. 7.
Spontaneous calcium oscillations are dependent on action potentials (A) and N-type calcium channels (B) and are driven by glutamatergic transmission (C and D), whereas the inhibitory influence of GABA and glycine are revealed with the antagonists bicuculline and strychnine, respectively (E and F). Spontaneous calcium oscillations are not dependent on L-type calcium channels (G). H, summary histogram of these data. Data are from a representative experiment performed in quadruplicate. Data represent the mean ± S.E.M. (n = 4) of calcium oscillations before (empty bars) and after (filled bars) compound addition. Addition of TTX, MK-801, ω-conotoxin, and NBQX significantly suppressed the occurrence of calcium oscillations, whereas addition of strychnine significantly increased the frequency of calcium oscillations. *, p < 0.05, paired t test between pre- and postaddition oscillations for each compound.
Spontaneous Spinal Neuron Calcium Oscillations Are Dependent on Glutamatergic Neurotransmission.
As the principal excitatory neurotransmitter in the central nervous system, we next sought to determine whether spontaneous calcium oscillations were driven by glutamatergic neurotransmission in spinal neurons. To assess the role of glutamate receptors, we used the noncompetitive antagonist of NMDA receptors, MK-801 (3 μM), and the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor antagonist, NBQX (1 μM; Fig. 7, C and D). Spontaneous calcium oscillations were completely abolished by addition of either of these glutamate receptor antagonists. A similar elimination of calcium transients was produced by the competitive NMDA receptor antagonist APV (100 μM) (data not shown). The complete suppression of calcium oscillations by the glutamate receptor antagonists is consistent with glutamatergic neurotransmission serving as the primary driver for the spontaneous activity. Bicuculline (GABAA antagonist) and strychnine (glycine receptor antagonist) increased the amplitude and frequency of calcium oscillations, respectively (Fig. 7, E and F). The effect of bicuculline and strychnine on spontaneous calcium oscillations suggests that in DIV 8 spinal neurons, the chloride gradient is similar to that of mature neurons, allowing GABA and glycine to exert an inhibitory tone. These data indicate that in DIV 8 spinal neurons, spontaneous calcium oscillations are a manifestation of excitatory neurotransmission driven primarily by glutamate.
Colocalization of KOR and Vesicular Glutamate Transporter-2 in Spinal Cord Neurons.
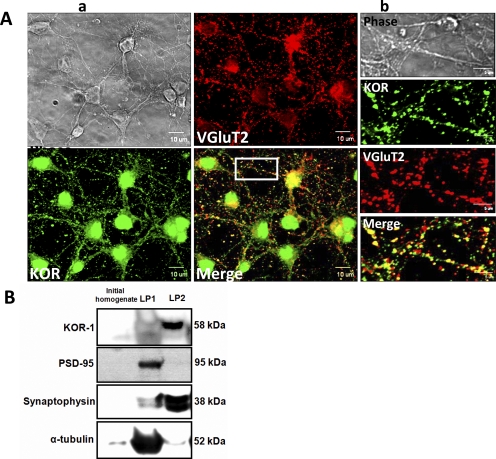
In the spinal cord, glutamate is the transmitter in all primary afferents and in most intraspinal neurons that project to the brain (Broman, 1994). There are also glutamatergic interneurons in the spinal cord and antibodies against the vesicular glutamate transporters, VGlut1 and VGlut2, have been demonstrated to label these glutamatergic neurons (Todd et al., 2003). We used an antibody to VGlut2 to quantify the fraction of glutamatergic neurons in DIV 8 embryonic spinal cord cultures. Approximately 60% of the total neuronal population was labeled with the VGlut2 antibody indicating the relative abundance of glutamatergic neurons in this culture system (Fig. 8A). More than 90% of the spinal cord neurons were labeled with anti-KOR and a substantial proportion of the KOR immunolabeled neurons were colocalized with VGlut2 suggesting the prevalent expression of KORs in glutamatergic neurons. This localization of KOR in embryonic spinal glutamatergic neurons is consistent with a presynaptic modulation of glutamate release.
Fig. 8.
KORs are primarily localized presynaptically in embryonic spinal neurons. A, a large proportion of KORs are colocalized with VGlut2 in spinal neurons, demonstrating the localization of KORs in glutamatergic neurons. a, confocal images show phase, VGlut2 (red), and KOR (green) immunolabeling separately with regions of colocalization that appear as yellow puncta (scale bar, 10 μm). b, boxed region shown in higher magnification (scale bar, 5 μm) separately and overlaid. B, subcellular fractionation of embryonic spinal neurons in culture shows the relative abundance of KORs in the crude synaptic vesicle fraction (LP2 fraction). Data are from a representative experiment performed.
Subcellular Fractionation Demonstrates the Presynaptic Localization of KORs.
The degree of enrichment of LP1 and LP2 fractions separated by subcellular fractionation was confirmed by immunoblotting with anti-PSD-95 and antisynaptophysin antibodies, respectively. PSD-95 was enriched in LP1 fraction characteristic of the postsynaptic component and synaptophysin relative abundance was elevated in the LP2 fraction characteristic of the presynaptic fraction. Densitometric analysis of immunoblots from subcellular fractionation showed the relative abundance of KORs in crude synaptic vesicle fraction (LP2) as opposed to the PSD-95 enriched fraction (LP1) (Fig. 8B).
An additional functional consequence of exposure of spinal neurons to the KOR ligand U69593 was the suppression of neurite outgrowth (Supplemental Fig. 1). Given the known trophic actions of endogenous glutamate (Mattson, 2008), we suspect that the suppression of neurite extension involves κ opioid receptor inhibition of glutamatergic transmission. These preliminary data warrant further investigation to explore the role of κ opioids in neurite outgrowth and development.
Discussion
We have demonstrated that mouse embryonic spinal cord neurons in culture exhibit spontaneous calcium oscillations. These spontaneous calcium oscillations are driven primarily by glutamatergic neurotransmission. An interesting feature of our results is that κ opioid receptors exert an inhibitory influence on spontaneous calcium oscillations in spinal cord neurons. We found that these spontaneous calcium oscillations were inhibited by exogenously applied κ agonists [Dyn-A (1–13) and U69593]. Opioid regulation of spontaneous calcium oscillations has been previously reported but with discordant results. Spontaneous calcium oscillations exhibited by organotypic cultures of embryonic rat hippocampi were shown to be enhanced by the application of the μ opioid agonist DAMGO (Przewlocki et al., 1999), whereas DAMGO was shown to inhibit this phenomenon in GH3 cells expressing only μ opioid receptors (GH3MOR) (Charles et al., 2003). However, in GH3MORDOR cells (expressing μ and δ opioid receptors in a 1:11 ratio), DAMGO's response changed from inhibitory to excitatory, and this was attributed to the differential coupling of μ opioid receptors from Gi to Gq as a result of preferential δ opioid receptor occupancy of Gi proteins (Charles et al., 2003). In a previous report, the δ-selective ligand DPDPE was shown to inhibit spontaneous calcium transients in cells expressing μ and δ opioid receptors (Piros et al., 2000). Here, we provide compelling evidence to demonstrate that the opioid receptor subtype involved in suppression of spontaneous calcium oscillations in spinal neurons is the κ receptor. Spinal neuron spontaneous calcium oscillations were not affected by DAMGO or DPDPE. The inhibitory effect of κ agonists on calcium oscillations, moreover, was completely blocked by the selective κ receptor antagonists nor-BNI and zyklophin.
Both KOR and μ opioid receptor have been reported to be present from early fetal life in the rat and human brain and spinal cord (Xia and Haddad, 1991). Our results indicate that KORs become functional during embryonic development of spinal cord. This is in agreement with a report that KOR expression in spinal cord appears in the ventral cord at embryonic day 15.5 and extends to the dorsal cord by embryonic day 17.5 (Zhu et al., 1998). Given the evidence supporting the role of endogenous opioids in early development of the CNS, opioid receptors may be required for maturation of synapses and fine tuning of spinal circuitry (Hauser et al., 1987). Although no tonic κ receptor-mediated inhibition of calcium oscillations was observed at this developmental stage in vitro, the possibility of endogenous dynorphins regulating oscillations in vivo cannot be ruled out. Several lines of evidence indicate that spontaneous calcium oscillations exhibited by DIV 6 to 8 embryonic spinal cord neurons were generated by network synaptic activity and are dependent on excitatory neurotransmission. TTX inhibited spontaneous calcium oscillations, suggesting their dependence on action potentials. Calcium oscillations were also blocked by the NMDA receptor antagonist MK-801 and the α-amino-3-hydroxy-5-methyl-4- isoxazole-propionic acid receptor antagonist NBQX, suggesting a dependence on glutamatergic neurotransmission. These findings are consistent with previous reports in cultured cerebrocortical neurons (Murphy et al., 1992; Dravid and Murray, 2004), organotypic hippocampal slices (Przewlocki et al., 1999), and organotypic spinal cord slices (Sibilla et al., 2009). The observation that ω-conotoxin inhibited spontaneous calcium oscillations suggests that this phenomenon was also dependent on calcium influx through N-type calcium channels. Synapses between primary sensory afferents and intrinsic dorsal horn neurons are primarily glutamatergic (Jessell et al., 1986), and N-type calcium channels contribute to glutamate release at these synapses. These findings reinforce the dependence of spontaneous calcium oscillations on glutamatergic transmission. By blocking N-type calcium channels, ω-conotoxin may inhibit glutamate release and suppress spontaneous calcium oscillations. In contrast, L-type voltage-gated calcium channels did not seem to play a role in the generation of spontaneous calcium oscillations. This finding is consistent with other studies monitoring spontaneous calcium oscillations in the presence of physiological concentrations of Mg2+ (Dravid and Murray, 2004). Previous reports examining calcium oscillations in organotypic spinal cord slices have also demonstrated that neither L- nor T-type channels were responsible for calcium oscillations (Sibilla et al., 2009). Our results are consistent with these earlier findings and point to N-type calcium channel involvement in the generation of spontaneous calcium oscillation activity triggered by the release of glutamate.
Opioids are known to depress synaptic neurotransmission either by inhibition of presynaptic calcium channels (Macdonald and Werz, 1986) or by activation of postsynaptic potassium channels (North et al., 1987; Wimpey and Chavkin, 1991), thereby hyperpolarizing neurons. A recent report, however, ruled out the involvement of either calcium or potassium channels in κ receptor-mediated inhibition of dopamine release (Ford et al., 2007). Another study has moreover proposed that, once activated, κ opioid receptors directly modulate presynaptic release machinery to reduce vesicular glutamate release (Iremonger and Bains, 2009). Consistent with the presynaptic mechanism of action reported for KORs, we found that KOR expression was enriched in spinal glutamatergic neurons. From both colocalization and subcellular fractionation studies, it is evident that KORs are present in spinal glutamatergic neurons with a relative abundance in the presynaptic compartment. This localization of KORs in embryonic spinal neurons is consistent with the ability of KOR ligands to modulate neurotransmitter release via a presynaptic mechanism of action (Rusin et al., 1997; Ford et al., 2007; Iremonger and Bains, 2009).
A compelling body of evidence has implicated the inhibitory effects of exogenously applied dynorphin on excitatory neurotransmission (Castillo et al., 1996; Simmons and Chavkin, 1996; Iremonger and Bains, 2009). Given the requirement of glutamatergic neurotransmission for spontaneous calcium oscillations, and the evidence that KORs are localized presynaptically in spinal glutamatergic neurons, a κ opioid receptor inhibition of excitatory synaptic transmission represents the most parsimonious explanation for KOR regulation of spinal neuron calcium oscillations.
Significance.
An increased production of the opioid peptide dynorphin has been reported after spinal cord trauma, and this has been implicated in neuropathic pain and pathophysiology associated with spinal injury (for review, see Hauser et al., 2005). Recovery from such injuries is thought to recapitulate ontogeny and is facilitated by unmasking of subthreshold inputs to the surrounding healthy tissue (for review, see Murphy and Corbett, 2009). This has been proposed to result from decreased inhibitory transmission, increased glutamate transmission, and increased spontaneous activity, thus promoting rewiring of lost synaptic connections. Accumulation of dynorphin in a spinal trauma site could therefore produce an inhibition of spontaneous activity and impede rewiring of synaptic connections.
Supplementary Material
Acknowledgments
We thank Bridget Leuschen for support in preparing spinal cord neuronal cultures.
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grant R01-DA023924].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.071456.
- CNS
- central nervous system
- KOR
- κ opioid receptors
- DIV
- day(s) in vitro
- nor-BNI
- norbinaltorphimine
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- DPDPE
- [d-Pen2,d-Pen5]-enkephalin
- NMDA
- N-methyl d-aspartate
- AM
- acetoxymethyl ester
- PSD
- postsynaptic density
- NBQX
- 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
- MK-801
- (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo-[a,d] cyclohepten-5,10-imine hydrogen (dizocilpine) maleate
- Dyn
- dynorphin
- U69593
- (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide
- TBST
- Tris-buffered saline-Tween 20
- PBS
- phosphate-buffered saline
- Cy-3
- cyanine 3
- TTX
- tetrodotoxin
- CI
- confidence interval.
Authorship Contributions
Participated in research design: Kelamangalath, George, Aldrich, and Murray.
Conducted experiments: Kelamangalath, Dravid and George.
Performed data analysis: Kelamangalath, Dravid and George.
Wrote or contributed to writing of the manuscript: Kelamangalath, Aldrich, and Murray.
References
- Aldrich JV, McLaughlin JP. (2009) Peptide kappa opioid receptor ligands: potential for drug development. AAPS J 11:312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Murray TF, DeLander GE, Aldrich JV. (1995) Synthesis and opioid activity of conformationally constrained dynorphin A analogues. 1. Conformational constraint in the “message” sequence. J Med Chem 38:2410–2417 [DOI] [PubMed] [Google Scholar]
- Birch PJ, Hayes AG, Sheehan MJ, Tyers MB. (1987) Norbinaltorphimine: antagonist profile at kappa opioid receptors. Eur J Pharmacol 144:405–408 [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. (2010) Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 11:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brana C, Charron G, Aubert I, Carles D, Martin-Negrier ML, Trouette H, Fournier MC, Vital C, Bloch B. (1995) Ontogeny of the striatal neurons expressing neuropeptide genes in the human fetus and neonate. J Comp Neurol 360:488–505 [DOI] [PubMed] [Google Scholar]
- Broman J. (1994) Neurotransmitters in subcortical somatosensory pathways. Anat Embryol (Berl) 189:181–214 [DOI] [PubMed] [Google Scholar]
- Castillo PE, Salin PA, Weisskopf MG, Nicoll RA. (1996) Characterizing the site and mode of action of dynorphin at hippocampal mossy fiber synapses in the guinea pig. J Neurosci 16:5942–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Mostovskaya N, Asas K, Evans CJ, Dankovich ML, Hales TG. (2003) Coexpression of delta-opioid receptors with micro receptors in GH3 cells changes the functional response to micro agonists from inhibitory to excitatory. Mol Pharmacol 63:89–95 [DOI] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. (1981) Specific receptor for the opioid peptide dynorphin: structure-activity relationships. Proc Natl Acad Sci USA 78:6543–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. (1982) Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215:413–415 [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P. (2007) Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 56:880–892 [DOI] [PubMed] [Google Scholar]
- Dravid SM, Murray TF. (2004) Spontaneous synchronized calcium oscillations in neocortical neurons in the presence of physiological [Mg2+]: involvement of AMPA/kainate and metabotropic glutamate receptors. Brain Res 1006:8–17 [DOI] [PubMed] [Google Scholar]
- Feller MB. (1999) Spontaneous correlated activity in developing neural circuits. Neuron 22:653–656 [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT. (2007) Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol 97:883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. (2003) Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci 23:587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, et al. (2005) Pathobiology of dynorphins in trauma and disease. Front Biosci 10:216–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, McLaughlin PJ, Zagon IS. (1987) Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res 416:157–161 [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. (2005) Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8:1319–1328 [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. (2009) Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci 29:7349–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. (2007) Integration of asynchronously released quanta prolongs the postsynaptic spike window. J Neurosci 27:6684–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Yoshioka K, Jahr CE. (1986) Amino acid receptor-mediated transmission at primary afferent synapses in rat spinal cord. J Exp Biol 124:239–258 [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Werz MA. (1986) Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J Physiol 377:237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. (2008) Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann NY Acad Sci 1144:97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Wier WG, Baraban JM. (1992) Spontaneous synchronous synaptic calcium transients in cultured cortical neurons. J Neurosci 12:4834–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. (2009) Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10:861–872 [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ. (1987) Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA 84:5487–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan MJ. (1999) The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol 9:94–104 [DOI] [PubMed] [Google Scholar]
- Patkar KA, Yan X, Murray TF, Aldrich JV. (2005) [Nalpha-benzylTyr1,cyclo(d-Asp5,Dap8)]-dynorphin A-(1–11)NH2 cyclized in the “address” domain is a novel kappa-opioid receptor antagonist. J Med Chem 48:4500–4503 [DOI] [PubMed] [Google Scholar]
- Piros ET, Charles RC, Song L, Evans CJ, Hales TG. (2000) Cloned delta-opioid receptors in GH3 cells inhibit spontaneous Ca2+ oscillations and prolactin release through KIR channel activation. J Neurophysiol 83:2691–2698 [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Parsons KL, Sweeney DD, Trotter C, Netzeband JG, Siggins GR, Gruol DL. (1999) Opioid enhancement of calcium oscillations and burst events involving NMDA receptors and L-type calcium channels in cultured hippocampal neurons. J Neurosci 19:9705–9715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin KI, Giovannucci DR, Stuenkel EL, Moises HC. (1997) κ-opioid receptor activation modulates Ca2+ currents and secretion in isolated neuroendocrine nerve terminals. J Neurosci 17:6565–6574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilla S, Fabbro A, Grandolfo M, D'Andrea P, Nistri A, Ballerini L. (2009) The patterns of spontaneous Ca2+ signals generated by ventral spinal neurons in vitro show time-dependent refinement. Eur J Neurosci 29:1543–1559 [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. (1996) k-Opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol Pharmacol 50:80–85 [PubMed] [Google Scholar]
- Spitzer NC, Olson E, Gu X. (1995) Spontaneous calcium transients regulate neuronal plasticity in developing neurons. J Neurobiol 26:316–324 [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgár E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. (2003) The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17:13–27 [DOI] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. (2006) Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci 26:1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. (1993) Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature 363:451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpey TL, Chavkin C. (1991) Opioids activate both an inward rectifier and a novel voltage-gated potassium conductance in the hippocampal formation. Neuron 6:281–289 [DOI] [PubMed] [Google Scholar]
- Xia Y, Haddad GG. (1991) Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res 549:181–193 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS, Pintar JE. (1998) Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci 18:2538–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.