Abstract
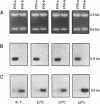
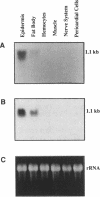
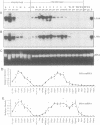
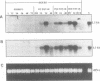
Two gene-specific probes were generated from the unique sequences in the 3' non-coding regions of the two insecticyanin genes, ins-a and ins-b to study the developmental expression of these genes in Manduca sexta. Both genes were initially transcribed in the freshly hatched first instar larvae and then expressed in the epidermis and to a lesser degree in the fat body during every larval feeding stage. In the epidermis of the 4th and 5th instar larvae, both mRNAs appeared shortly before ecdysis and accumulated to maximal levels within a day. As the larval epidermis became pupally committed on day 3 of the 5th (final) instar, INS-a mRNA quickly decreased, whereas INS-b mRNA showed a second peak of accumulation. In the fat body, both genes showed a similar expression pattern within the 4th instar to that of the epidermis except that levels were lower and ins-b mRNA dominated. In the final instar, only ins-b mRNA was present in significant amount. These findings not only reveal that the two duplicated insecticyanin genes have diverged in their expression pattern but also demonstrate, for the first time, that fat body also expresses insecticyanin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Anderson R. A reproducible microanalytical method for the detection of specific RNA sequences by dot-blot hybridization. Anal Biochem. 1984 Feb;137(1):15–19. doi: 10.1016/0003-2697(84)90339-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goodman W. G., Adams B., Trost J. T. Purification and characterization of a biliverdin-associated protein from the hemolymph of Manduca sexta. Biochemistry. 1985 Feb 26;24(5):1168–1175. doi: 10.1021/bi00326a017. [DOI] [PubMed] [Google Scholar]
- Hiruma K., Hardie J., Riddiford L. M. Hormonal regulation of epidermal metamorphosis in vitro: control of expression of a larval-specific cuticle gene. Dev Biol. 1991 Apr;144(2):369–378. doi: 10.1016/0012-1606(91)90429-7. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Spoerel N., Mitsialis S. A., Nguyen H. T., Romano C., Lingappa J. R., Mariani B. D., Rodakis G. C., Lecanidou R., Tsitilou S. G. Developmental control and evolution in the chorion gene families of insects. Adv Genet. 1987;24:223–242. doi: 10.1016/s0065-2660(08)60009-7. [DOI] [PubMed] [Google Scholar]
- Law J. H., Wells M. A. Insects as biochemical models. J Biol Chem. 1989 Oct 5;264(28):16335–16338. [PubMed] [Google Scholar]
- Li W., Riddiford L. M. Two distinct genes encode two major isoelectric forms of insecticyanin in the tobacco hornworm, Manduca sexta. Eur J Biochem. 1992 Apr 15;205(2):491–499. doi: 10.1111/j.1432-1033.1992.tb16805.x. [DOI] [PubMed] [Google Scholar]
- Ohta T. Multigene families and the evolution of complexity. J Mol Evol. 1991 Jul;33(1):34–41. doi: 10.1007/BF02100193. [DOI] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987 Sep;1(3):209–214. doi: 10.1096/fasebj.1.3.3622999. [DOI] [PubMed] [Google Scholar]
- Riddiford L. M. Changes in translatable mRNAs during the larval-pupal transformation of the epidermis of the tobacco hornworm. Dev Biol. 1982 Aug;92(2):330–342. doi: 10.1016/0012-1606(82)90179-8. [DOI] [PubMed] [Google Scholar]
- Riddiford L. M. Ecdysone-induced change in cellular commitment of the epidermis of the tobacco hornworm, Manduca sexta, at the initiation of metamorphosis. Gen Comp Endocrinol. 1978 Apr;34(4):438–446. doi: 10.1016/0016-6480(78)90284-8. [DOI] [PubMed] [Google Scholar]
- Riddiford L. M., Palli S. R., Hiruma K., Li W., Green J., Hice R. H., Wolfgang W. J., Webb B. A. Developmental expression, synthesis, and secretion of insecticyanin by the epidermis of the tobacco hornworm, Manduca sexta. Arch Insect Biochem Physiol. 1990;14(3):171–190. doi: 10.1002/arch.940140305. [DOI] [PubMed] [Google Scholar]
- Riley C. T., Barbeau B. K., Keim P. S., Kézdy F. J., Heinrikson R. L., Law J. H. The covalent protein structure of insecticyanin, a blue biliprotein from the hemolymph of the tobacco hornworm, Manduca sexta L. J Biol Chem. 1984 Nov 10;259(21):13159–13165. [PubMed] [Google Scholar]
- Schmidt F. S., Skerra A. The bilin-binding protein of Pieris brassicae. cDNA sequence and regulation of expression reveal distinct features of this insect pigment protein. Eur J Biochem. 1994 Feb 1;219(3):855–863. doi: 10.1111/j.1432-1033.1994.tb18567.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]