Abstract
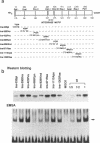
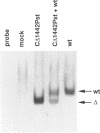
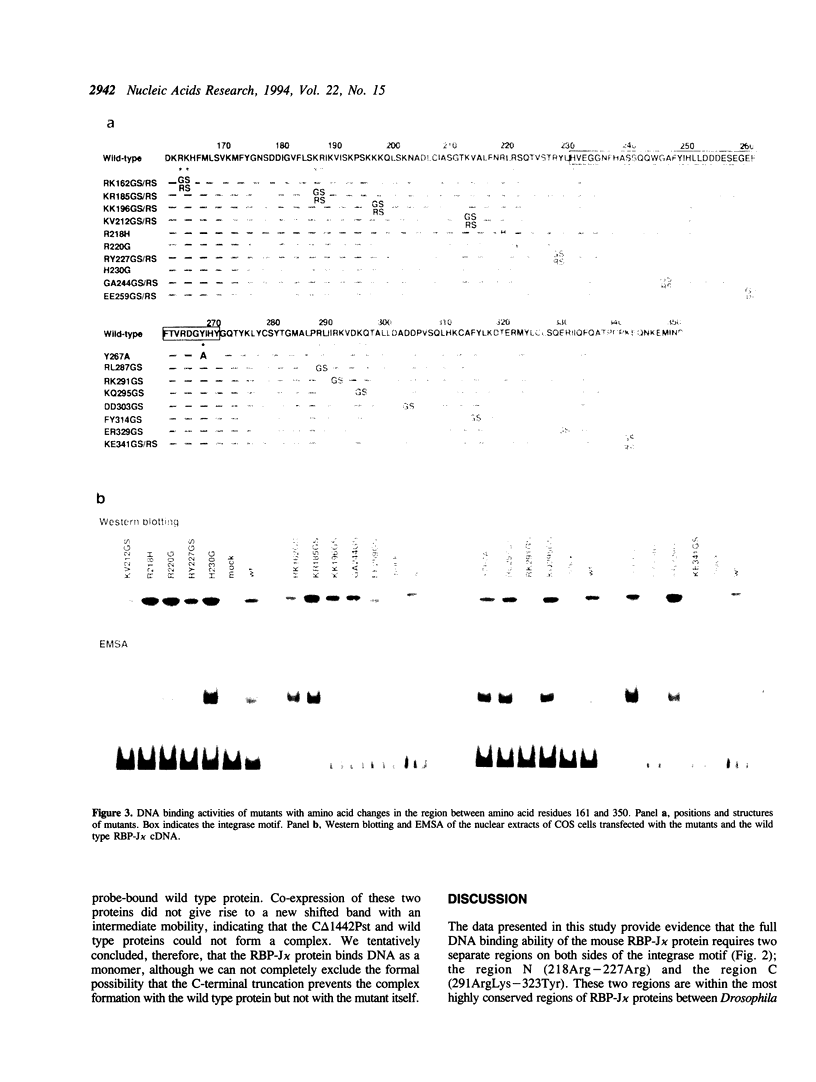
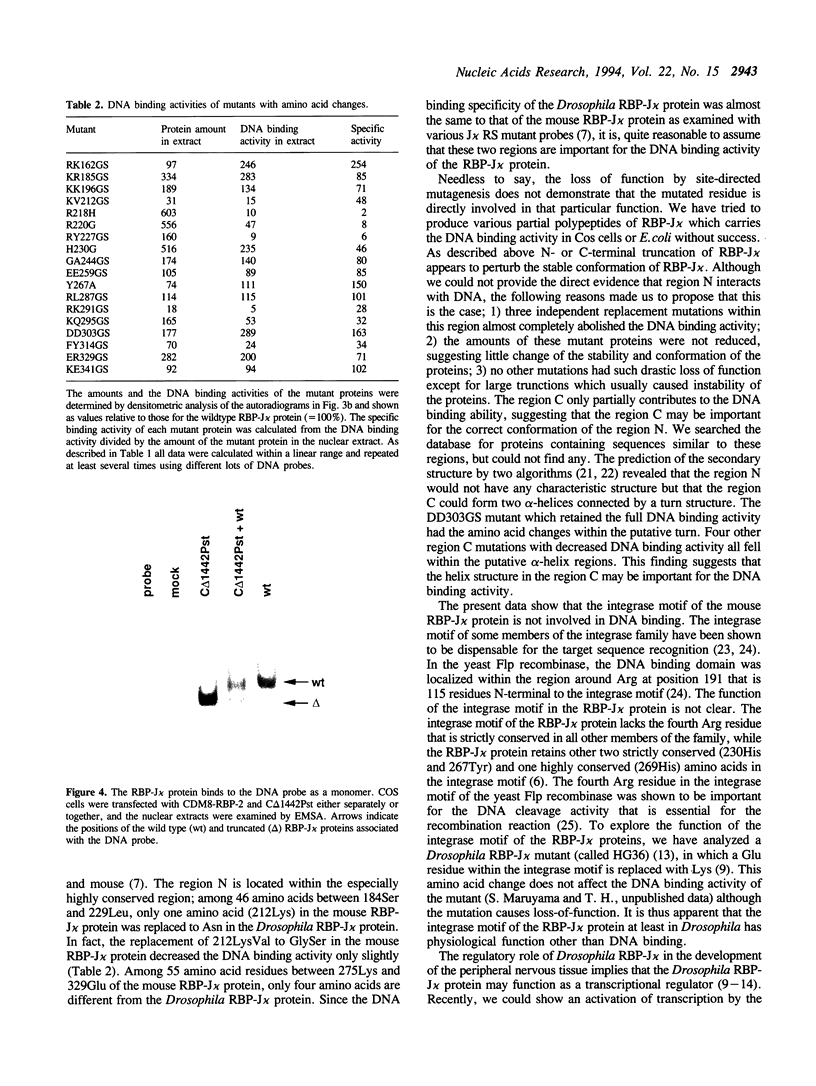
To map regions important for DNA binding of the mouse homologue of Suppressor of Hairless or RBP-J kappa protein, mutated mouse RBP-J kappa cDNAs were made by insertion of oligonucleotide linkers or base replacement. DNA binding assays using the mutated proteins expressed in COS cells showed that various mutations between 218 Arg and 227 Arg decreased the DNA binding activity drastically. The DNA binding activity was not affected by amino acid replacements within the integrase motif of the RBP-J kappa protein (230His-269His). Replacements between 291Arg and 323Tyr affected the DNA binding activity slightly but reproducibly. These results indicate that the region encompassing 218Arg-227Arg is critical for the DNA binding activity of RBP-J kappa. This region did not show any significant homology to motifs or domains of the previously described DNA binding proteins. Using a truncation mutant protein RBP-J kappa was shown to associate with DNA as a monomer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amakawa R., Jing W., Ozawa K., Matsunami N., Hamaguchi Y., Matsuda F., Kawaichi M., Honjo T. Human Jk recombination signal binding protein gene (IGKJRB): comparison with its mouse homologue. Genomics. 1993 Aug;17(2):306–315. doi: 10.1006/geno.1993.1326. [DOI] [PubMed] [Google Scholar]
- Amin A. A., Sadowski P. D. Synthesis of an enzymatically active FLP recombinase in vitro: search for a DNA-binding domain. Mol Cell Biol. 1989 May;9(5):1987–1995. doi: 10.1128/mcb.9.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Landy A., Abremski K., Egan J. B., Haggard-Ljungquist E., Hoess R. H., Kahn M. L., Kalionis B., Narayana S. V., Pierson L. S., 3rd The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986 Feb;5(2):433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. III. Hypomorphic and hypermorphic mutations affecting the expression of hairless. Genetics. 1982 Jul-Aug;101(3-4):447–459. doi: 10.1093/genetics/101.3-4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang A. G., Hartenstein V., Posakony J. W. Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development. 1991 Jan;111(1):89–104. doi: 10.1242/dev.111.1.89. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen H., Sadowski P. D. Mutagenesis of a conserved region of the gene encoding the FLP recombinase of Saccharomyces cerevisiae. A role for arginine 191 in binding and ligation. J Mol Biol. 1992 May 20;225(2):313–326. doi: 10.1016/0022-2836(92)90924-9. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Kawaichi M., Matsunami N., Ryo H., Nishida Y., Honjo T. The Drosophila RBP-J kappa gene encodes the binding protein for the immunoglobulin J kappa recombination signal sequence. J Biol Chem. 1991 Dec 5;266(34):23334–23340. [PubMed] [Google Scholar]
- Furukawa T., Maruyama S., Kawaichi M., Honjo T. The Drosophila homolog of the immunoglobulin recombination signal-binding protein regulates peripheral nervous system development. Cell. 1992 Jun 26;69(7):1191–1197. doi: 10.1016/0092-8674(92)90640-x. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Matsunami N., Yamamoto Y., Honjo T. Purification and characterization of a protein that binds to the recombination signal sequence of the immunoglobulin J kappa segment. Nucleic Acids Res. 1989 Nov 25;17(22):9015–9026. doi: 10.1093/nar/17.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi Y., Yamamoto Y., Iwanari H., Maruyama S., Furukawa T., Matsunami N., Honjo T. Biochemical and immunological characterization of the DNA binding protein (RBP-J kappa) to mouse J kappa recombination signal sequence. J Biochem. 1992 Sep;112(3):314–320. doi: 10.1093/oxfordjournals.jbchem.a123898. [DOI] [PubMed] [Google Scholar]
- Kammann M., Laufs J., Schell J., Gronenborn B. Rapid insertional mutagenesis of DNA by polymerase chain reaction (PCR). Nucleic Acids Res. 1989 Jul 11;17(13):5404–5404. doi: 10.1093/nar/17.13.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaichi M., Oka C., Shibayama S., Koromilas A. E., Matsunami N., Hamaguchi Y., Honjo T. Genomic organization of mouse J kappa recombination signal binding protein (RBP-J kappa) gene. J Biol Chem. 1992 Feb 25;267(6):4016–4022. [PubMed] [Google Scholar]
- Knust E., Tietze K., Campos-Ortega J. A. Molecular analysis of the neurogenic locus Enhancer of split of Drosophila melanogaster. EMBO J. 1987 Dec 20;6(13):4113–4123. doi: 10.1002/j.1460-2075.1987.tb02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami N., Hamaguchi Y., Yamamoto Y., Kuze K., Kangawa K., Matsuo H., Kawaichi M., Honjo T. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature. 1989 Dec 21;342(6252):934–937. doi: 10.1038/342934a0. [DOI] [PubMed] [Google Scholar]
- NASH D. THE EXPRESSION OF "HAIRLESS" IN DROSOPHILA AND THE ROLE OF TWO CLOSELY LINKED MODIFIERS OF OPPOSITE EFFECT. Genet Res. 1965 Jul;6:175–189. doi: 10.1017/s0016672300004079. [DOI] [PubMed] [Google Scholar]
- Nash D. The Mutational Basis for the "Allelic" Modifier Mutants, ENHANCER and SUPPRESSOR OF HAIRLESS, of DROSOPHILA MELANOGASTER. Genetics. 1970 Mar;64(3-4):471–479. doi: 10.1093/genetics/64.3-4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Parsons R. L., Evans B. R., Zheng L., Jayaram M. Functional analysis of Arg-308 mutants of Flp recombinase. Possible role of Arg-308 in coupling substrate binding to catalysis. J Biol Chem. 1990 Mar 15;265(8):4527–4533. [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. Molecular characterization of two proteins involved in the excision of the conjugative transposon Tn1545: homologies with other site-specific recombinases. EMBO J. 1989 Aug;8(8):2425–2433. doi: 10.1002/j.1460-2075.1989.tb08373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth F., Posakony J. W. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992 Jun 26;69(7):1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- Struhl K. Promoters, activator proteins, and the mechanism of transcriptional initiation in yeast. Cell. 1987 May 8;49(3):295–297. doi: 10.1016/0092-8674(87)90277-7. [DOI] [PubMed] [Google Scholar]
- Tun T., Hamaguchi Y., Matsunami N., Furukawa T., Honjo T., Kawaichi M. Recognition sequence of a highly conserved DNA binding protein RBP-J kappa. Nucleic Acids Res. 1994 Mar 25;22(6):965–971. doi: 10.1093/nar/22.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]