Abstract
Purpose
To determine whether hyperopia aggregates in families in an older mixed-race population.
Design
Cross-sectional familial aggregation study using sibships.
Methods
We recruited 759 subjects (mean age, 73.4 years) in 241 families through the population-based Salisbury Eye Evaluation study. Subjects underwent noncycloplegic refraction if best-corrected visual acuity (BCVA) was ≤20/40, had lensometry to measure their currently worn spectacles if BCVA was >20/40 with spectacles, or were considered to be plano (refraction of zero) if the BCVA was >20/40 without spectacles. Preoperative refraction from medical records was used for bilaterally pseudophakic subjects.
Results
Utilizing hyperopia cutoffs from 1.00 to 2.50 diopters, age-, race-, and gender-adjusted odds ratios for hyperopia with an affected sibling ranged from 2.72 (95% confidence interval [CI], 1.84–4.01) to 4.87 (95% CI, 2.54–9.30). The odds of hyperopia increased with age until 75 years, after which they remained relatively constant. Black men were significantly less likely to be hyperopic than white men, white women, or black women.
Conclusions
Hyperopia appears to be under strong genetic control in this older population.
Hyeropia, or farsightedness, describes the refractive state where the focal point of light entering the eye is behind the retina, due to a short axial length relative to the eye’s optical system. Hyperopia of >3.0 diopters (D) is estimated to affect 11.8 million persons over the age of 40 years in the United States, or 9.9% of the U.S. population.1 Though the process of accommodation allows a number of these individuals to see normally without refractive correction, many others require spectacle, contact lens, or surgical correction, with the attendant risk of bacterial keratitis2 or infection due to surgical correction of refractive error.3–5
Additionally, children with high levels of hyperopia are at risk for accommodative esotropia, a condition that, if untreated, may result in amblyopia, impaired stereoscopic vision, and a significant reduction in vision-related functional status.6 Although the number of children with accommodative esotropia is not well known, strabismus has been estimated to affect between 0.5% and 4% of children.7–10 Esodeviations comprise >50% of this,11 with refractive accommodative esotropia clearly playing a significant role.
The small, crowded anterior segment of the hyperopic eye places individuals at greatly increased risk for angle-closure glaucoma (ACG).12 This condition is highly prevalent among the very large populations of East Asia, and is associated with a 3-fold risk of blindness at the time of diagnosis when compared to open-angle glaucoma.13 Angle-closure glaucoma may in fact constitute the world’s second-leading cause of blindness after cataract.13
Evidence exists suggesting that hyperopia may be under strong genetic control.14,15 Twin studies in England have suggested that hyperopia, treated as a binary trait, has a heritability of 89%, virtually the same as myopia.14 Biometric traits such as axial length and radius of corneal curvature, which determine hyperopia, also demonstrated heritability in the 80% to 90% range in the British twin study. However, genetic studies of hyperopia have been far fewer than for myopia. A small number of genetic conditions have been identified that are characterized by hyperopia in conjunction with ocular16 or systemic17 abnormalities. To the best of our knowledge, though, only a single genetic locus has been identified in conjunction with isolated hyperopia: autosomal dominant nanophthalmos on chromosome 11.18 Given the importance of hyperopia as a determinant of both refractive accommodative esotropia and ACG, further genetic study of this entity is clearly warranted.
We report on the familial aggregation of hyperopia in a cohort of elderly sibships drawn from the population-based Salisbury Eye Evaluation (SEE) project on Maryland’s Eastern Shore. Subjects were selected for inclusion in the study without regard to refractive error. The familial aggregation of myopia and heritability of refractive error in this cohort are described elsewhere.19
Subjects and Methods
Subjects
All participants in rounds 3 and 4 of the SEE project, a study of aging and visual function, were administered a short family history questionnaire. Participants were asked whether they had any living siblings, where these siblings lived, and whether they had a history of cataracts or cataract surgery. Those with ≥1 locally resident siblings were invited to participate in genetic studies of ocular disease and refractive error. Contact information and permission to initiate contact were sought for all locally resident siblings of eligible SEE participants. Informed consent was obtained from all subjects before initiating any study procedures. The protocol was approved in its entirety by the Institutional Review Board of the Johns Hopkins University School of Medicine and was carried out in full accord with the principles laid out in the Declaration of Helsinki.
Methods
The study methods have been described in detail elsewhere.19–21 Refractive error data were obtained for each phakic eye of all subjects as noncycloplegic automatic refraction (Humphrey Auto-refractometer 595, Zeiss-Humphrey Systems, Dublin, CA) followed by subjective refinement, if presenting vision using habitual correction in either eye was 20/40 or worse on an Early Treatment Diabetic Retinopathy Study chart22 under standardized lighting conditions, or the spectacle lensometry reading if the vision was better than 20/40 in both eyes with habitual spectacle correction in place. Participants whose uncorrected distance visual acuity (VA) was better than 20/40 in both eyes did not undergo refraction and were assigned a refractive error of zero (plano).
Cataract grading was attempted for all phakic eyes of all participants, based on digital retroillumination and slit-lens photographs obtained according to a previously described protocol.23 All photographs were graded according to the Wilmer Cataract Grading System,24 which distinguishes separate grades for nuclear (0.1–4.0), cortical (0/16–16/16), and posterior subcapsular (present or absent) cataract.
Subjects also underwent a slit-lamp examination with dilated pupil by study personnel (HB), to identify conditions (media opacity, unrecognized pseudophakia, etc.) that might preclude obtaining an accurate refraction in either eye. For unilaterally pseudophakic or otherwise unrefractable subjects, refraction for the phakic eye was used. For bilaterally pseudophakic subjects, refraction data for one or both eyes were obtained from medical records before surgery. In individuals for whom refractive error could be measured in both eyes, the value for the individual was defined as the mean between the two eyes.
Statistical Methods
We used the sibling recurrence odds ratio (OR) as the measure of familial aggregation of hyperopia. This quantity was defined as the ratio of the odds of hyperopia in a sibling of a hyperopic individual relative to the odds of hyperopia given a nonhyperopic sibling. The sibling recurrence OR is similar to the commonly cited sibling recurrence risk (λs), which measures the risk of being affected given an affected sibling relative to the risk of being affected in the general population (i.e., the population prevalence). Because the definition of hyperopia is based on a continuous trait (i.e., refractive error), we used several cutoffs to classify hyperopes and nonhyperopes. These thresholds were set at spherical equivalent (SE) refractive errors of +1.00, +1.50, +2.00, and +2.50 D.
Sibling recurrence ORs for hyperopia were estimated using multivariate logistic regression models and extended generalized estimating equations.25 Generalized estimating equations provide unbiased estimates of sibling recurrence ORs while allowing for the inclusion of covariates and accommodating varying family sizes by using an exchangeable correlation coefficient in the correlation matrix. The statistical analyses were performed in R26,27 using the geepack library,28 which was extended to accommodate logistic models and provide sibling recurrence ORs. Our main covariates of interest were age, gender, and race. Age was determined as the age at examination or, for bilaterally pseudophakic participants, as the age of last refraction before cataract surgery. A linear spline term with a node at 75 years of age was incorporated in our logistic regression models to better fit the appearance of our data. In addition, we included first-order interactions between age, race, and gender to test for effect modification. We report only race–gender interactions, because the other interaction terms were not statistically significant.
Results
Of the 523 SEE participants who had locally residing siblings, 307 elected to participate in the study. The total number of siblings residing within 100 miles of the study site was 1069. Of these, 452 agreed to participate in the study, for a total of 759 participants. Nonparticipation was due either to refusal or our inability to contact or coordinate transport for participants. Participating siblings did not differ significantly from all locally resident siblings with respect to age (± standard deviation) (70.2±7.7 and 71.1±9.7 years for participants and all locally resident siblings, respectively), gender (59% women for participants, vs. 60% for all eligible siblings), or race (26.8% of persons in both groups were black). Sibship sizes ranged from 2 to 8 siblings per family, and the total number of sibling pairs was 845.
The prevalence of SE refractive errors of ≥+1.00, ≥+1.50, ≥+2.00, and ≥+2.50 D (the cutoffs used to define hyperopia in the study) were 45.3%, 32.7%, 22.8%, and 14.6%, respectively. Numbers of hyperopia-concordant sibling pairs corresponding to thresholds of +1.00, +1.50, +2.00, and +2.50 D were 188, 109, 66, and 37, respectively (Table 1).
Table 1.
Number of Concordant Affected and Discordant Sibling Pairs for 4 Thresholds of Hyperopia
| Threshold (D) | Concordant Affected Pairs | Discordant Pairs |
|---|---|---|
| +1.00 | 188 | 433 |
| +1.50 | 109 | 432 |
| +2.00 | 66 | 306 |
| +2.50 | 37 | 189 |
D = diopters.
The total number of participants was 759, in 241 sibships
The results of our multivariate logistic regression analyses for 4 definitions of hyperopia are displayed in Table 2. The gender-and race-corrected odds of hyperopia increased significantly with age until 75 in all our models (mean OR, 1.89/decade). After age 75, the odds of hyperopia decreased as a function of age (mean OR, 0.63/decade). However, this latter trend was statistically significant only for a hyperopia threshold of +2.00 D (OR, 0.52/decade; P = 0.046).
Table 2.
Odds Ratios (ORs) of Hyperopia in Multiple Logistic Regression Models Using Different Thresholds for Hyperopia
| Threshold (D) | +1.00
|
+1.50
|
+2.00
|
+2.50
|
||||
|---|---|---|---|---|---|---|---|---|
| OR | P Value | OR | P Value | OR | P Value | OR | P Value | |
| Sibling recurrence | 2.72 | <0.001 | 3.40 | <0.001 | 3.91 | <0.001 | 4.87 | <0.001 |
| White females* | 1.10 | 0.60 | 1.25 | 0.19 | 1.14 | 0.49 | 1.26 | 0.26 |
| Black males* | 0.25 | <0.001 | 0.16 | 0.002 | 0.15 | 0.01 | 0.12 | 0.04 |
| Black females* | 0.91 | 0.70 | 0.99 | 0.99 | 0.76 | 0.35 | 0.68 | 0.42 |
| Age before 75 (per decade) | 1.68 | 0.005 | 2.06 | 0.001 | 2.06 | 0.01 | 1.75 | 0.055 |
| Age after 75 (per decade) | 0.79 | 0.41 | 0.62 | 0.10 | 0.52 | 0.046 | 0.58 | 0.14 |
D = diopters.
Odds ratios are relative to white males. The coefficient for black females is a linear combination of the coefficients for female gender and the gender–race interaction term.
Black men were statistically significantly less likely to be hyperopic than white men, white women, or black women (mean age- and gender-adjusted OR, 0.17, compared with white males). None of the cataract subtypes were significantly associated with hyperopia in the multivariate model. Hence, measures of lens opacity were not included in the final model.
The age-, gender-, and race-adjusted sibling recurrence ORs for various definitions of hyperopia were 2.72 (95% confidence interval [CI], 1.84–4.01) for a cutoff of +1.00, 3.40 (95% CI, 2.18–5.21) for +1.50, 3.91 (95% CI, 2.25–6.75) for +2.00, and 4.87 (95% CI, 2.54–9.30) for a threshold of +2.50 D (Table 1). The sibling recurrence OR of hyperopia increased monotonically as the threshold for hyperopia was raised incrementally from +1.00 to +2.50 D (Fig 1).
Figure 1.
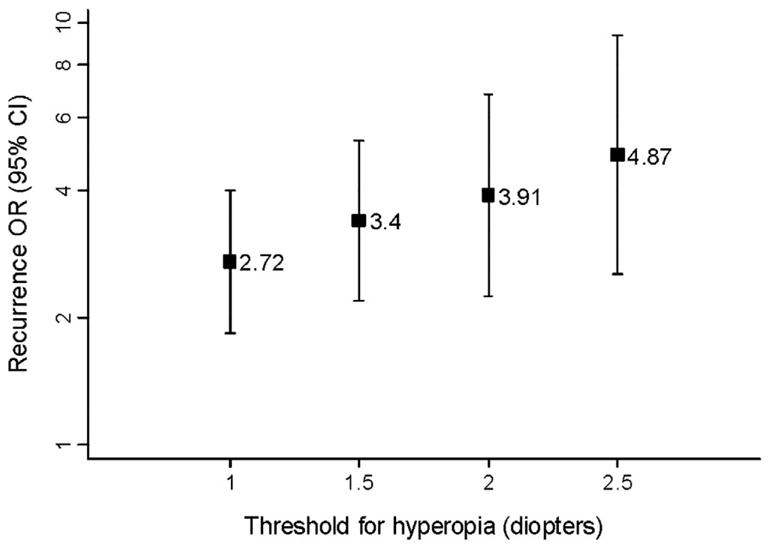
Sibling recurrence odds ratios (ORs) of hyperopia for different thresholds of refractive error.
We also investigated the effects of height, weight, and body mass index on the odds of hyperopia. However, because none of these variables was found to be statistically significantly associated with the odds of hyperopia in multivariate analyses, they are not reported here.
Discussion
Hyperopia shows strong familial aggregation in this older population, with the odds associated with having an affected sibling somewhat higher than we reported for myopia.19 This is in keeping with the few other published studies that have examined the heritability of hyperopia, including twin studies by Hammond et al14 and Teikari et al,29 who reported figures of 89% and 75%, respectively. It would seem that this high figure reflects the heritability of the various biometric factors, such as axial length and radius of corneal curvature, that determine hyperopia: Lyhne et al have reported heritability figures of 89% to 94% for various biometric determinants of refractive error in a Danish twin study.30
Our results are consistent with those of Lee et al,15 who, using similar statistical methods, reported a sibling recurrence OR of 2.87 for hyperopia. It should be noted that this group defined hyperopia as a mean SE refractive error of +0.50 D. Our estimated sibling recurrence OR for a threshold of +1.00 D, the lowest threshold we used, was 2.72.
It is interesting that our estimates of sibling recurrence ORs for hyperopia increased monotonically as the threshold for hyperopia increased. This can, in part, be explained by higher misclassification rates at lower thresholds for hyperopia, biasing our estimates towards the null. Specifically, participants with low refractive errors would be more likely to be misclassified by phenotype than individuals with more extreme refractive errors. It is also possible that the underlying genetic factors responsible for more extreme phenotypes have greater penetrance and/or expressivity than those controlling less severe hyperopic grades. This finding could be important in genetic linkage studies of hyperopia because the power to detect significant genetic linkage to a binary trait locus is strongly influenced by the relative-pair recurrence risk of the disease.31 Researchers wanting to increase the likelihood of identifying chromosomal regions linked to hyperopia in binary trait analyses should therefore either focus on recruiting participants with more extreme refractive errors or set higher thresholds to define the phenotype. Nevertheless, the true statistical significance of this finding is difficult to evaluate, given that the sibling recurrence ORs for varying thresholds were obtained from the same population sample and were therefore not independent.
In this study, the odds of hyperopia increased with age until age 75, after which they remained relatively constant. A similar trend has been noted in previous studies.32–36 A number of studies have suggested both cross-sectional37,38 and longitudinal33,39,40 associations between various cataract types and refractive error. The directionality of this association is not entirely established, as nuclear cataract clearly causes myopic refractive shifts, but it is possible that myopia may also be a risk factor for incident nuclear and/or posterior subcapsular cataract. We could not confirm a statistical link between lens opacification and hyperopia in the present study.
Although evidence for a gene tic role in determining refractive error is strong, as outlined above, environmental and behavioral determinants also seem to play a role. Studies in populations of Asian and European descent have demonstrated that higher socioeconomic status38,41,42 and greater educational attainment43,44 are independently predictive of higher levels of myopia. However, we did not include indices of socioeconomic status and education in the current study. Hence, it is possible that part of the familial aggregation of hyperopia in this population is the result of a shared familial environment that was unaccounted for in our analyses. It will be important in subsequent refractive error genetic studies to include information on socioeconomic status, profession, years of education, and, probably, some index of time spent in near vision work, particularly during childhood.45–49
Other limitations of the current study must be acknowledged. Our choice of an older population presumably will tend to accentuate the impact of personal and nonfamilial environmental factors that accumulate over time, thus leading to relatively lower estimates of familial aggregation. The older age of the cohort is also associated with a high prevalence of pseudophakia (prevalence of bilateral pseudophakia was 13%), necessitating the use of possibly inaccurate measurements of refractive error from previous medical records, and rendering data from persons without prepseudophakic refractions unusable.
Finally, the measurement of refractive error was subject to some inaccuracies under our protocol. Persons with presenting vision better than 20/40 in both eyes and not wearing spectacles were presumed to be plano (zero refraction). It is possible that some of these persons may have had small degrees of hyperopia, leading to misclassification, and, perhaps, lower point estimates of sibling recurrence ORs. A total of 127 (127/759 = 16.7%) persons were assigned a refraction of plano in both eyes in this fashion. However, this potential bias is unlikely to have affected ORs for higher thresholds of hyperopia because older individuals with significant refractive errors would be unlikely to have had distance VAs better than 20/40. Our use of noncycloplegic refractions may have contributed marginally to this inaccuracy in the measurement of refractive error as well, though levels of latent hyperopia would be expected to be low in this population with a mean age in the 70s.
Additionally, because we calculated refractive error on the basis of a mean between two eyes, some persons with myopia in one eye and hyperopia in the other might have been treated as hyperopic under our study definition. Conversely, other such subjects with monocular hyperopia may have been excluded from our analysis of hyperopes based on their mean refractive error. Unfortunately, there is no way to avoid this misclassification problem, short of censoring such anisometropes from analysis. The total number of bilaterally phakic subjects within our cohort with myopia of more than −1 D in one eye and hyperopia beyond +1 D in the fellow eye was 2 (0.3%). The results of our familial aggregation calculations were not significantly affected when these subjects were excluded.
Though a combination of accommodation (depending on age) and spectacle wear can treat hyperopia in the large majority of subjects, the condition remains of importance to blindness prevention workers. In the first place, numerous population-based studies43,50–54 confirm that uncorrected refractive error is the most common cause of impaired vision in many areas. Secondly, as noted above, hyperopia is an important risk factor for 2 common ophthalmic conditions: accommodative esotropia9 and ACG.12 Medical and surgical treatments exist for both of these entities, but effective and efficient screening strategies for either have yet to be devised. The insights into the regulation of refractive error progression and ocular growth throughout life that refractive genetic studies may bring could lend critical insights into the mechanism of these 2 diseases and, perhaps, even contribute to better detection and treatment strategies.
Acknowledgments
This work was supported by the National Institute on Aging, Bethesda, Maryland (grant no.: R-01 16294); National Eye Institute, Bethesda, Maryland (grant no.: K-23 EY00388); and Research to Prevent Blindness, New York, New York (NC).
Footnotes
The authors have no financial interest in the material presented here.
References
- 1.Kempen JH, Mitchell P, Lee KE, et al. The prevalence of refractive error among adults in the United States, Western Europe, and Australia. Arch Ophthalmol. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- 2.Schein OD, Poggio EC. Ulcerative keratitis in contact lens wearers. Incidence and risk factors. Cornea. 1990;9(suppl):S55–8. doi: 10.1097/00003226-199010001-00023. [DOI] [PubMed] [Google Scholar]
- 3.Hersh PS, Stulting RD, Steinert RF, et al. Summit PRK Study Group. Results of phase III excimer laser photorefractive keratectomy for myopia. Ophthalmology. 1997;104:1535–53. doi: 10.1016/s0161-6420(97)30073-6. [DOI] [PubMed] [Google Scholar]
- 4.Stulting RD, Carr JD, Thompson KP, et al. Complications of laser in situ keratomileusis for the correction of myopia. Ophthalmology. 1999;106:13–20. doi: 10.1016/S0161-6420(99)90000-3. [DOI] [PubMed] [Google Scholar]
- 5.Waring GO, III, Lynn MJ, Culbertson W, et al. Three-year results of the Prospective Evaluation of Radial Keratotomy (PERK) Study. Ophthalmology. 1987;94:1339–54. doi: 10.1016/s0161-6420(87)80021-0. [DOI] [PubMed] [Google Scholar]
- 6.Katz J, Tielsch JM. Visual function and visual acuity in the Baltimore Eye Survey. J Vis Impair Blind. 1996;90:367–77. [Google Scholar]
- 7.Friedman Z, Neumann E, Hyams SW, Peleg B. Ophthalmic screening of 38,000 children, age 1 to 2 1/2 years, in child welfare clinics. J Pediatr Ophthalmol Strabismus. 1980;17:261–7. doi: 10.3928/0191-3913-19800701-16. [DOI] [PubMed] [Google Scholar]
- 8.Macfarlane DJ, Fitzgerald WJ, Stark DJ. The prevalence of ocular disorders in 1000 Queensland primary school children. Aust N Z J Ophthalmol. 1987;15:161–74. doi: 10.1111/j.1442-9071.1987.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 9.Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996;103:105–9. doi: 10.1016/s0161-6420(96)30753-7. [DOI] [PubMed] [Google Scholar]
- 10.Roberts J, Rowland M. Refraction status and motility defects of persons 4–74 years, United States. 1971-72. Vital Health Stat. 1978;11(206):1–124. [PubMed] [Google Scholar]
- 11.Pediatric Ophthalmology and Strabismus. San Francisco: American Academy of Ophthalmology; 2001. [Google Scholar]
- 12.Congdon NG, Wang F, Tielsch JM. Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv Ophthalmol. 1992;36:411–23. doi: 10.1016/s0039-6257(05)80022-0. [DOI] [PubMed] [Google Scholar]
- 13.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277–82. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the Twin Eye Study. Invest Ophthalmol Vis Sci. 2001;42:1232–6. [PubMed] [Google Scholar]
- 15.Lee KE, Klein BE, Klein R, Fine JP. Aggregation of refractive error and 5-year changes in refractive error among families in the Beaver Dam Eye Study. Arch Ophthalmol. 2001;119:1679–85. doi: 10.1001/archopht.119.11.1679. [DOI] [PubMed] [Google Scholar]
- 16.Kato K, Miyake Y, Kachi S, et al. Axial length and refractive error in X-linked retinoschisis. Am J Ophthalmol. 2001;131:812–4. doi: 10.1016/s0002-9394(00)00923-5. [DOI] [PubMed] [Google Scholar]
- 17.Brown DJ, Kim TB, Petty EM, et al. Autosomal dominant stapes ankylosis with broad thumbs and toes, hyperopia, and skeletal anomalies is caused by heterozygous nonsense and frameshift mutations in NOG, the gene encoding noggin. Am J Hum Genet. 2002;71:618–24. doi: 10.1086/342067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Othman MI, Sullivan SA, Skuta GL, et al. Autosomal dominant nanophthalmos (NNO1) with high hyperopia and angle-closure glaucoma maps to chromosome 11. Am J Hum Genet. 1998;63:1411–8. doi: 10.1086/302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojciechowski R, Congdon NG, Bowie H, et al. Heritability of refractive error and familial aggregation of myopia in an elderly population of siblings in Salisbury, Maryland. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.04-0740. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38:72–82. [PubMed] [Google Scholar]
- 21.Rubin GS, West SK, Munoz B, et al. A comprehensive assessment of visual impairment in a population of older Americans. The SEE Study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 1997;38:557–68. [PubMed] [Google Scholar]
- 22.Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–6. [PubMed] [Google Scholar]
- 23.Congdon NG, Broman K, Lai H, et al. Nuclear cataract shows significant familial aggregation in an older population after adjustment for possible shared environmental factors. Invest Ophthalmol Vis Sci. 2004;45:2182–6. doi: 10.1167/iovs.03-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West SK, Munoz B, Wang F, Taylor H. Measuring progression of lens opacities for longitudinal studies. Curr Eye Res. 1993;12:123–32. doi: 10.3109/02713689308999480. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY, Beaty TH. Measuring familial aggregation by using odds-ratio regression models. Genet Epidemiol. 1991;8:361–70. doi: 10.1002/gepi.1370080602. [DOI] [PubMed] [Google Scholar]
- 26.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 27.R [computer program]. Version 1.7.1. Vienna: R Foundation for Statistical Computing; 2003. [Google Scholar]
- 28.geepack [computer program]. Version 0.2-4. Iowa City, IA: Jun Yan; 2003. [Google Scholar]
- 29.Teikari J, Koskenvuo M, Kaprio J, O’Donnell J. Study of gene-environment effects on development of hyperopia: a study of 191 adult twin pairs from the Finnish Twin Cohort Study. Acta Genet Med Gemellol (Roma) 1990;39:133–6. doi: 10.1017/s0001566000005651. [DOI] [PubMed] [Google Scholar]
- 30.Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol. 2001;85:1470–6. doi: 10.1136/bjo.85.12.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. Am J Hum Genet. 1990;46:229–41. [PMC free article] [PubMed] [Google Scholar]
- 32.Fledelius HC. Refraction and eye size in the elderly. A review based on literature, including own results. Acta Ophthalmol (Copenh) 1988;66:241–8. doi: 10.1111/j.1755-3768.1988.tb04592.x. [DOI] [PubMed] [Google Scholar]
- 33.Lavery JR, Gibson JM, Shaw DE, Rosenthal AR. Refraction and refractive errors in an elderly population. Ophthalmic Physiol Opt. 1988;8:394–6. doi: 10.1111/j.1475-1313.1988.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee KE, Klein BE, Klein R. Changes in refractive error over a 5-year interval in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1999;40:1645–9. [PubMed] [Google Scholar]
- 35.Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994;35:4344–7. [PubMed] [Google Scholar]
- 36.Wojciechowski R, Congdon N, Anninger W, Broman AT. Age, gender, biometry, refractive error, and the anterior chamber angle among Alaskan Eskimos. Ophthalmology. 2003;110:365–75. doi: 10.1016/S0161-6420(02)01748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dandona R, Dandona L, Naduvilath TJ, et al. Refractive errors in an urban population in Southern India: the Andhra Pradesh Eye Disease Study. Invest Ophthalmol Vis Sci. 1999;40:2810–8. [PubMed] [Google Scholar]
- 38.Shimizu N, Nomura H, Ando F, et al. Refractive errors and factors associated with myopia in an adult Japanese population. Jpn J Ophthalmol. 2003;47:6–12. doi: 10.1016/s0021-5155(02)00620-2. [DOI] [PubMed] [Google Scholar]
- 39.Wong TY, Klein BE, Klein R, et al. Refractive errors and incident cataracts: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2001;42:1449–54. [PubMed] [Google Scholar]
- 40.Younan C, Mitchell P, Cumming RG, et al. Myopia and incident cataract and cataract surgery: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2002;43:3625–32. [PubMed] [Google Scholar]
- 41.Saw SM, Chia SE, Chew SJ. Relation between work and myopia in Singapore women. Optom Vis Sci. 1999;76:393–6. doi: 10.1097/00006324-199906000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Wong TY, Foster PJ, Johnson GJ, Seah SK. Education, socioeconomic status, and ocular dimensions in Chinese adults: the Tanjong Pagar Survey. Br J Ophthalmol. 2002;86:963–8. doi: 10.1136/bjo.86.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999;106:1066–72. doi: 10.1016/S0161-6420(99)90251-8. [DOI] [PubMed] [Google Scholar]
- 44.Guzowski M, Wang JJ, Rochtchina E, et al. Five-year refractive changes in an older population: the Blue Mountains Eye Study. Ophthalmology. 2003;110:1364–70. doi: 10.1016/S0161-6420(03)00465-2. [DOI] [PubMed] [Google Scholar]
- 45.Kinge B, Midelfart A, Jacobsen G, Rystad J. The influence of near-work on development of myopia among university students. A three-year longitudinal study among engineering students in Norway. Acta Ophthalmol Scand. 2000;78:26–9. doi: 10.1034/j.1600-0420.2000.078001026.x. [DOI] [PubMed] [Google Scholar]
- 46.Mutti DO, Mitchell GL, Moeschberger ML, et al. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–40. [PubMed] [Google Scholar]
- 47.Saw SM, Hong RZ, Zhang MZ, et al. Near-work activity and myopia in rural and urban schoolchildren in China. J Pediatr Ophthalmol Strabismus. 2001;38:149–55. doi: 10.3928/0191-3913-20010501-08. [DOI] [PubMed] [Google Scholar]
- 48.Saw SM, Zhang MZ, Hong RZ, et al. Near-work activity, night-lights, and myopia in the Singapore-China study. Arch Ophthalmol. 2002;120:620–7. doi: 10.1001/archopht.120.5.620. [DOI] [PubMed] [Google Scholar]
- 49.Tan GJ, Ng YP, Lim YC, et al. Cross-sectional study of near-work and myopia in kindergarten children in Singapore. Ann Acad Med Singapore. 2000;29:740–4. [PubMed] [Google Scholar]
- 50.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–66. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 51.Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–25. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 52.Munoz B, West SK, Rodriguez J, et al. Blindness, visual impairment and the problem of uncorrected refractive error in a Mexican-American population: Proyecto VER. Invest Ophthalmol Vis Sci. 2002;43:608–14. [PubMed] [Google Scholar]
- 53.Tielsch JM, Sommer A, Witt K, et al. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–90. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 54.VanNewkirk MR, Weih L, McCarty CA, Taylor HR. Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the Visual Impairment Project. Ophthalmology. 2001;108:960–7. doi: 10.1016/s0161-6420(01)00554-1. [DOI] [PubMed] [Google Scholar]


