Abstract
The increasing exposure to low-dose radiation from diagnostic testing has prompted renewed interest in evaluating its carcinogenic risk, but quantifying health risk from low-dose radiation exposure remains controversial. The current approach is to adopt the linear non-threshold model, which is commonly applied to high-dose exposure, and apply it to assess risk from low-dose exposure. However, existing data are conflicting and limited to epidemiological studies and/or in vitro analyses. In this article, we will discuss the potential cancer risk from low- and high-dose radiation, their effects on DNA repair response pathways, and the best course of action for patients and providers to minimize risk.
Keywords: cancer risk, diagnostic imaging, DNA damage, ionizing radiation, low-dose radiation
Growing concern over exposure to low-dose radiation from imaging tests
Radiation exposure from medical procedures is a potential carcinogen affecting millions worldwide. A major concern is that the total exposure to ionizing radiation in the USA has nearly doubled over the past 20 years [1], based on a recent report by the National Council on Radiation Protection and Measurements (NCRP). Unfortunately, the incidence of radiation exposure from imaging will continue to rise exponentially for several reasons. First, advances in imaging technology have enabled physicians to evaluate both the anatomy and function using x-ray and nuclear medicine-based techniques, both of which are significant sources of radiation. Second, more physicians place a greater reliance on imaging tests for patient management. Finally, patients are demanding more testing for reassurance of accurate diagnosis and treatment.
Imaging procedures such as computed tomography (CT), single-photon emission computed tomography (SPECT) and positron emission tomography (PET) account for major sources of ionizing radiation. In a study of 952,420 non-elderly adults [2], approximately 75% of the cumulative effective dose was accounted for by CT and nuclear imaging procedures (i.e., SPECT and PET). The annual mean (± standard deviation) effective dose from imaging procedures was 2.4 ± 6.0 millisieverts (mSv) per subject [3]. By comparison, an average US citizen receives approximately 3.6 mSv of background radiation annually.
Similarly, there has been a dramatic rise in the number of cardiac imaging tests ordered in recent years [2]. For example, in 1990 fewer than 3 million nuclear medicine studies were performed in the USA, compared with 9.9 million in 2002 [4]. Between 2002 and 2003, the number of cardiac CT scans doubled [5]. In addition, the number of cardiac catheterizations increased from 2.45 million in 1993 to 3.85 million in 2002 [6]. Each cardiac imaging test that uses x-rays or radioactive agents can increase exposure to radiation. Estimated exposures range from approximately 10–20 mSv per procedure, depending on the type of imaging test, and multiple tests can result in cumulative exposures of more than 100 mSv [2]. A recent study showed that among patients who underwent more than one cardiac imaging procedure, the mean cumulative effective dose over 3 years was 16.4 mSv (range: 1.5–189.5 mSv) [7]. Of note, 3.3% of patients received more than 20 mSv per year, which is the maximal annual occupational dose limit for radiation workers recommended by the International Commission on Radiological Protection (ICRP).
Not only is the radiation dose per procedure and the cumulative radiation exposure a concern, but the rate of exposure must be considered in estimating cancer risk. Similar to evaluating the effects of dose on cancer risk, estimates of cancer risk from low and moderate dose-rate exposures are based on the risk coefficients derived from atomic bomb survivors with high dose-rate exposure. Risk coefficients are combined with a dose and dose-rate effectiveness factor, which is deduced from experiments with laboratory animals and from radiobiological measurements [8,9]. The Biological Effects of Ionizing Radiation (BEIR) VII Committee on the US National Research Council reduces the corresponding risk value for atomic bomb survivors by a dose and dose-rate effectiveness factor of 2.0 to estimate risk from low dose-rate exposure. However, this estimate of risk may not be accurate. For example, based on a recent metaanalysis of 12 epidemiological studies, the cancer risk from occupational exposure with low and moderate dose-rate exposure was not lower than atomic bomb survivors with high dose-rate exposure [10].
Debate over the effects of low-dose radiation
Despite the growing concern of the public and federal regulators, it remains unclear whether low-dose radiation causes an increased risk of cancer. By contrast, it is well known and generally accepted that exposure to high-dose radiation increases the risk of solid cancers and leukemias, based on evidence from epidemiological studies in atomic bomb survivors and radiation workers [11–14]. These data suggest that the risk of cancer from high-dose radiation is proportional to the dose, following the linear nonthreshold (LNT) model.
Currently, the LNT model is used to extrapolate risk from low-dose radiation, an approach that is endorsed by the BEIR report of the US National Academy of Sciences and the ICRP. Based on this model, even the very lowest dose of radiation poses an increased risk that is proportional to the dose, and there is no safe exposure level. Epidemiologic studies of atomic bomb survivors have shown an increased cancer risk, even in those exposed to low-dose radiation (5–100 mSv) [11–13]. In addition, studies in radiation workers also support this premise. An international study in over 400,000 radiation workers with an average dose of radiation of approximately 20 mSv and cumulative doses of less than 150 mSv showed increased cancer mortality [14]. Consistent with these findings, a second study found that radiation workers followed by a national registry had increased cancer mortality associated with low-dose radiation [15].
Recent studies have also applied the LNT model to estimate cancer risk from low-dose radiation from imaging tests [16–19]. Using the LNT model, the lifetime attributable risk (LAR) of cancer is calculated based on age, sex and radiation dose. Based on the LNT model, the LAR is adjusted proportionally by the dose of radiation to calculate the excess cancer risk from low-dose radiation. For example, the LAR of having lung cancer after a 100-mSv exposure is 240/100,000 in a 40-year-old woman [16]. Thus, a single exposure of 74 mSv to the lungs after a CT angiography will give her a 0.178% LAR of lung cancer (calculated as 74 mSv/100 mSv multiplied by 240/100,000 multiplied by 100 for the percentage risk). This means that 1 in 562 women who have a CT angiography at 40 years of age are likely to have lung cancer in their lifetime, which is not negligible.
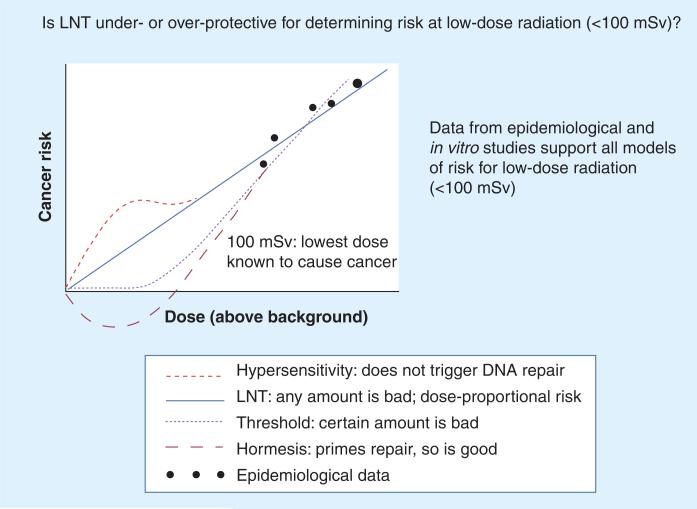
Epidemiologic studies, however, may not adequately control for other risk factors for cancer such as smoking, ultraviolet radiation exposure and genetic susceptibility. Furthermore, it may be difficult to discern a small excess in cancer incidence or mortality from the natural incidence of cancer in the population. Studies that extrapolate cancer risk from the LNT model assume that it accurately estimates cancer risk at low dose [16–19]. Finally, other data suggest that the biological effects of low-dose radiation are more complex than the predictions from the LNT model. Some studies indicate that the LNT model may be over protective, causing unnecessary concern, while others imply it may be underprotective, prompting the need for more stringent regulation. Alternative models include threshold, hormesis (adaptive responses) and hypersensitivity models, as shown in Figure 1.
Figure 1. The four models of estimating increased cancer risk from radiation exposure.
In the LNT model, risk is proportional to the dose, so even the smallest dose induces cancer. In the threshold model, a certain threshold needs to be reached before the risk of cancer increases. In the hypersensitivity model, risk at lower doses is even higher than predicted by the LNT model, because at low doses (<100 mSv) DNA repair systems are not triggered. In the hormesis model, risk at low doses (<100 mSv) is less than predicted by the LNT model because chronic exposure to low-dose radiation stimulates DNA repair mechanisms.
LNT: Linear non-threshold; mSv: Millisieverts.
Adapted with permission from the Canadian Nuclear Safety Commission.
Unlike the LNT model, the threshold model suggests that there may be a level of radiation exposure that is harmless [20]. This has been supported by evidence from the analysis of studies in atomic bomb survivors, which cannot exclude a threshold effect at 60 mSv, although data at higher doses suggest linearity [11]. In addition, mice that lack an enzyme that excises single base mutations, which is a common injury from low-dose radiation exposure, display only a moderate increase in spontaneous mutation rate and are not prone to have malignancies or pathological findings [21]. Finally, in vitro studies show that exposure of cells to radiation doses of less than 100 milligray (mGy; equivalent to mSv for γ- and x-rays) resulted in only approximately 10% rate of base mutations. These findings suggest that alternative repair systems may minimize the effects of low-dose radiation [22], and at a low threshold of radiation exposure there may not be a measurable increased health risk [20,23].
An alternative model, termed hormesis (also referred to as adaptive responses), postulates that low-dose radiation may actually be protective [20,24]. In this model, exposure to low-dose radiation conditions DNA repair systems so that they can more effectively respond to a second, higher dose of radiation. In vitro and in vivo studies using various indicators of cellular damage (i.e., cell lethality, chromosomal aberrations, mutation induction, radio-sensitivity and DNA repair) have demonstrated reduced damage after priming with low-dose radiation [25]. For example, in a previous study, mice that received a priming dose of 10 mGy had lower recombination events after exposure to a 1-Gy challenge than those without prior exposure [26]. Furthermore, long-term exposure to low-dose radiation in mice appears to prevent the development of lymphoma after high-dose exposure [27]. The effects of hormesis, however, shows a high degree of variation, which may depend on factors such as dose rate, time lapse between doses, genetic variation and experimental conditions [25].
In contrast to the threshold and adaptive response models, the hypersensitivity model suggests that the LNT model may underestimate rather than overestimate radiation risk. In the hyper-sensitivity model, radiation induces DNA repair pathways in nonirradiated cells in addition to irradiated cells, thereby increasing the number of damaged cells. Thus, this model would predict that the degree of harm from radiation exceeds the amount predicted by the LNT. Previous studies have shown an increase in the frequency of mutations, apoptosis, DNA damage and DNA repair induction in vitro after co-culture of nonirradiated and irradiated cells, and after transfer of medium from irradiated cells to nonirradiated cells [28,29]. A recent study has also demonstrated that human fibroblasts irradiated with doses as low as 1.2 mGy displayed a stronger than expected induction of genes involved in DNA repair [30]. Although the mechanisms underlying the hypersensitivity model are still uncertain, data support a role for cell–cell signaling, possibly mediated by macrophages. In one study, chromosomal instability was induced in nonirradiated hematopoietic cells after transfer of macrophages from mice receiving 4 Gy of total body radiation [31].
Relationship between radiation damage, DNA repair response pathways & cancer
The conflicting data supporting each of the four models underscore the need for a better understanding of the interactions between radiation damage at low doses, DNA repair response pathways, and the development of cancer [32]. It is well known that ionizing radiation causes DNA damage by base modification and strand breaks. Exposure to radiation leads to DNA double-strand breaks (DSBs), the most serious and potentially lethal type of cellular damage that can result in carcinogenesis. A recent in vitro study using x-rays has shown that the number of DSBs is linear with doses ranging from 1 mGy to 1 Gy in cultured cells [33]. Similar in vivo findings have been found in mice and humans exposed to x-rays with radiation doses of less than 100 mSv [33–35]. In addition, even low-dose radiation (i.e., 50 mSv) can result in loss of heterozygosity and telomere impairment that can result in chromosomal damage, leading to cancer [36,37].
To protect against the development of mutations, DNA damage can be detected by sensors that are transmitted by transducers and controlled by various effector pathways, resulting in the following possible outcomes:
Failure of the DNA damage response pathway to detect and repair mutations can lead to the accumulation of genetic damage and the development of cancer. For example, ataxia telangiectasia mutated (ATM) kinase is crucial in signaling DNA damage [39]. Phosphorylation of ATM activates ATM-dependent signaling that is critical for phosphorylation of H2AX, p53 and checkpoint kinases, which are all involved in DNA repair, cell cycle arrest and chromatin remodeling [40]. Mutations in ATM result in increased radiosensitivity and cancer susceptibility in affected [41]. Similarly, patients with mutations in Artemis, an endonuclease required for repair of DSBs, have a severe immunodeficiency and an increased predisposition to developing lymphomas [42].
An important question to consider is whether a threshold dose of radiation is required to activate DNA damage response pathways. Initial evidence from an in vitro study suggested that ATM-dependent effector pathways, including p53, Check 1 and Check 2, were not threshold dependent, but only radiation doses higher than 200 mGy were evaluated [43]. More recently, inefficient and even absent DNA repair measured by phosphorylation of H2AX (an ATM-mediated pathway) was noted at very low doses of radiation (<5 mGy) [33]. Furthermore, activation of cell cycle checkpoints (specifically, the G2/M checkpoint) that monitor chromosomal integrity before progression to replication and mitosis requires the presence of at least 10–20 DSBs, which entails exposure to at least 200 mGy of radiation [44]. Failure of checkpoint activation increases radiosensitivity and thus cancer susceptibility [45]. Apoptosis, however, appears to be activated even at doses as low as 2 mGy [46]. A recent study in patients undergoing CT, however, suggests that complete DNA repair occurs in vivo, even at very low doses (~5 mSv), as measured by phosphorylation of H2AX [47]. Further in vivo studies are needed to evaluate whether a threshold for activation of DNA repair exists.
It will also be essential to compare the efficiency of DNA damage response activated at low versus high doses. In vitro studies have shown that dose and dose rate affect radiation-induced gene-expression profiles [48–51]. One study found that only 34 common genes, out of a total of 208 genes changed, were modulated in lymphocytes after exposure to 100, 250 and 500 mGy. Changes in gene expression have been noted at doses as low as 20 mGy [49,51]. Finally, not only is gene expression dose dependent, but it may also be dose-rate dependent. One study found that induction of one group of genes is dose-rate dependent while another set of genes is dose-rate independent [51]. These findings suggest that the response to damage may vary at different doses and at different delivery rates. Whether the DNA damage response is more or less effective at low versus high doses thus remains unclear and warrants further investigation.
Minimizing risk to low-dose radiation exposure from imaging tests
Although not currently regulated, the increasing exposure to low-dose radiation (<100 mSv) associated with diagnostic testing has been met with growing concern among medical professionals and patients. Exposure to low-dose radiation may not cause immediate harm to patients but may potentially have long-term biological effects, which has prompted the US FDA to announce a three-point initiative to ensure radiation protection for patients. The program's objectives are to:
Promote the safe use of medical imaging devices;
Support informed clinical decision-making;
Increase patient awareness of their own exposure [52].
In addition to the FDA's initiative, the US Department of Energy has also started the Low-Dose Radiation Research Program to promote investigation into this important area.
The first objective involves promoting the safe use of medical imaging devices. The FDA intends to issue requirements for manufacturers that include safeguards in their machines to minimize radiation risk and to provide appropriate training to support safe use by practitioners. The FDA also encourages providers to develop diagnostic radiation dose reference levels and to develop registries for radiation dose. Another strategy to minimize dose is to modify existing scanning protocols, which has been achieved in several studies using coronary computed tomographic angiography (CTA). In a recent prospective, controlled, nonrandomized study, it was shown that a best-practice protocol for coronary CTA, which minimized scan range, heart rate reduction, electronic-gated tube current modification and reduced tube voltage, decreased the estimated median radiation dose in the follow-up period by 53.3% without compromising image quality [53]. Importantly, a second study found no difference in diagnostic accuracy compared with invasive angiography after using a standardized radiation reduction coronary CT angiography protocol [54]. A radiation dose reduction of 16% was also achieved by using the calcium scoring images instead of the scout view to plan acquisition [55]. Another study found that using minimal padding (i.e., the surrounding x-ray beam on-time) results in reduced radiation time without compromising image quality. Increased padding was associated with greater radiation dose (45% increase per 100-ms increase in padding; p < 0.001) [56]. In addition, a recent study showed that tube current adaption based on anterior–posterior diameter rather than stepwise adaptation based on BMI improved radiation dose optimization in patients with diverse body habitus and significantly improved image quality [57]. This is a simple and practical way to maintain constant image quality, irrespective of body habitus. The addition of a 320-multidetector row CT may further reduce radiation dose, although further study is needed [58].
The second objective advocates informed clinical decision making, which can be achieved by notifying clinicians of the dose administered to patients at the time of acquisition and encouraging provider adherence to published guidelines. The FDA has proposed that all manufacturers have devices that display, record and transmit the dose to the patient electronic record. An alarm to alert providers when the optimal dose is exceeded should also be installed. The NIH has already mandated manufacturers producing scanners at their clinics to have software to track a patient's radiation dose and log it into the medical record, which will support informed clinical decision making.
Cardiac imaging studies should also be ordered only after evaluating the risks and benefits to the patient, as defined by the ‘appropriateness criteria’ [59,60]. Unfortunately, recent studies have shown that providers do not always follow these guidelines. For example, a recent study found that 12% of the nuclear SPECT tests were still inappropriate [61]. Similarly, another study found that 46% of CT studies were ordered for indications not established by these criteria [62]. Encouragingly, a recent study showed that institution of these criteria has had a positive effect [63]. For example, the number of appropriate CT examinations increased from 69.5 to 78.5%, whereas the number of inappropriate examinations decreased from 11.5 to 4.6%. Of note, cardiologists were more likely to order appropriate CT examinations than noncardiologists.
Furthermore, the increasing use of radiation-based imaging tests for prevention in asymptomatic individuals should be discouraged. The risks often outweigh the benefits in these patients who are typically younger, who have a low likelihood of having disease, and who would require testing at regular intervals. In addition, the utility of these tests remains contentious [64]. For example, the efficacy of lung cancer screening by CT and its effects on mortality are debatable, although results from a recent study funded by the NIH showed improvement in mortality in smokers screened by spiral CT compared with chest x-rays [65]. For atherosclerosis screening, coronary CT is only useful in those patients whose management would be changed (i.e., prescribed more aggressive treatment) if the patient had significant coronary calcium.
The third objective is to increase patients’ awareness of their own exposure. Patients should be encouraged to keep a medical imaging history card that will allow them to track their own medical imaging history and share it with their providers. The FDA is also collaborating with the American College of Radiology and Radiologic Society of North America joint task force to develop and disseminate a patient medical imaging record card that will be available on their website [52].
Conclusion
Epidemiological and experimental data suggest that the relationship between dose and cancer risk may not be adequately explained by the LNT model. It is likely that cellular and tissue response to radiation, including damage and subsequent repair, are also modulated by specific trigger thresholds, hypersensivity and hormesis. Furthermore, individual genetic susceptibility is an important factor that is not incorporated into current models of cancer risk related to radiation. Overall, at present there is insufficient evidence to jettison the LNT model. The LNT model is used because it follows the precautionary principle. For the time being, the best and safest recommendation is to use caution when ordering any imaging tests and to be cognizant of the risks and benefits to the patient. Radiation exposure, however, is an unavoidable risk of imaging and should not be considered in isolation when ordering imaging procedures. Both providers and patients should be informed of their reasons for the test and the potential risks and benefits of the test.
Expert commentary & five-year view
Without changes in federal regulation and/or the medical reimbursement system, radiation exposure from diagnostic testing will continue to increase exponentially over the next decade. Unfortunately, our current understanding of the carcinogenic risk from low-dose radiation is limited by conflicting data from epidemiological and in vitro studies. These inconsistencies may diminish if future studies detail such factors as the radiation source, dose, dose rate, dose frequency, tissue type/cell irradiated and time of analysis postradiation in order to ensure that valid comparisons of data can be performed. Furthermore, a better understanding of the DNA repair response pathways and difficulties in detecting mutations will enable a more accurate estimate of radiation risk. Improvement in the sensitivity and specificity of molecular and cellular techniques will facilitate the identification of biomarkers of radiation injury that may indicate increased carcinogenic risk. Although estimating the health risk from low-dose radiation remains controversial, the most prudent recommendation is to minimize exposure to all sources of radiation, especially those from unnecessary medical imaging tests. In general, decisions regarding the use of imaging tests should be evaluated based on individualized risk–benefit analysis for each patient. Radiation exposure should not be considered as a contraindication for testing if the procedure is clinically indicated and appropriate for the management of patients.
Key issues.
There is growing concern over the risk of low-dose radiation from diagnostic imaging tests.
Evidence suggests that there is no single model that fully explains the carcinogenic risk from low-dose radiation.
Further research is needed to evaluate the relationship between radiation damage and the DNA repair response pathways in the development of cancer.
The best and safest recommendation at this time is for patients and physicians to minimize exposure to all forms of radiation and to discourage unnecessary testing.
Financial & competing interests disclosure
This work was supported by grants from the ACC-GE Healthcare Career Development Award (PKN), and NIH grants EB009689 and HL093172 (Joseph C Wu). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.National Council on Radiation Protection and Measurements . Ionizing radiation exposure of the population of the United States. National Council on Radiation Protection and Measurements; MD, USA: 2009. National Council on Radiation Protection report no. 160. [Google Scholar]
- 2.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 3.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N. Engl. J. Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2003 Nuclear Medicine Census Market Summary Report. IMV Medical Information Division; IL, USA: 2003. No authors listed. [Google Scholar]
- 5.2004 CT Census Market Summary Report. IMV Medical Information Division; IL, USA: 2005. No authors listed. [Google Scholar]
- 6.2003 Cardiac Catheterization Lab Census Market Summary Report. IMV Medical Information Division; IL, USA: 2004. No authors listed. [Google Scholar]
- 7••.Chen J, Einstein AJ, Fazel R, et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures a population-based analysis. J. Am. Coll. Cardiol. 2010;56:702–711. doi: 10.1016/j.jacc.2010.05.014. [A retrospective study of more than 90,000 patients describing radiation exposure from cardiac imaging procedures from 2005 to 2007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP. 2007;37(2–4):1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation NRC . Health Risks from Exposures to Low Levels of Ionizing Radiation. BEIR VII Phase 2. National Academic Press; Washington, DC, USA: 2006. [PubMed] [Google Scholar]
- 10.Jacob P, Ruhm W, Walsh L, Blettner M, Hammer G, Zeeb H. Is cancer risk of radiation workers larger than expected? Occup. Environ. Med. 2009;66:789–796. doi: 10.1136/oem.2008.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat. Res. 2000;154:178–186. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Preston DL, Pierce DA, Shimizu Y, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat. Res. 2004;162:377–389. doi: 10.1667/rr3232. [DOI] [PubMed] [Google Scholar]
- 13.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 14.Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk Among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat. Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 15.Muirhead CR, O'Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br. J. Cancer. 2009;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 17.Faletra FF, D'Angeli I, Klersy C, et al. Estimates of lifetime attributable risk of cancer after a single radiation exposure from 64-slice computed tomographic coronary angiography. Heart. 2010;96:927–932. doi: 10.1136/hrt.2009.186973. [DOI] [PubMed] [Google Scholar]
- 18.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Intern. Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br. J. Radiol. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- 21.Klungland A, Rosewell I, Hollenbach S, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl Acad. Sci. USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouget JP, Frelon S, Ravanat JL, Testard I, Odin F, Cadet J. Formation of modified DNA bases in cells exposed either to γ radiation or to high-LET particles. Radiat. Res. 2002;157:589–595. doi: 10.1667/0033-7587(2002)157[0589:fomdbi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23•.Wilson K, Sun N, Huang M, et al. Effect of ionizing radiation on self-renewal and pluripotency of human embryonic stem cells. Cancer Res. 2010;70:5539–5548. doi: 10.1158/0008-5472.CAN-09-4238. [Interesting study shows that, like somatic cells, a significant number of human embryonic stem cells die after high-dose irradiation. Surviving cells, however, retain their pluripotency] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders C. Radiation Hormesis and the Linear-No-Threshold Assumption. Springer; PA, USA: 2010. [Google Scholar]
- 25.Tapio S, Jacob V. Radioadaptive response revisited. Radiat. Environ. Biophys. 2007;46:1–12. doi: 10.1007/s00411-006-0078-8. [DOI] [PubMed] [Google Scholar]
- 26.Sykes PJ, Day TK, Swinburne SJ, et al. In vivo mutagenic effect of very low dose radiation. Dose Res. 2006;4:309–316. doi: 10.2203/dose-response.06-004.Sykes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat. Res. 2005;163:153–158. doi: 10.1667/rr3289. [DOI] [PubMed] [Google Scholar]
- 28.Wright EG, Coates PJ. Untargeted effects of ionizing radiation: implications for radiation pathology. Mutat. Res. 2006;597:119–132. doi: 10.1016/j.mrfmmm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 29••.Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. Assessing cancer risks of low-dose radiation. Nat. Rev. Cancer. 2009;9:596–604. doi: 10.1038/nrc2677. [Excellent review of the differences in biological responses to high- and low-dose radiation that may affect carcinogenic risk] [DOI] [PubMed] [Google Scholar]
- 30.Ojima M, Ban N, Kai M. DNA double-strand breaks induced by very low x-ray doses are largely due to bystander effects. Radiat. Res. 2008;170:365–371. doi: 10.1667/RR1255.1. [DOI] [PubMed] [Google Scholar]
- 31.Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008;68:8122–8126. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 33.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl Acad. Sci. USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rube CE, Grudzenski S, Kuhne M, et al. DNA double-strand break repair of blood lymphocytes and normal tissues analysed in a preclinical mouse model: implications for radiosensitivity testing. Clin. Cancer Res. 2008;14:6546–6555. doi: 10.1158/1078-0432.CCR-07-5147. [DOI] [PubMed] [Google Scholar]
- 35•.Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. γ-H2AX foci as a biomarker for patient x-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation. 2009;120:1903–1909. doi: 10.1161/CIRCULATIONAHA.109.880385. [This prospective study was conducted in 49 pediatric patients with congenital heart disease undergoing cardiac catheterization procedures using γ-H2AX as a biomarker for DNA damage. It demonstrated that the linear nonthreshold model may underestimate radiation risk] [DOI] [PubMed] [Google Scholar]
- 36.Sabatier L, Ricoul M, Pottier G, Murnane JP. The loss of a single telomere can result in instability of multiple chromosomes in a human tumor cell line. Mol. Cancer Res. 2005;3:139–150. doi: 10.1158/1541-7786.MCR-04-0194. [DOI] [PubMed] [Google Scholar]
- 37.Soler D, Genesca A, Arnedo G, Egozcue J, Tusell L. Telomere dysfunction drives chromosomal instability in human mammary epithelial cells. Genes Chromosomes Cancer. 2005;44:339–350. doi: 10.1002/gcc.20244. [DOI] [PubMed] [Google Scholar]
- 38•.Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [Excellent review of the DNA damage response pathways] [DOI] [PubMed] [Google Scholar]
- 39•.Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int. J. Radiat. Biol. 2006;82:605–639. doi: 10.1080/09553000600930103. [Excellent review of the studies detailing changes in gene expression after radiation exposure] [DOI] [PubMed] [Google Scholar]
- 40.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AM, Byrd PJ. Molecular pathology of ataxia telangiectasia. J. Clin. Pathol. 2005;58:1009–1015. doi: 10.1136/jcp.2005.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo Y, Wright SM, Maas SA, et al. The nonhomologous end joining factor Artemis suppresses multi-tissue tumor formation and prevents loss of heterozygosity. Oncogene. 2007;26:6010–6020. doi: 10.1038/sj.onc.1210430. [DOI] [PubMed] [Google Scholar]
- 43••.Short SC, Bourne S, Martindale C, Woodcock M, Jackson SP. DNA damage responses at low radiation doses. Radiat. Res. 2005;164:292–302. doi: 10.1667/rr3421.1. [This study evaluated changes in gene expression after an acute exposure to x-ray radiation in two cell lines, one that shows sensitivity to low radiation dose and one that does not] [DOI] [PubMed] [Google Scholar]
- 44.Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat. Rev. Cancer. 2007;7:861–869. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 45.Marples B, Wouters BG, Joiner MC. An association between the radiation-induced arrest of G2-phase cells and low-dose hyper-radiosensitivity: a plausible underlying mechanism? Radiat. Res. 2003;160:38–45. doi: 10.1667/rr3013. [DOI] [PubMed] [Google Scholar]
- 46.Portess DI, Bauer G, Hill MA, O'Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67:1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- 47.Lobrich M, Rief N, Kuhne M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc. Natl Acad. Sci. USA. 2005;102:8984–8989. doi: 10.1073/pnas.0501895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amundson SA, Do KT, Vinikoor LC, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68:415–424. doi: 10.1158/0008-5472.CAN-07-2120. [DOI] [PubMed] [Google Scholar]
- 49.Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJ., Jr Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat. Res. 2001;156:657–661. doi: 10.1667/0033-7587(2001)156[0657:iogeaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Fachin AL, Mello SS, Sandrin-Garcia P, et al. Gene expression profiles in human lymphocytes irradiated in vitro with low doses of γ rays. Radiat. Res. 2007;168:650–665. doi: 10.1667/RR0487.1. [DOI] [PubMed] [Google Scholar]
- 51.Amundson SA, Lee RA, Koch-Paiz CA, et al. Differential responses of stress genes to low dose-rate γ irradiation. Mol. Cancer Res. 2003;1:445–452. [PubMed] [Google Scholar]
- 52.US FDA . Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging. US FDA; MD, USA: 2010. [Google Scholar]
- 53.Raff GL, Chinnaiyan KM, Share DA, et al. Radiation dose from cardiac computed tomography before and after implementation of radiation dose-reduction techniques. JAMA. 2009;301:2340–2348. doi: 10.1001/jama.2009.814. [DOI] [PubMed] [Google Scholar]
- 54.LaBounty TM, Leipsic J, Mancini GB, et al. Effect of a standardized radiation dose reduction protocol on diagnostic accuracy of coronary computed tomographic angiography. Am. J. Cardiol. 2010;106:287–292. doi: 10.1016/j.amjcard.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 55.Leschka S, Kim CH, Baumueller S, et al. Scan length adjustment of CT coronary angiography using the calcium scoring scan: effect on radiation dose. AJR Am. J. Roentgenol. 2010;194:W272–W277. doi: 10.2214/AJR.09.2970. [DOI] [PubMed] [Google Scholar]
- 56.Labounty TM, Leipsic J, Min JK, et al. Effect of padding duration on radiation dose and image interpretation in prospectively ECG-triggered coronary CT angiography. AJR Am. J. Roentgenol. 2010;194:933–937. doi: 10.2214/AJR.09.3371. [DOI] [PubMed] [Google Scholar]
- 57.Rogalla P, Blobel J, Kandel S, et al. Radiation dose optimisation in dynamic volume CT of the heart: tube current adaptation based on anterior–posterior chest diameter. Int. J. Cardiovasc. Imaging. 2010;26(8):933–940. doi: 10.1007/s10554-010-9630-3. [DOI] [PubMed] [Google Scholar]
- 58.Dewey M, Zimmermann E, Deissenrieder F, et al. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation. 2009;120:867–875. doi: 10.1161/CIRCULATIONAHA.109.859280. [DOI] [PubMed] [Google Scholar]
- 59.Brindis RG, Douglas PS, Hendel RC, et al. ACCF/ASNC appropriateness criteria for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI): a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group and the American Society of Nuclear Cardiology endorsed by the American Heart Association. J. Am. Coll. Cardiol. 2005;46:1587–1605. doi: 10.1016/j.jacc.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 60.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/ NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J. Am. Coll. Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Hendel RC, Cerqueira M, Douglas PS, et al. A multicenter assessment of the use of single-photon emission computed tomography myocardial perfusion imaging with appropriateness criteria. J. Am. Coll. Cardiol. 2010;55:156–162. doi: 10.1016/j.jacc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Miller JA, Raichlin E, Williamson EE, et al. Evaluation of coronary CTA appropriateness criteria in an academic medical center. J. Am. Coll. Radiol. 2010;7:125–131. doi: 10.1016/j.jacr.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Ayyad AE, Cole J, Syed A, et al. Temporal trends in utilization of cardiac computed tomography. J. Cardiovasc. Comput. Tomogr. 2009;3:16–21. doi: 10.1016/j.jcct.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Committee on the Medical Aspects of Radiation in the Environment. Twelfth Report. The impact of personally linked x-ray computed tomography scanning for the health assessment of asymptomatic individuals. Produced by the Health Protection Agency for the Committee on Medical Aspects of Radiation in the Environment. Publisher: Crown; 2007. [Google Scholar]
- 65.National Lung Screening Trial Research Team National Lung Screening Trial. Radiology: overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.Canadian Nuclear Safety Commission: Low-level radiation: how the linear no-threshold model keeps canadians safe. http://nuclearsafety.gc.ca/eng/mediacentre/perspectives/linear_no_threshold_model.cfm.