Abstract
Objectives
To determine whether older adults (age≥60 years) experience less improvement in disability and pain with nonsurgical treatment of lumbar disk herniation (LDH), as compared to younger adults (age<60 years).
Design
Prospective longitudinal comparative cohort study.
Setting
Outpatient specialty spine clinic
Participants
133 consecutive patients with radicular pain and MR-confirmed acute LDH (89 younger adults and 44 older adults).
Intervention
Nonsurgical treatment tailored to the individual patient.
Measurements
Patient-reported disability on the Oswestry Disability Index (ODI), leg pain intensity, and back pain intensity were recorded at baseline, 1, 3, and 6 months. The primary outcome was the ODI change score at 6 months. Secondary longitudinal analyses examined rates of change over the follow-up period.
Results
Older adults demonstrated improvements in ODI(range 0-100) and pain intensity(range 0-10) with nonsurgical treatment that were not significantly different from those seen in younger adults at 6 month follow-up, either with or without adjustment for potential confounders. Adjusted mean improvements in older adults as compared to younger adults were 31 vs. 33 (p=0.63) for ODI, 4.5 vs. 4.5 (p=0.99) for leg pain, and 2.4 vs. 2.7 for back pain (p=0.69). A greater amount of the total improvement in leg pain and back pain in older adults was noted in the first month of follow-up, as compared to younger adults.
Conclusion
These preliminary findings suggest that the outcomes of LDH with nonsurgical treatment were not worse in older adults (age≥60 years) as compared to younger adults (age<60 years). Future research is warranted to examine nonsurgical treatment for LDH in older adults.
Keywords: herniation, Iitervertebral disk displacement, geriatrics, outcomes
INTRODUCTION
Lower extremity pain in the setting of low back pain affects 12 % of older males in the community-based population1, and 21% of older adults in retirement communities2. Lumbar disk herniation (LDH) is a common cause of these symptoms, and most typically manifests as a lumbosacral radicular syndrome: a combination of radicular pain, paresthesias, sensory changes, motor weakness, and/or impaired reflexes in the distribution of one or more lumbosacral spinal nerve roots in the lower extremity3-4. A classical dichotomy has been prominent in spine care whereby LDH is considered a clinical entity common mainly to younger adults, with a shift to a predominance of degenerative lumbar spinal stenosis (LSS) in older adults5. The view that LDH is rare in older adults is echoed in scientific reports and textbooks of spine care6-9. However, other reports caution that LDH in older adults is more common than previously believed10-12. The prevalence of LDH in older adults is of particular importance, because the outcomes with nonsurgical treatment of LDH are favorable in the majority of individuals13, while dramatic improvements in LSS with nonsurgical treatment are seen less commonly12.
Decompressive lumbar spinal surgery for LDH and LSS typically involves removal of a portion of the intervertebral disk (diskectomy) and/or removal of the spinal lamina (laminectomy/laminotomy). Rates of laminectomy/diskectomy in the Medicare population have shown steady increases over recent decades, and exceed the rates of growth in younger populations14. Higher rates of increase in surgical procedures in older adults may be due to the increasing recognition that spine surgery in older adults can be performed safely in properly selected patients11, 15. On the other hand, increasing rates of surgical procedures may be related to the perception by clinicians that the outcomes of nonsurgical treatment of LDH are poor in older adults as compared to younger adults. Various reports in the surgical literature have suggested poor outcomes with nonsurgically treated LDH in older adults16-18. Indeed, in the landmark study of LDH by Weber, increased age was the only characteristic associated with a poor outcome at multiple follow-up time points19. Poor outcomes in older adults may be due to age-related histologic and inflammatory changes in the lumbar intervertebral disk8-9, 20-22. Furthermore, concomitant age-related degenerative changes, such as a decrease in reserve spinal canal space due to osteoarthritic joint hypertrophy, may impede the natural history of improvement as typically seen in younger adults. However, no prior study has examined the outcomes of LDH with nonsurgical treatment in older adults.
We conducted a prospective cohort study of the outcomes of the nonsurgical treatment of LDH in adults age 60 and older, as compared to younger adults age <60. The objective of this study was to determine whether older adults experienced less improvement in back-related disability and pain over a six month follow-up period, as compared to younger adults. We hypothesized that the outcomes of nonsurgical treatment of LDH in older adults would be poor as compared to the outcomes of treatment in younger adults. In order to characterize rates of recovery over time, we utilized longitudinal outcome data at multiple time points to conduct secondary analyses examining possible differences between older adults and younger adults.
METHODS
Study Participants
Participants were recruited from a hospital-based outpatient spine center between January 2008 and March 2009. The study was approved by the Institutional Review Board of New England Baptist Hospital. All consecutive patients age 18 and older with lumbosacral radicular pain for < 12 weeks were evaluated for participation. All patients received a standardized history and physical examination. Inclusion criteria were recent onset radicular pain (<12 weeks) in an L2, L3, L4, L5, or S1 dermatome, with or without neurologic changes, and available magnetic resonance imaging (MRI) demonstrating LDH corresponding with the neurologic level and side suggested by the clinical presentation. Exclusion criteria were known pregnancy; severe active medical or psychiatric comorbidities that would limit study participation; the presence of significant central canal or neuroforaminal stenosis from reasons other than LDH as the likely cause of radicular pain; infectious, inflammatory, or neoplastic cause of radiculopathy; significant degenerative or isthmic spondylolisthesis suspected of contributing to symptoms; and prior lumbar spine surgery at the affected level. Some patients met clinical criteria for study participation, but had not yet undergone MR imaging to confirm whether LDH was present at the baseline evaluation. These patients were offered informed consent at the baseline evaluation for practical reasons, but did not formally enter the study unless their subsequent MRI imaging met study criteria. Subjects who went on to receive surgical treatment during the 6-month follow up period were excluded from analysis.
Participant Demographics and Historical Features
We prospectively collected information on participant age, gender, race, comorbidity, duration of symptoms, prior history of low back pain, prior lumbar spine surgery, tobacco use, employment status, and worker’s compensation status. Race was categorized as ‘white’ and ‘non-white’. Medical and psychiatric comorbidity burden was measured using the Self-Administered Comorbidity Questionnaire (SACQ). The SACQ is widely used in orthopedic research, and has previously demonstrated reliability and validity. Employment status was categorized as part-time employment, full-time employment, student, retired, disabled, and unemployed.
Physical Examination Characteristics
Each participant received a comprehensive physical examination for the evaluation of lumbar radiculopathy by one of six board-certified physiatrists specializing in spine care. Specific physical examination tests received emphasis in this analysis due to their common usage, or because deficits on these tests were felt to have relatively greater clinical or functional importance. The provocative tests of the straight leg raise (SLR) and the femoral stretch test (FST) are used commonly for the diagnosis of LDH. These tests elicit symptoms of neural tension affecting the low lumbar and midlumbar nerves roots, respectively, and have been well described previously23. Motor strength testing included functional tests designed to use the patients’ own body weight as the measure of resistance, in order to provide a physical challenge sufficient to detect subtle losses in strength, and to facilitate reproducibility24. Knee extension strength was measured with the single leg sit-to-stand test, and ankle plantarflexion strength was measured with the heel-raise test24-25. Any deficits were further characterized using manual muscle testing26.
Magnetic Resonance Imaging Characteristics
MRI imaging scans consisted at minimum of T1 and T2 weighted images of the lumbar spine in the sagittal and axial planes. For the purposes of this study, features of LDH and nerve root impingement were evaluated by the recruiting physician, who in most cases also had access to the official report by the interpreting neuroradiologist, and/or direct consultation with the neuroradiologist. The recruiting physician recorded herniation level, nerve root impingement level, herniation morphology, and herniation location. Herniation level was classified as midlumbar (L1-L2, L2-L3, or L3-L4) or low lumbar (L4-L5 or L5-S1) disk herniation. Herniation morphology was classified as protrusion, extrusion, or sequestration27. Herniation location was classified as central (central, paracentral, or subarticular/lateral recess location) or foraminal (foraminal or extraforaminal location)27.
Outcomes
Patient-reported disability and pain intensity were recorded at the baseline clinic visit. The primary outcome of this study was the patient-reported change in functional limitations and disability at 6 month follow-up, as measured by the Oswestry Disability Index (ODI). The ODI is a condition-specific measure of disability, which has been used extensively in prior studies of low back pain and radiculopathy, and has demonstrated validity and reliability in these contexts28. ODI scores range from 0 to 100, with higher scores indicating greater disability The secondary outcomes of this study were change in leg pain and change in back pain at 6 month follow-up, as measured by a 0-10 visual analogue scale (VAS)29. Follow-up information was obtained by mailed questionnaire at 1, 3, and 6 months. Each questionnaire consisted of the ODI, VAS for leg pain, a VAS for back pain, and questions regarding nonsurgical and surgical treatments received.
Statistical Analysis
To characterize the study population at baseline, we calculated means and standard deviations (SD) for continuous variables, medians and interquartile ranges (IQR) for ordinal variables, and frequencies and proportions for categorical variables. We used the chi-square test for categorical variables, and the Student’s T-test or Wilcoxon Signed-Rank test for continuous variables, to compare the baseline characteristics of older adults with those of younger adults. Due to small numbers in individual cells, the employment status categories of ‘unemployed’ and ‘student’ were combined as one category. Herniation morphology was dichotomized as ‘protrusion’ vs. ‘extrusion/sequestration’ due to small numbers of individuals with sequestered disks. We then examined bivariate associations between age group and the 6 month change scores for ODI, VAS leg pain, and VAS back pain. Statistical significance was determined using a threshold of p=0.05. For associations that demonstrated at least a trend towards statistical significance (p ≤ 0.15) in the bivariate analyses, we created multivariate regression models including as covariates those baseline characteristics that 1) demonstrated a statistical trend towards between-age group differences (p ≤ 0.15), or 2) were felt to have a conceptual basis for explaining the observed differences. The method of last value carried forward was used to account for missing outcome data. To examine whether age was related to outcome when treated as a continuous variable, we repeated the multivariate analyses replacing age group with age in years. Last, given the absence of any prior literature on differences in rates of recovery from LDH by age, we conducted secondary longitudinal analyses of outcomes by age group at baseline, 1 month, 3 months, and 6 months, while adjusting for covariates, using generalized estimating equations. All analyses were performed using SAS software, version 9.0 (SAS Institute., Cary, NC).
RESULTS
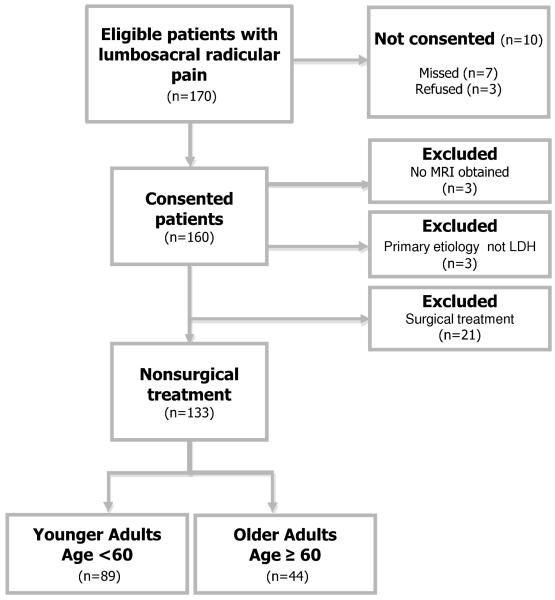
Figure 1 presents a flowchart of study recruitment. One hundred and seventy patients were eligible to participate in this observational study. Of these, seven patients were not offered informed consent due to failure on the part of the recruiting physicians, and an additional three patients refused to participate. Of 160 consented patients, three patients experienced clinical improvement and did not go on to receive MRI, and three patients were excluded for having nerve root impingement due primarily to causes other than LDH. One hundred fifty four subjects met initial criteria, including 106 subjects in the younger group (age <60) and 48 subjects in the older group (age ≥ 60). 21 subjects went on to receive lumbar decompression surgery, and were excluded from this analysis of nonsurgical outcomes. Individuals who underwent surgery were younger than those who did not (48.4 ± 13.2 vs. 53.6 ± 13.5; p=0.05), were less likely to be retired, and were more likely to be disabled or unemployed. There were otherwise no demographic or clinical factors that were significantly associated with surgical treatment over the follow-up period (data not shown).
Figure 1. Flowchart of Recruitment.
The study sample had a mean age ± SD of 53.6 ± 13.5 years, with 33% of participants of female gender, and 93.8% of white race. The age of older adults ranged from 60 to 87. 60% of older adults were age 60-69, 27% were age 70-79, and 13% were 80 or older. Patients who were eligible to participate but were missed or refused were not materially different from study participants with respect to demographic features. Baseline characteristics of the study sample by age group are presented in Table 1. Older adults had higher comorbidity burden (median [IQR] of 1 [0,3] vs. 4 [2,6]; p= <0.0001) and a shorter duration of symptoms (4.2 ± 3.4 vs. 5.2 ± 2.8; p=0.006) at clinical presentation as compared to younger adults. Employment status was significantly different in older adults (p<0.0001). Some physical examination and MRI characteristics differed by age group. A positive straight leg raise test (SLR) was significantly less common in older adults, and conversely, a positive femoral stretch test (FST) was significantly more common in older adults. Midlumbar disk herniation and foraminal disk herniations were more common in older adults. Baseline ODI scores and VAS leg pain were comparable in younger adults and older adults. Baseline VAS back pain, however, was slightly lower in older adults as compared to younger adults (4.2 vs. 5.4; p=0.07).
Table 1. Baseline Characteristics of the Study Sample by Age Group.
| Characteristics | Younger Adults* Age < 60 (N=89) |
Older Adults* Age ≥ 60 (N=44) |
p-value |
|---|---|---|---|
| Demographics and Medical History | |||
|
| |||
| Age (yrs.) | 46.3 (8.8) | 68.2 (7.9) | - |
| Female | 33 (37.1%) | 12 (27.2%) | 0.26 |
| Race (White) | 81 (91.0%) | 43 (97.7%) | 0.27 |
| SACQ (0-45) | 1 (0,3) † | 4 (2,6) † | <0.0001‡ |
| Duration of symptoms (wks.) | 5.2 (2.8) | 4.2 (3.4) | 0.006‡ |
| Prior low back pain history | 61 (68.5%) | 36 (81.8%) | 0.10 |
| Prior lumbar spine surgery | 6 (6.7%) | 4 (9.1%) | 0.73 |
| Tobacco use | 21 (24.4%) | 5 (11.4%) | 0.08 |
| Employment status | |||
| Current part-time employment | 6 (6.9%) | 6 (13.6%) | . <0.0001‡ |
| Current full-time employment | 54 (62.1%) | 11 (25.0%) | |
| Unemployed§ | 6 (6.9%) | 0 (0%) | |
| Retired | 2 (2.3%) | 23 (52.3%) | |
| Disabled | 19 (21.8%) | 4 (9.1%) | |
| Worker’s compensation | 9 (12.2%) | 1 (3.1%) | 0.28 |
|
| |||
| Physical Examination Findings | |||
|
| |||
| Straight Leg Raise Test (+) | 38 (42.7%) | 11 (25%) | 0.05‡ |
| Femoral Stretch Test (+) | 14 (16.7%) | 16 (38.1%) | 0.008‡ |
| Knee Extension Weakness | 24 (27%) | 13 (29.6%) | 0.75 |
| Ankle Plantarflexion Weakness | 13 (14.6%) | 5 (11.6%) | 0.64 |
|
| |||
| MRI Characteristics | |||
|
| |||
| Midlumbar or High Lumbar Disk Herniation (L1-L2, L2-L3, L3-L4 levels) |
28 (31.5%) | 20 (45.5%) | 0.11 |
| Foraminal or Extraforaminal Herniation |
29 (32.6%) | 21 (47.7%) | 0.09 |
| Disk Extrusion/Sequestration | 60 (67.4%) | 37 (84.1%) | 0.04 |
|
| |||
| Severity of the Clinical Presentation | |||
|
| |||
| Oswestry Disability Index (0-100) |
52 (21) | 48 (18) | 0.37 |
| Visual Analogue Scale Leg Pain (0-10) |
6.7 (2.5) | 7.1 (2.3) | 0.46 |
| Visual Analogue Scale Back Pain (0-10) |
5.4 (3.1) | 4.2 (3.3) | 0.07 |
Mean (standard deviation) or N (%)
Median (interquartile range)
Statistically significant (p ≤ 0.05)
Includes ‘unemployed’ and ‘student’ status
SACQ – Self-administered Comorbidity Questionnaire
Associations between age group and outcomes of nonsurgical treatment at six-month follow-up are presented in Table 2. The proportion of missing data for the outcomes of change in the ODI, leg pain, and back pain was 8%, 4%, and 4%, respectively. There were no statistically significant bivariate associations between age group and the primary outcome of ODI change score at 6 months. In multivariate analysis including the covariates of gender, race, employment status, prior LBP, tobacco history, comorbidity (SACQ), duration of symptoms, baseline ODI, herniation level, herniation location, and herniation morphology, the association of age group with ODI remained nonsignificant. Up to 3% of data were missing for some covariates. Age group was not significantly associated with the secondary outcome of leg pain in bivariate analyses. In multivariate analysis including all covariates used in the full model for ODI described above (with adjustment for baseline leg pain), age group continued to not be associated with leg pain change scores. Older adults showed significantly less improvement in back pain as compared to younger adults (2.0 ± 4.1 vs. 3.2 ± 3.1; p = 0.04) in bivariate analysis. However, older adults had reported less back pain at baseline as compared to younger adults (see Table 1). In multivariate analysis including all covariates used in the full models described above (with adjustment for baseline back pain), adjusted back pain improvement was not significantly different in older adults as compared to younger adults (2.4 vs. 2.7; p=0.69). When the outcomes of change in ODI, leg pain and back pain at 6 months were expressed instead as % change from baseline, age group was not significantly associated with any outcome in both bivariate and multivariate analyses (data not shown). When age was treated instead as a continuous variable in a secondary analysis, age remained not significantly associated with change in ODI, leg pain, or back pain (data not shown).
Table 2. Associations between Age Group and Outcomes (Change Scores) at 6 month Follow-up.
| Crude Associations | Multivariate-adjusted Associations* |
|||||
|---|---|---|---|---|---|---|
| Outcome (6-month change scores) |
Younger Adults* Age < 60 |
Older Adults* Age ≥ 60 |
p- value |
Younger Adults* Age < 60 |
Older Adults* Age ≥ 60 |
p- value |
| Primary Outcome | ||||||
| Oswestry Disability Index (0-100) |
−37 ± 22 | −34 ± 25 | 0.63 | −33 | −31 | 0.63 |
| Secondary Outcomes | ||||||
| Visual Analogue Scale Leg Pain (0-10) |
−5.0 ± 3.0 | −5.2± 3.0 | 0.73 | −4.5 | −4.5 | 0.99 |
| Visual Analogue Scale Back Pain (0-10) |
−3.2 ± 3.1 | −2.0 ± 4.1 | 0.04 | −2.7 | −2.4 | 0.69 |
Adjusted for gender, race, work status, prior LBP, tobacco history, comorbidities, duration of symptoms, baseline severity, midlumbar disk herniation, foraminal/extraforaminal disk herniation, and disk extrusion/sequestration.
Table 3 describes treatments utilized by study participants over the 6-month follow-up period. Oral corticosteroid tapers were utilized less frequently in older adults than in younger adults (16% vs. 29 %; p=0.09). Physical therapy was utilized more frequently in older adults than in younger adults (79.6% vs. 59.6 %; p=0.02), as were transforaminal ESIs (37.5% vs. 22.6 %; p=0.06). To account for the influence of treatments received, we conducted secondary analyses of the associations between age group and 6-month change scores for disability and pain, while adjusting for the utilization of oral corticosteroids, physical therapy, transforaminal ESI, and baseline covariates. Our findings were not materially changed by accounting for these treatments. No single treatment was significantly associated with outcomes for disability and pain (data not shown).
Table 3. Treatments Utilized During the Follow-up Period.
| Treatment | Younger Adults* Age < 60 |
Older Adults* Age ≥ 60 |
p-value |
|---|---|---|---|
| Any oral medication | 60 (67.4%) | 30 (68.2%) | 0.93 |
| Acetaminophen | 25 (28.1%) | 18 (40.9%) | 0.14 |
| Non-steroid anti-inflammatory drugs (NSAIDS |
59(66.3%) | 26 (59.1%) | 0.42 |
| Oral corticosteroid taper | 26 (29.2%) | 7 (15.9%) | 0.09 |
| Muscle relaxants | 23 (25.8%) | 9 (20.5%) | 0.49 |
| Tramadol | 6 (6.7%) | 2 (4.6%) | 1.00 |
| Narcotics | 44 (49.4%) | 22 (50.0%) | 0.95 |
| Other medications | 2 (2.3%) | 4 (9.1%) | 0.09 |
| Physical therapy | 53 (59.6%) | 35 (79.6%) | 0.02 |
| Chiropractic | 19 (21.4%) | 13 (29.6%) | 0.30 |
| Acupuncture | 10 (11.2%) | 8 (18.2%) | 0.27 |
| Massage Therapy | 23 (25.8%) | 9 (20.5%) | 0.49 |
| Interlaminar ESI | 41 (46.1%) | 19 (43.2%) | 0.75 |
| Transforaminal ESI | 20 (22.5%) | 15 (34.1%) | 0.15 |
| Comprehensive Pain Clinic Management |
12 (13.5%) | 9 (20.5%) | 0.30 |
N (%)
ESI – Epidural steroid Injection
SNRB – Selective nerve root block
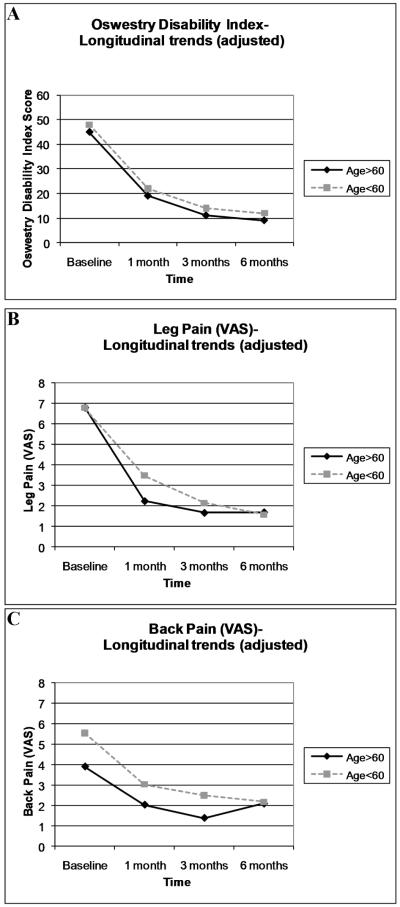
In longitudinal analyses, we examined the outcomes of disability and pain by age group at 1 month, 3 months, and 6 months, while adjusting for demographic and historical features which were significant in our primary analyses: comorbidity score, duration of symptoms, and work status. Specific herniation characteristics were not adjusted for, because we did not wish to ‘adjust out’ for age-related anatomic factors. Figure 2 depicts outcome scores over time, adjusted for comorbidity, duration of symptoms, and work status. Both groups demonstrated the largest improvements in ODI and pain scores over the first month of follow-up, with a slower rate of improvement thereafter. In longitudinal analyses, no age group*time interaction was found for disability on the ODI, indicating no differences in rates of improvement between older and younger individuals. Significant age group*time interactions were noted, however, for the outcomes of leg pain (p=0.02) and back pain (p=0.04). The meaning of this interaction can be easily appreciated by simple visual inspection of longitudinal trends for adjusted pain scores in Figure 2. This figure demonstrates that trajectories of improvement between age groups were similar for the outcome of ODI. However, a greater amount of the total improvement in leg pain and back pain intensity in older adults was noted in the first month of follow-up, as compared to younger adults.
Figure 2. Longitudinal Outcomes*.
*adjusted for comorbidity burden, duration of symptoms, and work status
DISCUSSION
The primary finding of this study was that older adults demonstrated improvements in disability and pain with nonsurgical treatment that were not significantly different from those seen in younger adults over 6 months of follow-up, either with or without adjustment for potential confounders. No prior studies of nonsurgical treatment of MR-confirmed acute LDH have utilized repeated assessment with validated outcome measures at fixed intervals30-32. This has left a notable gap in our knowledge base regarding the course of improvement early in nonsurgically treated LDH. A secondary finding of this study was that while rates of improvement in disability were not significantly different in older adults as compared to younger adults, a greater amount of the total improvement in pain intensity occurred in the first month of follow-up in older adults. However, this difference with respect to rate of improvement in pain intensity was quite small, and not likely to be clinically significant.
Although some authors have asserted that the outcomes of LDH with nonsurgical treatment are poor in older adults16-18, to our knowledge, only one prospective study including nonsurgically treated patients has reported a negative influence of age on outcomes with LDH. In Weber’s randomized trial of surgical vs. nonsurgical treatment of LDH, increased age was found to be correlated with poor long-term outcome19. The findings of our study contradict Weber’s observation. The lack of concordance between our findings and Weber’s may be due to the fact that the study by Weber did not include adults over the age of 60, since Weber’s study was performed during a time when LDH was generally considered to be a problem exclusively of younger adults. On the contrary, the current study demonstrates that in a nonsurgical specialty spine clinic, the diagnosis of LDH is quite common, corroborating prior reports from surgical clinics. Indeed, almost 1/3 of all adult disk herniations presenting to this nonsurgical clinic during the study period were seen in adults age 60 or older. The overly simplistic paradigm of LDH as a disorder primarily of younger adults, and LSS as a disorder primarily of older adults, may potentially lead to misdiagnosis if relied upon heavily.
This study has limitations. First, the relatively small sample size of this study may have limited our statistical power to detect differences between the two age groups with respect to the study outcomes. For this reason, we were unable to examine whether different age cutpoints (below age 60) were associated with clinical outcomes. Second, our findings may not be generalizable to older adults with severe bony stenosis in addition to stenosis secondary to LDH, since these adults may have been excluded due to our predefined study criteria. However, many subjects in this sample had moderate bony stenosis at one or more levels. Third, this study does not allow us to assess whether differences in outcomes affecting older adults may have emerged after longer than 6 months of follow-up. We believe this to be highly unlikely, in light of multiple studies which document that the vast majority of improvement in LDH occurs over the first 6 months of recovery3, 33. Fourth, the influence of some important psychological factors such as treatment expectancy, coping, self-efficacy, and fear avoidance beliefs were not examined in this study. Future studies may wish to examine the effects of these factors in older adults with LDH. Last, formal testing for cognitive impairments using mental status examination was not performed on all subjects. Although subjects with severe medical/psychiatric comorbidities that would limit study participation were excluded according to our study criteria, the unintentional inclusion of subjects with cognitive impairments is possible, and could potentially have affected our findings.
The well-documented increase in the utilization of spinal decompression procedures for older adults over recent decades is likely driven by many different factors. The current study offers no evidence to support the notion that outcomes of LDH with nonsurgical treatment are different in older adults as compared to younger adults. Other explanations for increasing rates of spine surgery in older adults include an increasing prevalence of spinal disorders in the community, surgical advancements in patient selection and technique allowing safer procedures for older adults, or a lack of consensus on indications for surgery. Further research is warranted to investigate the reasons behind increased surgical rates, and to determine whether this translates into better patient outcomes, at a reasonable cost to society.
ACKNOWLEDGMENTS
The authors wish to thank the study participants for their time and effort.
Dr. Suri is funded by the Rehabilitation Medicine Scientist Training Program (RMSTP) and the National Institutes of Health (K12 HD001097-12).
Sponsor’s role: None.
Footnotes
Conflict of Interest: The authors declare that they have no potential financial and personal conflict of interests in the publication of this study.
REFERENCES
- 1.Vogt MT, Cawthon PM, Kang JD, et al. Prevalence of symptoms of cervical and lumbar stenosis among participants in the Osteoporotic Fractures in Men Study. Spine. 2006;31:1445–1451. doi: 10.1097/01.brs.0000219875.19688.a6. [DOI] [PubMed] [Google Scholar]
- 2.Hicks GE, Gaines JM, Shardell M, et al. Associations of back and leg pain with health status and functional capacity of older adults: Findings from the retirement community back pain study. Arthritis Rheum. 2008;59:1306–1313. doi: 10.1002/art.24006. [DOI] [PubMed] [Google Scholar]
- 3.Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 4.Peul WC, van Houwelingen HC, van der Hout WB, et al. Prolonged conservative treatment or ‘early’ surgery in sciatica caused by a lumbar disc herniation: rationale and design of a randomized trial. BMC Musculoskelet Disord. 2005;6:8. doi: 10.1186/1471-2474-6-8. ISRCT 26872154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson B, Stromqvist B. Influence of age on symptoms and signs in lumbar disc herniation. Eur Spine J. 1995;4:202–205. doi: 10.1007/BF00303410. [DOI] [PubMed] [Google Scholar]
- 6.Bono CM. Lumbar Disc Herniations. In: Herkowitz HN, Garfin SR, Eismont FJ, et al., editors. The Spine. Elsevier, Inc.; Philadelphia: 2006. p. 986. [Google Scholar]
- 7.Maistrelli GL, Vaughan PA, Evans DC, et al. Lumbar disc herniation in the elderly. Spine. 1987;12:63–66. doi: 10.1097/00007632-198701000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Yasuma T, Koh S, Okamura T, et al. Histological changes in aging lumbar intervertebral discs. Their role in protrusions and prolapses. J Bone Joint Surg. 1990;72:220–229. [PubMed] [Google Scholar]
- 9.St. John TA, Handling MA, Daffner SD, Vaccaro A. The Aging Lumbar Spine: The Pain Generator. In: Vaccaro A, Betz RR, Zeidman SM, editors. Principles and Practice of Spine Surgery. Mosby; 2002. pp. p83–95. [Google Scholar]
- 10.Akagi S, Saito T, Kato I, et al. Clinical and pathologic characteristics of lumbar disk herniation in the elderly. Orthopedics. 2000;23:445–448. doi: 10.3928/0147-7447-20000501-12. [DOI] [PubMed] [Google Scholar]
- 11.An HS, Vaccaro A, Simeone FA, et al. Herniated lumbar disc in patients over the age of fifty. J Spinal Disord. 1990;3:143–146. [PubMed] [Google Scholar]
- 12.Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358:818–825. doi: 10.1056/NEJMcp0708097. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch JA. Focus issue on lumbar disc herniation: macro- and microdiscectomy. Spine (Phila Pa 1976) 1996;21:45S–56S. doi: 10.1097/00007632-199612151-00005. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Mirza SK. Trends and variations in the use of spine surgery. Clin Orthop Relat Res. 2006;443:139–146. doi: 10.1097/01.blo.0000198726.62514.75. [DOI] [PubMed] [Google Scholar]
- 15.Best NM, Sasso RC. Outpatient lumbar spine decompression in 233 patients 65 years of age or older. Spine. 2007;32:1135–1139. doi: 10.1097/01.brs.0000261486.51019.4a. discussion 40. [DOI] [PubMed] [Google Scholar]
- 16.Fujii K, Henmi T, Kanematsu Y, et al. Surgical treatment of lumbar disc herniation in elderly patients. J Bone Joint Surg Br. 2003;85:1146–1150. doi: 10.1302/0301-620x.85b8.14625. [DOI] [PubMed] [Google Scholar]
- 17.Gembun Y, Nakayama Y, Shirai Y, et al. Surgical results of lumbar disc herniation in the elderly. J Nippon Med Sch = Nihon Ika Daigaku zasshi. 2001;68:50–53. doi: 10.1272/jnms.68.50. [DOI] [PubMed] [Google Scholar]
- 18.Kulali A, von Wild K. Lumbar spinal surgery for sciatica due to intervertebral disc disease in the elderly. Neurosurg Rev. 1996;19:157–162. doi: 10.1007/BF00512045. [DOI] [PubMed] [Google Scholar]
- 19.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131–140. [PubMed] [Google Scholar]
- 20.Hasegawa T, An HS, Inufusa A, et al. Compositional influences for regression of the sequestered lumbar disc hernia in dogs. Neuro-Orthop. 1998;22:69–75. [Google Scholar]
- 21.Hasegawa T, An HS, Inufusa A, et al. The effect of age on inflammatory responses and nerve root injuries after lumbar disc herniation: An experimental study in a canine model. Spine. 2000;25:937–940. doi: 10.1097/00007632-200004150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Katsuno R, Hasegawa T, Iwashina T, et al. Age-related effects of cocultured rat nucleus pulposus cells and macrophages on nitric oxide production and cytokine imbalance. Spine (Phila Pa 1976) 2008;33:845–849. doi: 10.1097/BRS.0b013e31816b4685. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760–765. [PubMed] [Google Scholar]
- 24.Suri P, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine (Phila Pa 1976) 2010 Jun 10; doi: 10.1097/BRS.0b013e3181c953cc. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rainville J, Jouve C, Finno M, et al. Comparison of four tests of quadriceps strength in L3 or L4 radiculopathies. Spine. 2003;28:2466–2471. doi: 10.1097/01.BRS.0000090832.38227.98. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien MD, editor. Aids to the Examination of the Peripheral Nervous System. 4 ed. WB Saunders for The Guarantors of Brain; London: 2000. Brain. Introduction; pp. 1–2. [DOI] [PubMed] [Google Scholar]
- 27.Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine. 2001;26:461–462. doi: 10.1097/00007632-200103010-00007. [DOI] [PubMed] [Google Scholar]
- 28.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 52. [DOI] [PubMed] [Google Scholar]
- 29.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72:95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 30.Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine. 1992;17:1205–1212. doi: 10.1097/00007632-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine. 1989;14:431–437. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine. 1993;18:1433–1438. [PubMed] [Google Scholar]
- 33.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]