Abstract
Background
The prevalence of angle-closure glaucoma (ACG) is greater for Eskimos/Inuit than it is for any other ethnic group in the world. Although it has been suggested that this prevalence may be due to a population tendency toward shallower anterior chamber angles, available evidence for other populations such as Chinese with high rates of ACG has not consistently demonstrated such a tendency.
Methods
A reticule, slit-lamp, and standard Goldmann one-mirror goniolens were used to make measurements in the anterior chamber (AC) angle according to a previously reported protocol for biometric gonioscopy (BG) (Ophthalmology 1999;106:2161–7). Measurements were made in all four quadrants of one eye among 133 phakic Alaskan Eskimos aged 40 years and older. Automatic refraction, dilated examination of the anterior segment and optic nerve, and A-scan measurements of AC depth, lens thickness, and axial length were also carried out for all subjects.
Results
Both central and peripheral AC measurements for the Eskimo subjects were significantly lower than those previously reported by us for Chinese, blacks, and whites under the identical protocol. Eskimos also seemed to have somewhat more hyperopia. There were no differences in biometric measurements between men and women in this Eskimo population. Angle measurements by BG seemed to decline more rapidly over life among Eskimos and Chinese than blacks or whites. Although there was a significant apparent decrease in AC depth, increase in lens thickness, and increase in hyperopia with age among Eskimos, all of these trends seemed to reverse in the seventh decade and beyond.
Conclusions
Eskimos do seem to have shallower ACs than do other racial groups. Measurements of the AC angle seem to decline more rapidly over life among Eskimos than among blacks or whites, a phenomenon also observed by us among Chinese, another group with high ACG prevalence. This apparent more rapid decline may be due to a cohort effect with higher prevalence of myopia and resulting wider angles among younger Eskimos and Chinese.
Prevalence figures for angle-closure glaucoma (ACG) have been reported for Eskimos in Greenland,1–3 Canada,4 and Alaska,5 which are at least an order of magnitude higher than those for persons of European or African descent. The prevalence of angle-closure seems to be in the range of 2% to 4% for subjects aged 40 years and older, the highest rate in the world.6 The reasons for this are not well understood. Careful work by Alsbirk7,8 and others9 has suggested that Eskimos may have shallower anterior chamber (AC) depths on a population basis compared with those of persons of Caucasian descent and that hyperopia may be very common, particularly among older Eskimos.10 However, no data have been reported regarding population measurements of the AC angle itself among Eskimos. It is the narrowness of this structure that is generally understood to make up the single most important risk factor for ACG. Furthermore, population biometry among other groups with a high prevalence of ACG, such as East Asians, has provided conflicting evidence of any population trend toward shallow central AC depth.11,12 The full explanation for high prevalence of ACG among Eskimos and, to a lesser extent, other racial groups has not yet been entirely explained.
We have previously reported a novel technique for making simple and noninvasive measurements of the AC angle.13 This technique has been applied to making measurements for a group of blacks, whites, and Chinese of both genders ranging in age from 40 to 80 years and older.14 Chinese have been reported to have rates of ACG that are as much as 10 times higher than those of whites.6 Our study suggested that Chinese did not have significantly narrower angles than those of blacks or whites, but that the apparent rate of narrowing of the angles of Chinese over time was significantly steeper.14 However, longitudinal inferences drawn from cross-sectional data are fraught with the potential for misinterpretation. In the case of our study, it seemed that a cohort effect resulting from the recent reported increase in the prevalence of myopia among younger Chinese15–17 was an alternative explanation for the apparently more rapid decrease in AC angle depth over life among Chinese persons: the younger cohort with higher rates of myopia might have deeper ACs than those of the older cohort who had been unaffected by increased access to education or other factors associated with increased myopia prevalence among younger subjects. Distinguishing definitively between a true longitudinal tendency toward more rapid shallowing of the AC angle and a cohort effect would require a prospective study following the biometry of a single cohort over time. In the absence of such prospective data, measurement of the central and peripheral AC among a population of Eskimos offers the potential both to better understand the trends observed in our studies among Chinese and to explain the reasons for high primary ACG prevalence among Eskimos. Comparison will also be made with refractive and biometric measurements from our previously published studies on other races,12,14 made using identical instruments and protocols.
Material and Methods
The study was approved by the Johns Hopkins University Joint Committee on Clinical Investigations and by the Yukon-Kuskokwim Health Corporation (YKHC) Human Studies Committee. The tribal councils of the villages in which the studies were conducted also approved the study protocol. The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association regarding scientific research on human patients.
All patients were screened for ocular abnormalities requiring treatment or follow-up. Affected patients were referred to the YKHC regional hospital in Bethel, Alaska, where secondary care could be provided. Participants requiring surgical care were referred to the Alaska Native Medical Corporation hospital in Anchorage, Alaska.
Patients
All patients were self-declared Yup’ik Eskimos from two villages in the Yukon-Kuskokwim Delta of Western Alaska. All patients who reported being of at least 50% Yup’ik Eskimo descent were included in the data analysis. To maximize participation, the study was conducted during the regular YKHC Eye Clinic field trip to these villages. The field trips are timed to coincide with the winter season when most village residents are at home and have easy access to the village clinic.
We attempted to recruit all residents older than 40 years of age in village A. (The identity of the villages has been masked at the request of the YKHC Human Studies Committee). This village was chosen because of its relatively large population. A small number of additional patients aged 60 years and older were examined in Village B because of the difficulty in obtaining data from older Eskimos. Medical records for all residents in both villages are maintained in the local village clinics by community health aide practitioners. These records were used to acquire census data for the study and to recruit study participants. We attempted to telephone or radio all potential patients and invite them to participate in the study. The purpose of the study was explained in English or in Yup’ik through an interpreter to all patients. Written informed consent was obtained from all study participants.
Methods
All patients underwent a standard complete eye examination, including the measurement of distance visual acuity, objective and subjective refraction, tonometry, slit-lamp biomicroscopy, and dilated fundus evaluation (unless contraindicated). In addition, A-scan ultrasonography, keratometry, and biometric gonioscopy (BG) were performed on the left eyes of all patients. The right eye was substituted for the left eye in cases in which the latter was ineligible because of previous intraocular surgery, including cataract extraction, trabeculectomy, or peripheral iridectomy; media opacity precluding clear visualization of the AC angle structures; or infectious or ocular surface conditions precluding safe gonioscopy. If both eyes of a subject were ineligible, the subject was not eligible for the study. All examinations took place in the local clinics of the respective villages.
A-scan ultrasonography to measure AC depth, lens thickness, and axial length was performed with a Sonomed 1500 (Sonomed Inc., Lake Success, NY). Measurements were repeated until three successive values within 0.1 mm were obtained for AC depth and within 0.3 mm for axial length, with the mean value being recorded. Immersion was not practical under these conditions, and care was taken not to exert pressure on the cornea. The identical protocol and model of A-scan machine were used in our previously published report on ocular biometry among Chinese, blacks, and whites.12
Radius of corneal curvature and refraction were measured for each subject using an automatic keratometer/refractometer (Nikon Retinomax K-plus 2, Nikon Instruments, Melville, NY). Intraocular pressure was measured using a Tono-Pen handheld tonometer (Mentor Ophthalmic Instruments, Santa Barbara, CA) after induction of corneal anesthesia with topical proparacaine. Recordings were made until a single value within the instrument’s 5% accuracy range was obtained. Care was taken not to exert pressure on the eyes or eyelids while making intraocular pressure measurements.
Monocular distance visual acuities were measured using a projected Snellen chart calibrated for the distance of the examination room. Retinoscopy and subjective refraction were performed, and best-corrected visual acuities were recorded. After subjective refraction, slit-lamp biomicroscopy with Biometric Gonioscopy (BG) measurements of the AC angle (see below) was performed on all study participants.
A dilated fundus examination was performed on all patients, unless the angle was considered occludable by the study optometrist (RW). Cup-to-disc ratios were graded empirically using non-contact fundus biomicroscopy, and the peripheral retina was visualized with binocular indirect ophthalmoscopy.
Protocol for Biometric Gonioscopy
All BG measurements were made by a single investigator (RW), using a one-mirror Goldmann gonioscopic lens (Charles Bell Instruments, Westville, NY) and slit lamp equipped with a Haag-Streit measuring eyepiece (Haag-Streit AG, Liebefeld-Berne, Switzerland), both identical to those used in our previous studies.13,14 The principal examiners of the previous (NGC) and current (RW) studies were standardized against one another by examining patients with varying AC depth in a glaucoma specialty clinic until measurements within one unit could be obtained by both observers on 10 successive patients.
The protocol for BG has been described in detail elsewhere13 and is reviewed here. BG involves measuring the maximum extent of the visible angle structures using a reticule in the slit-lamp eyepiece. Conditions were standardized as follows: ambient light from a small indirect source, total magnification of 16× and a power of 6W, using the middle filter setting and a slit beam of 4 × 1 mm. No attempt was made to standardize the subject’s position of gaze, angle of the slit beam, or placement of the goniolens on the eye; these were allowed to vary so as to maximize the measurement in each quadrant.
BG measurements were made using the slit-lamp–mounted reticule in arbitrary units (although the reticule was ruled in millimeters, the magnification of the slit lamp meant that the true unit of measurement was in fact smaller) from the first point of contact between the iris and the eye wall to the farthest anterior extent of pigmented trabecular meshwork. Each quadrant was measured separately, with the average being recorded as the grade for a given individual. A measurement of 0 was recorded in the case of a completely closed angle.
Statistical Analysis
In the Eskimo data set, linear models were used to study the association of age and gender with six measurements believed to be of importance in determining risk of angle closure: AC central depth, axial length, refractive error, lens thickness, radius of corneal curvature, and measured depth of the peripheral AC angle using BG. To allow for a possible nonlinearity in the models, a second model was analyzed in which the linear relationship between the eye measurements and age was allowed to change after age 65 years (spline regression).
In comparisons between racial groups, the linear relationship of age with eye measurement was allowed to change after age 65, based on an initial graphical analysis of smoothed plots. The interaction between age and race was included in the models. To compute P values for comparisons between racial groups, simultaneous interval estimation was used.18 For large numbers of observations, the combined estimates squared multiplied by the inverse of the covariance follows a chi-square test distribution (Wald’s chi-square test) with degrees of freedom equal to the number of estimates tested.
When comparison was made between multiple racial groups (as for BG and AC depth), the Bonferroni correction was used. Under these conditions, there were five possible comparisons of interest to us (Eskimos to Chinese, whites, and blacks; Chinese to whites and blacks), and thus a P value of 0.05/5 = 0.01 was considered significant for such multiple comparisons.
Results
In village A, we examined 132 of 150 (88.0%) Eskimo residents aged 40 years and older. One hundred sixteen (87.9%) of these reported being full-blooded Eskimo, with the remaining 16 (12.1%) reporting being of at least 50% Eskimo heritage. Among these patients, seven (5.1%) had undergone previous intraocular laser or incisional surgery in both eyes and were excluded from further analyses, leaving 125 eligible subjects from Village A. Another, smaller village was used to supplement village A data. Because we had gathered sufficient data on the younger than 60 age group, only patients age 60 or older were recruited in Village B. We examined 9 of 15 (60%) such patients in village B, with one subject being ineligible because of corneal disease. All of the patients in village B were self-reported full-blooded Yup’ik Eskimos. Thus, eight eligible patients were recruited in Village B, for a total of 133 eligible Eskimo patients reported in this study; 54.9% of eligible patients were female, and the mean age of patients included was 56.1 years (Table 1).
Table 1.
Participant Characteristics in a Study of Ocular Biometry of Alaskan Eskimos
| Village A N
|
Village B N
|
Total N (%)
|
Total | ||||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| Age | |||||||
| 40–49 | 17 | 31 | 0 | 0 | 17 | 31 | 48 |
| 50–59 | 24 | 13 | 0 | 0 | 24 | 13 | 37 |
| 60–69 | 8 | 17 | 4 | 2 | 12 | 19 | 31 |
| 70–79 | 6 | 9 | 1 | 1 | 7 | 10 | 17 |
| Total | 55 | 70 | 5 | 3 | 60 | 73 | 133 |
| Total potentially eligible | 165 | ||||||
| Total number presenting | 140 | ||||||
| Total number eligible | 133 | ||||||
Among the Eskimo patients, the age-adjusted mean BG measurement for women (2.76, standard error = 0.11) was lower than that for men (2.99, standard error = 0.12), but not significantly so (P = 0.17). AC depth, spherical equivalent, lens thickness, radius of corneal curvature, and axial length also did not differ significantly by gender, after adjusting for age.
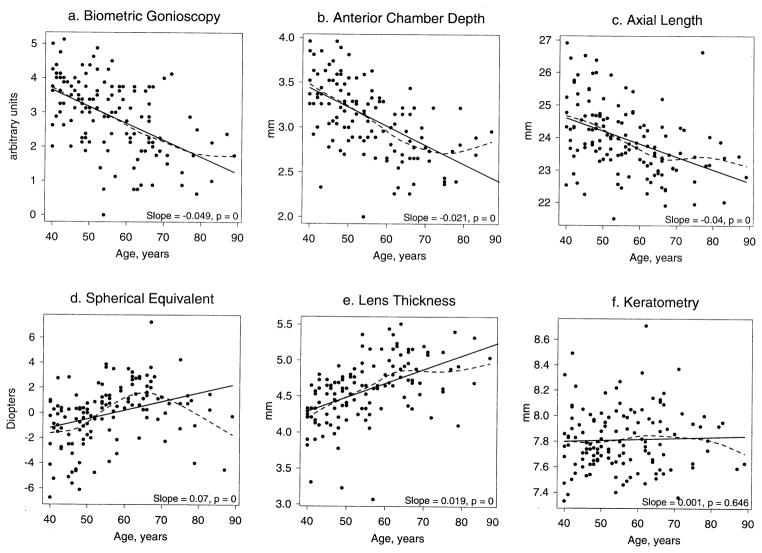
All of the measured refractive and biometric parameters except radius of corneal curvature varied significantly with age among Eskimos. BG, AC depth, and axial length fell significantly with age, spherical equivalent refraction became significantly more hyperopic, and lens thickness increased significantly with age. However, in smoothed plots of the Eskimo biometric data by age (dotted lines in Fig 1a–f), an apparent change in the slope later in life was observed for many of the measurements. The change in the slope after 65 years was significant for AC depth (slope change = 0.02, P = 0.03), lens thickness (slope change = −0.03, P = 0.01), refractive error (slope change = −0.3, P < 0.0001), and axial length (slope change = 0.06, P = 0.04). The slope change was not significant for BG (slope change = 0.02, P = 0.38) or radius of corneal curvature (slope change = −0.0068, P = 0.34).
Figure 1.
Relationship between ocular biometric parameters and age among Eskimos. Both linear (solid line) and smoothed curve (dashed line) models are shown. mm = millimeters.
The age-adjusted mean BG measurement for Eskimo patients reported in this study was significantly lower than previously reported by us14 for Chinese, black, and white patients measured using the same device and protocol (Table 2). AC depth was also significantly shallower and lens thickness greater for Eskimos than for the other racial groups, also using the same protocol and measurement devices.12 Eskimos seemed also to have more hyperopia, flatter radius of corneal curvature, and slightly longer axial length, although differences with other racial groups were not all significant (Table 2).
Table 2.
Comparison of Ocular Parameters Among Eskimos, Chinese, Blacks, and Whites, Age-adjusted*
| Race | NBiometric Gonioscopy | Biometric Gonioscopy† (Standard Error) | NOther | Anterior Chamber Depth† (Standard Error) | Axial Length‡ (Standard Error) | Refraction§ (Standard Error) | Lens Thickness† (Standard Error) | Keratometry|| (Standard Error) |
|---|---|---|---|---|---|---|---|---|
| Eskimo | 133 | 2.65 (0.13) | 133 | 2.96 (0.04) | 23.7 (0.15) | +1.01 (0.29) | 4.74 (0.05) | 7.83 (0.03) |
| White | 114 | 3.66 (0.14) | 176 | 3.05 (0.03) | 23.4 (0.12) | +0.40 (0.23) | 4.45 (0.04) | 7.77 (0.03) |
| Black | 122 | 3.64 (0.14) | 190 | 3.01 (0.03) | 23.0 (0.10) | +0.53 (0.21) | 4.35 (0.04) | 7.74 (0.02) |
| Chinese | 124 | 3.68 (0.15) | 556 | 3.00 (0.02) | 23.3 (0.06) | +0.42 (0.13) | 4.55 (0.02) | 7.67 (0.02) |
All estimates are adjusted for age, interaction of age and race, and allow for a change in slope at age 65; P values for between-group comparisons were computed using simultaneous interval estimation as described in Methods; average age = 59.1 years for biometric gonioscopy comparison; average age = 59.5 years for anterior chamber depth, axial length, lens thickness, and reference comparison.
P < 0.01 difference between Eskimos and other races, no difference between Chinese and blacks/whites.
P < 0.01 difference between Eskimos and Chinese/blacks, no other significant racial differences.
P < 0.01 difference between Eskimos and Chinese, no other significant racial differences.
P < 0.001 difference between Eskimos and Chinese; P < 0.01 difference between Chinese and blacks; no other significant racial differences.
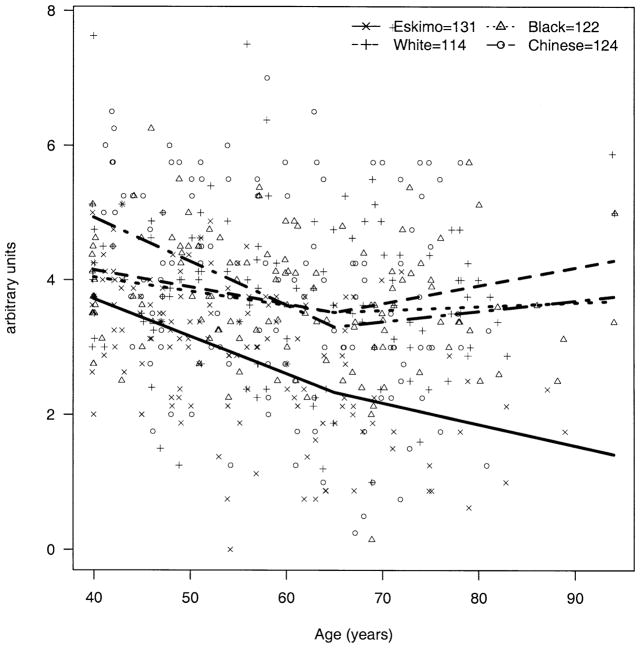
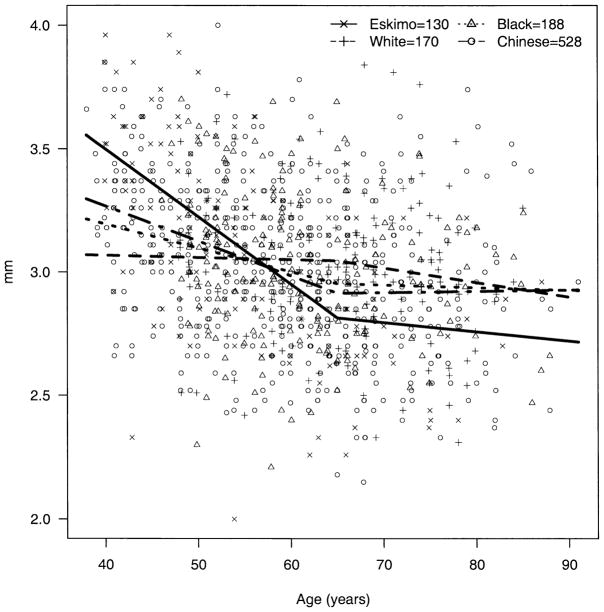
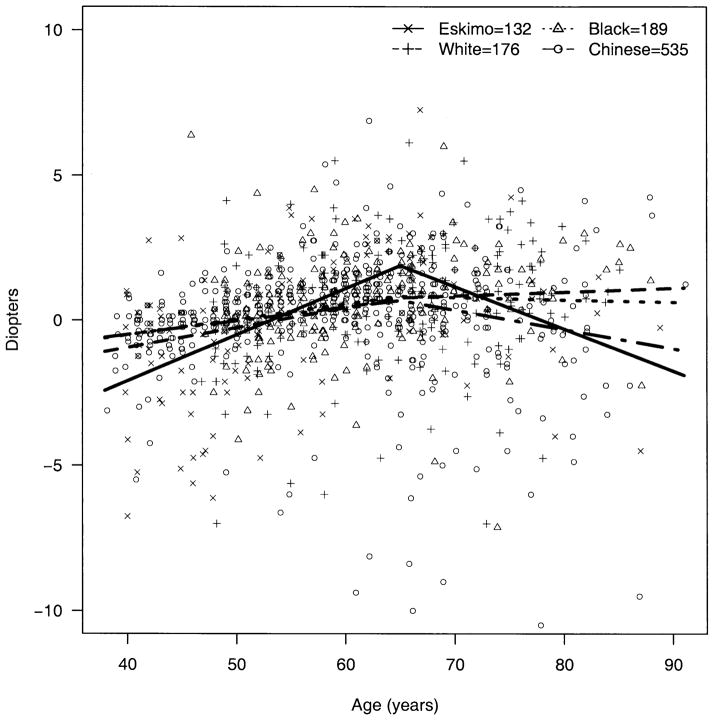
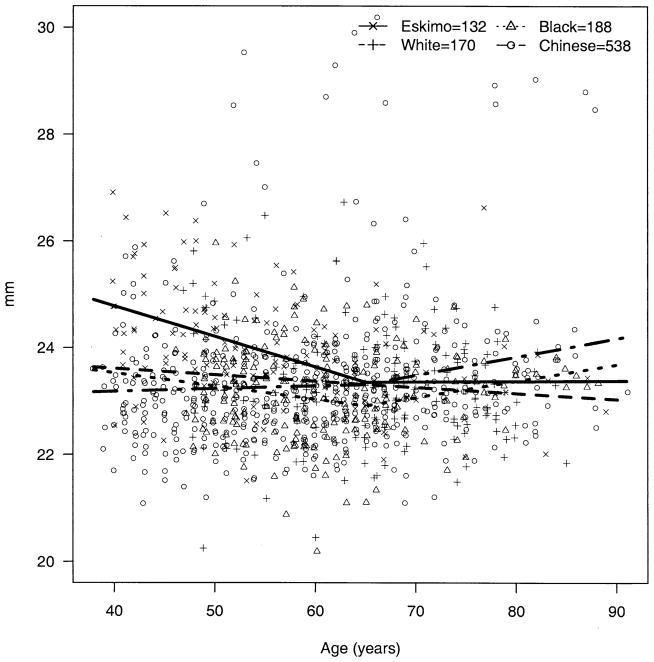
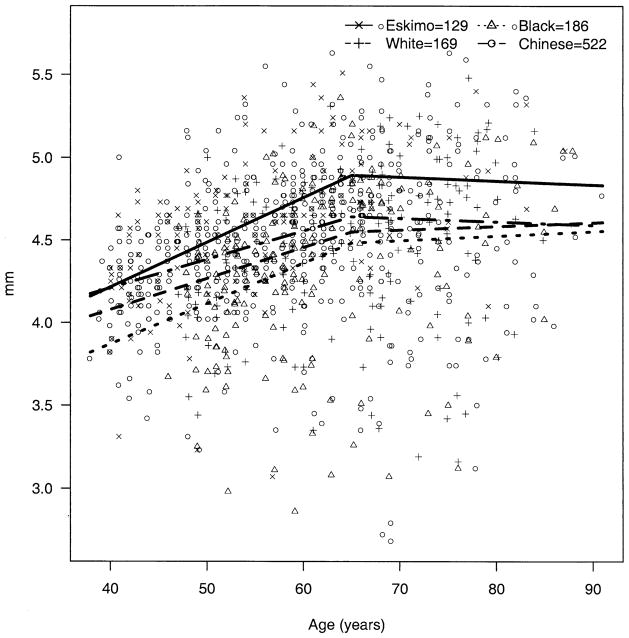
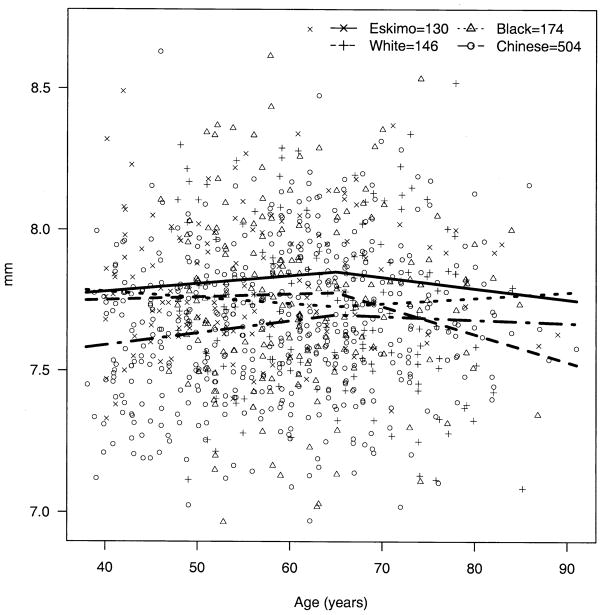
The apparent decline in BG over age was significantly steeper among Eskimos compared with blacks (P = 0.009) and whites (P = 0.003); the decline over age in Chinese was not significantly different from that of Eskimos (P = 0.49) but was significantly steeper than those of blacks (P = 0.009) and whites (P = 0.003) (Fig 2). The trend for AC depth over age (decline until age 65 and then rise) was also significantly steeper among Eskimos compared with blacks, whites, and Chinese (P < 0.002 for all three). The change in AC depth for Chinese did not differ significantly from that for blacks (P = 0.65) and whites (P = 0.04, note, nonsignificant with Bonferroni correction for multiple comparisons) (Fig 3). The apparent increase in hyperopia early in life and myopia later was more pronounced among Eskimos and least apparent for Chinese, with the difference between Eskimos and Chinese being significant (P = 0.001) (Fig 4). The age trend for axial length was significantly different for Eskimos compared with Chinese (P < 0.0001) but not whites (P = 0.12) or blacks (P = 0.07) (Fig 5). The age trend in lens thickness (thicker until age 65, and then thinner) was significantly different between Eskimos and whites, blacks, and Chinese (P < 0.001 for all) (Fig 6). Finally, the change in radius of corneal curvature did not differ significantly between the races (Fig 7).
Figure 2.
Biometric gonioscopy by age for Eskimos, Chinese, blacks, and whites. (In figures 2–7 the number of subjects receiving each test is slightly smaller than the total number of subjects participating in the study, due to a small number of subjects who were unable to complete some tests).
Figure 3.
Anterior chamber depth by age for Eskimos, Chinese, blacks, and whites.
Figure 4.
Spherical equivalent refractive error by age for Eskimos, Chinese, blacks, and whites.
Figure 5.
Axial length by age for Eskimos, Chinese, blacks, and whites.
Figure 6.
Lens thickness by age for Eskimos, Chinese, blacks, and whites.
Figure 7.
Radius of corneal curvature by age for Eskimos, Chinese, blacks, and whites.
Discussion
Racial differences in the prevalence of primary ACG have long been recognized, although the mechanisms underlying these differences are not well understood. We found shallower central and peripheral AC measurements among Eskimos compared with other racial groups previously measured using our techniques.14 The finding of narrower angles among Eskimos is consistent with their high reported prevalence of primary ACG1–5 and also with previous reports of markedly small ocular biometric parameters in this population.7–9
It is curious that we have not found shallower AC depths12 or narrower angle measurements14 among Chinese, another population with high rates of ACG. It may be that Chinese ACG prevalence is higher for some other reason, or that we simply failed to find a difference that does exist. Shallower AC depths have been reported for other East Asian populations with high ACG prevalence.11
It may also be that the apparent similarity between races of biometric measurements averaged across age obscures actual differences in these measurements, which become apparent when the measurements are stratified by age. In fact, the AC measurements of both Eskimos (AC depth, Fig 3) and Chinese (BG, Fig 2) seemed significantly higher for younger patients and lower for older patients compared with measurements for blacks and whites.
Although this finding suggests a more rapid shallowing of the AC over life among Chinese and Eskimos, it is also possible that some or all of the apparent shallowing of the AC observed in our studies may result from a cohort effect, whereby younger individuals exposed to greater opportunities for near work become more myopic, presumably with deeper AC depth and wider angles. Older cohorts who did not have such opportunities have less myopia and shallower AC depths as a result. This would tend to accentuate the apparent decline in AC depth over life as observed in a cross-sectional study such as ours.
In fact, our data are not entirely consistent with this alternative hypothesis: the slope of refractive error with age does not differ greatly between the races, and although the trend toward more myopia at younger ages is strongest for Eskimos, it is actually the least apparent for Chinese (Fig 4).
An apparent decline in ocular biometric parameters including AC depth and axial length throughout life was also reported recently for a Chinese population in Singapore.17 These results were also based on cross-sectional data and were interpreted by the authors of that study as evidence of a cohort effect related to increased myopia prevalence among younger patients. It is, however, interesting to note that the two populations in whom a high prevalence of primary ACG is best established, Eskimos1–5 and Chinese,6 should also demonstrate an apparent more rapid shift during aging toward biometric parameters known to be associated with primary ACG risk than that of other groups, such as blacks and whites. If, in fact, this phenomenon is due to a greater propensity toward acquired myopia among young Eskimos and Chinese, then the question to be answered is whether the factors underlying this propensity are in any way associated with those that contribute to the excess burden of primary ACG.
The possibility also exists that the apparently more rapid narrowing of the anterior segment observed in Eskimo and Chinese eyes represents, at least in part, a real phenomenon and that it contributes directly to the elevated prevalence of primary ACG observed in these two populations. Such rapid narrowing could be explained in several ways. The phenomenon of creeping angle closure, which has been described in Asian populations but never definitively demonstrated,19–21 could potentially lead to more rapid narrowing of the anterior segment throughout life.
Or, perhaps more rapid progression of lens opacity, because of factors such as diet, increased exposure to ultraviolet B light near the equator (among Singapore Chinese) or reflected from snow (among Alaskan Eskimos), or comparatively poor access to cataract surgical services among Eskimos, might lead to increased rates of lens thickening and thus shallowing of the anterior segment compared with those of groups such as blacks and whites resident in the continental United States. Alternatively, if lens opacities among the Eskimo and Singapore Chinese populations studied by us are, indeed, denser than those of the white and black populations, ultrasound waves would tend to travel more rapidly, resulting in an artificially decreased measurement of axial length for individuals with denser lens opacity.22,23 Our data do seem to be consistent with these lens-based hypotheses. Eskimos in this study had thicker lenses than those of other racial groups at all ages, and their pattern of thickening of the lens until the seventh decade, followed by later thinning, is consistent generally with observed changes in the AC depth (Figs 3 and 6).
It would only be possible to distinguish between a cohort effect and a true decline in biometric parameters throughout life by recourse to a prospective study over several years. This distinction has important programmatic implications. If increasing prevalence of myopia among Eskimos, and especially Chinese, is truly leading to deepened AC, wider angle, and presumably lower risk of ACG among the cohort now in their 40s, the burden of this common and important cause of blindness may be reduced in East Asia in the future. If the cohort explanation is not correct, and the more rapid shallowing of the anterior segment among populations with high prevalence of ACG is real, then research must be directed toward a better understanding of this phenomenon as a potential key to the etiology of ACG.
The fall of AC depth and increase in lens thickness throughout life that we observed have been reported by many other investigators, based on both cross-sectional24 and longitudinal3 data. The reversal of both of these tendencies in the seventh decade, and the reversal during the same period of the trend toward increasing hyperopia, which were observed in the current study among Eskimos and previously14 among Chinese, blacks, and whites, have not been widely reported. Foster et al11 did note a similar tendency among Mongolians. The declining prevalence of ACG seen in the seventh decade in several groups, including Mongolian women,25 Canadian Eskimos,4 Alaskan Eskimos,26 Thais,27 Israeli men,28 and a mixed racial group in Singapore,29 may be associated with this reduced tendency toward shallow AC depth late in life. However, the number of older patients in most of these studies is small, and prevalence figures correspondingly uncertain.
The possibility must be considered that bias could explain the appearance of increasing AC depth in the seventh decade in this cross-sectional study. Most obviously, it could be that eyes predisposed toward angle-closure attacks underwent peripheral iridectomy or cataract extraction in the 50s and 60s, leaving a group with relatively deeper angles remaining in the 70s. However, none of the Eskimo patients in this study, or the Chinese, blacks, or whites on whom we reported previously, were pseudophakic or aphakic at the time of examination. It is possible that the apparent decline in lens thickness observed beyond age 65 years among Eskimos in this study is an artifact of cataract extraction having removed the thickest lenses, so that only patients with less opacity and presumably thinner lenses remained phakic into their 70s. It is interesting to note, though, that access to cataract surgery is limited in this area, and that reports clearly suggest a continued thickening of the lens on a population basis until well into the 80s in areas of the United States where cataract surgery is widely available.30
As for the possibility that apparent deepening of the central AC is due largely to selective treatment of patients with more narrow angles with iridectomy before age 70, we have observed previously that among a group of Mongolian patients, all of whom had had previous iridectomy, the apparent deepening of the angle in the 70s and above was still observed (unpublished data).
Beyond issues of bias, other physiologic explanations are possible for the apparent reversals of various biometric trends observed in the seventh decade: lens thickness could seem to decrease because of increased lens density and more rapid speed of sound waves on A-scan (as described previously), and refraction could seem more myopic because of acquired myopia from increasingly dense nuclear sclerotic lens opacity later in life.31,32 A possible explanation for apparent deepening of the AC in the 70s and above could be atrophy and posterior rotation of the ciliary body with aging, as suggested by recent studies with ultrasound biomicroscopy33 and magnetic resonance imaging.34 However, it is not clear that any combination of these phenomena could explain the constellation of deepening AC depth, decreasing hyperopia, and decreasing lens thickness that we observed among Eskimos in this study.
The significance of a finding of decreased risk for ACG among the very old, if in fact it does turn out to be true, is that fewer resources would need to be focused on detection and prophylaxis of ACG and narrow angles among the oldest patients, traditionally the most resource-intensive group to reach.
The limitations of this study must be acknowledged. In the first place, the Eskimo population examined was small, consisting of only 133 eligible patients. Thus, our conclusions with regard to this group must be provisional, particularly insofar as statements are made about subgroups by age and gender, where the number of patients is smaller still. However, the logistics involved in studying this scattered and inaccessible population mandates that large studies will generally be impractical. Moreover, many of the trends we observed among Eskimos have been replicated in other populations using identical techniques and devices.12,14
Second, the fact that the different populations we are comparing were examined by different investigators leaves open the possibility of bias or other systematic error. Some or all of the observed differences in the BG measurements between Eskimos and other races could have been due to systematic differences in technique between the investigators who measured the Eskimos (RW) and black and white patients (NC). Attempts were made to reduce or eliminate such systematic differences by carrying out a process of standardization before the study, but the possibility that these differences persisted cannot be excluded.
Finally, and perhaps most importantly, these data and those for Chinese, blacks, and whites with which they are compared are cross-sectional. On several occasions, we have drawn longitudinal inferences from these data, which must be done only with caution. As noted previously, the possibility that these inferences are confounded by a cohort effect cannot be excluded. The strongest justification for drawing attention to these longitudinal comparisons is the fact that comparisons between parameters such as AC depth between races, which do not take account of different patterns with aging, may mask true differences. Although the mean AC depth did not differ between Eskimos and blacks and whites, even a brief examination of the graph of AC depth over age (Fig 3) reveals very significant differences between these groups. Whether these differences observed in this study are due to increased prevalence of myopia between younger Eskimos and Chinese or whether they are real trends that may help us better to understand the enhanced risk of ACG among these populations, remains to be proven by long-term prospective studies.
Acknowledgments
Supported in part by an NEI Career Development Award to Dr. Congdon (K23 EY 00388) and a Research to Prevent Blindness Career Development Award, to Dr. Congdon.
Footnotes
Presented in part at the meeting of the American Glaucoma Society in Anaheim, California, February 2001.
References
- 1.Alsbirk PH. Anterior chamber depth and primary angle-closure glaucoma. I. An epidemiologic study in Greenland Eskimos. Acta Ophthalmol (Copenh) 1975;53:89–104. doi: 10.1111/j.1755-3768.1975.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 2.Alsbirk PH. Primary angle-closure glaucoma. Oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol. 1976;127(Suppl):5–31. [PubMed] [Google Scholar]
- 3.Alsbirk PH. Anatomical risk factors in primary angle-closure glaucoma. A ten year follow up survey based on limbal and axial anterior chamber depths in a high risk population. Int Ophthalmol. 1992;16:265–72. doi: 10.1007/BF00917973. [DOI] [PubMed] [Google Scholar]
- 4.Drance SM. Angle closure glaucoma among Canadian Eskimos. Can J Ophthalmol. 1973;8:252–4. [PubMed] [Google Scholar]
- 5.Arkell SM, Lightman DA, Sommer A, et al. The prevalence of glaucoma among Eskimos of northwest Alaska. Arch Ophthalmol. 1987;105:482–5. doi: 10.1001/archopht.1987.01060040052031. [DOI] [PubMed] [Google Scholar]
- 6.Congdon N, Wang F, Tielsch JM. Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv Ophthalmol. 1992;36:411–23. doi: 10.1016/s0039-6257(05)80022-0. [DOI] [PubMed] [Google Scholar]
- 7.Alsbirk PH. Anterior chamber depth, genes and environment. A population study among long-term Greenland Eskimo immigrants in Copenhagen. Acta Ophthalmol (Copenh) 1982;60:223–4. doi: 10.1111/j.1755-3768.1982.tb08376.x. [DOI] [PubMed] [Google Scholar]
- 8.Alsbirk PH. Limbal and axial chamber depth variations. A population study in Eskimos. Acta Ophthalmol (Copenh) 1986;64:593–600. doi: 10.1111/j.1755-3768.1986.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 9.Drance SM, Morgan RW, Bryett J, Fairclough M. Anterior chamber depth and gonioscopic findings among the Eskimos and Indians in the Canadian Arctic. Can J Ophthalmol. 1973;8:255–9. [PubMed] [Google Scholar]
- 10.van Rens GH, Arkell SM. Refractive errors and axial length among Alaskan Eskimos. Acta Ophthalmol (Copenh) 1991;69:27–32. doi: 10.1111/j.1755-3768.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- 11.Foster PJ, Alsbirk PH, Baasanhu J, et al. Anterior chamber depth in Mongolians: variation with age, sex, and method of measurement. Am J Ophthalmol. 1997;124:53–60. doi: 10.1016/s0002-9394(14)71644-7. [DOI] [PubMed] [Google Scholar]
- 12.Congdon NG, Youlin Q, Quigley H, et al. Biometry and primary angle-closure glaucoma among Chinese, white, and black populations. Ophthalmology. 1997;104:1489–95. doi: 10.1016/s0161-6420(97)30112-2. [DOI] [PubMed] [Google Scholar]
- 13.Congdon NG, Spaeth GL, Augsburger J, et al. A proposed simple method for measurement in the anterior chamber angle: biometric gonioscopy. Ophthalmology. 1999;106:2161–7. doi: 10.1016/S0161-6420(99)90499-2. [DOI] [PubMed] [Google Scholar]
- 14.Congdon NG, Foster PJ, Wamsley S, et al. Biometric gonioscopy and the effects of age, race, and sex on the anterior chamber angle. Br J Ophthalmol. 2002;86:18–22. doi: 10.1136/bjo.86.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam CS, Goh WS, Tang YK, et al. Changes in refractive trends and optical components of Hong Kong Chinese aged over 40 years. Ophthalmic Physiol Opt. 1994;14:383–8. [PubMed] [Google Scholar]
- 16.Goh WS, Lam CS. Changes in refractive trends and optical components of Hong Kong Chinese aged 19–39 years. Ophthalmic Physiol Opt. 1994;14:378–82. [PubMed] [Google Scholar]
- 17.Wong TY, Foster PJ, Ng TP, et al. Variations in ocular biometry in an adult Chinese population in Singapore: the Tanjong Pagar Survey. Invest Ophthalmol Vis Sci. 2001;42:73–80. [PubMed] [Google Scholar]
- 18.Seber GAF. Linear Regression Analysis. New York: John Wiley and Sons; 1977. pp. 125–39. [Google Scholar]
- 19.Lim ASM. Primary angle-closure glaucoma in Singapore. Aust J Ophthalmol. 1979;7:23–30. [Google Scholar]
- 20.Lowe RF. Clinical types of primary angle-closure glaucoma. Aust N Z J Ophthalmol. 1988;16:245–50. doi: 10.1111/j.1442-9071.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowe RF. Treatment for primary creeping (chronic) angle closure glaucoma. Asia Pac J Ophthalmol. 1990;2:91–4. [Google Scholar]
- 22.Byrne SF. A-scan Axial Eye Length Measurements. Mars Hill, NC: Grove Park; 1995. pp. 2–5. [Google Scholar]
- 23.Byrne SF, Green RL. Ultrasound of the Eye and Orbit. St. Louis: Mosby–Year Book; 1992. pp. 2–3. [Google Scholar]
- 24.Tornquist R. Shallow anterior chambers in acute glaucoma. Acta Ophthalmol (Copenh) 1953;31:1–74. [PubMed] [Google Scholar]
- 25.Foster PJ, Baasanhu J, Alsbirk PH, et al. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol. 1996;114:1235–41. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- 26.Cox JE. Angle-closure glaucoma among the Alaskan Eskimos. Glaucoma. 1984;6:135–7. [Google Scholar]
- 27.Fujita K. Epidemiology of acute angle-closure glaucoma. Report I Jpn J Clin Ophthalmol. 1996;37:625–9. [Google Scholar]
- 28.David R, Tessler Z, Yassur Y. Epidemiology of acute angle-closure glaucoma: incidence and seasonal variations. Ophthalmologica. 1985;191:4–7. doi: 10.1159/000309530. [DOI] [PubMed] [Google Scholar]
- 29.Seah SK, Foster PJ, Chew PT, et al. Incidence of acute primary angle-closure glaucoma in Singapore. An island-wide survey. Arch Ophthalmol. 1997;115:1436–40. doi: 10.1001/archopht.1997.01100160606014. [DOI] [PubMed] [Google Scholar]
- 30.Klein BEK, Klein R, Moss SE. Correlates of lens thickness: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1998;39:1507–10. [PubMed] [Google Scholar]
- 31.Wong TY, Klein BEK, Klein R, et al. Refractive errors and incident cataracts: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2001;42:1449–54. [PubMed] [Google Scholar]
- 32.Lim R, Mitchell P, Cumming RG. Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 1999;40:3021–6. [PubMed] [Google Scholar]
- 33.Marchini G, Pagliarusco A, Toscano A, et al. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 1998;105:2091–8. doi: 10.1016/S0161-6420(98)91132-0. [DOI] [PubMed] [Google Scholar]
- 34.Strenk SA, Semmlow JL, Strenk LM, et al. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–9. [PubMed] [Google Scholar]