Abstract
Clinical question:
Is there any new knowledge about the pathogenesis and treatment of age-related macular degeneration (AMD)?
Results:
We now understand better the biochemical and pathological pathways involved in the genesis of AMD. Treatment of exudative AMD is based on intravitreal injection of new antivascular endothelial growth factor drugs for which there does not yet exist a unique recognized strategy of administration. No therapies are actually available for atrophic AMD, despite some experimental new pharmacological approaches.
Implementation:
strategy of administration, safety of intravitreal injection
Keywords: age-related macular degeneration, antivascular endothelial growth factor, choroidal neovascularization, drusen, geographic atrophy
Age-related macular degeneration
Definition:
Age-related macular degeneration (AMD) is a condition characterized, in the early stages, by slow development and progression, absence of symptoms over a number of years, and extensive retinal deposits called drusen, often associated with pigmentary abnormalities (early AMD). The advanced presentation of AMD also includes geographic atrophy and choroidal neovascularization. The patient complains of metamorphopsia, discromatopsia, vision loss, and central scotoma.
Incidence:
AMD is the leading cause of blindness and visual disability in patients over the age of 55 years in developed countries. The prevalence of early AMD is 18% in the population aged 65–74 years and 30% in the population older than 74 years. Approximately 10%–15% of patients with AMD have severe central vision loss. The prevalence of geographic atrophy (atrophic AMD) is 3.5% in subjects older than 75 years, and accounts for approximately half the prevalence of choroidal neovascularization (exudative or neovascular AMD). Atrophic AMD accounts for approximately 25% of cases with severe central vision loss. Exudative AMD accounts for approximately 75% of cases with severe central vision loss.
Search sources:
PubMed, Cochrane Library, Association for Research in Vision and Ophthalmology meeting abstracts, ClinicalTrial.gov.
Methods:
A search of the English language literature was carried out using the PubMed and Cochrane Library databases from January 2000 through to December 2010. For the same period, we referred to ClinicalTrial.gov and also analyzed in detail abstracts (sessions related to AMD, genetics, and new drugs) from the Association for Research in Vision and Ophthalmology meetings, looking for relevant studies in this field. The following keywords were used in the search: “genetic factors,” “pathogen,” “retinal pigment epithelium,” “Bruch’s membrane,” “choroid,” “drusen,” “immunol” (MESH), “anti-VEGF drugs,” “new antiangiogen” (MESH), “intravitreal,” “prn,” “verteporfin,” “geographic atrophy,” “vitamins,” “fluorophores,” “myocardial infarction,” “mortality,” “adverse events,” and “safe.” The reference sections of papers located in this way were then searched for other relevant studies. A few articles published before 2000 were included for historical purposes, but the present review is based mainly on articles published in the past decade.
Outcomes:
Improvement of knowledge about AMD pathology, efficacy of new therapies, and avoidance of complications.
Consumer summary:
AMD is an aging process of the central retina that may compromise high-resolution perception of images. There are new findings about the onset and development of the disease. There is good evidence that new intravitreal injection therapies are effective and safe.
The evidence
Is there any new information about how AMD arises and develops?
Systematic reviews: 4
Case-control studies: 2
Cohort study: 1
Case series: 1
Longitudinal prospective studies: 2
Preclinical studies: 3
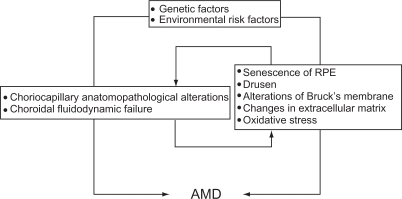
The systematic reviews concluded that, with advancing age, changes occur in macular structure, such as in the retinal pigment epithelium and Bruch’s membrane, as well as in ocular blood flow.1,2 Genetics may also play a role in the pathogenesis of AMD. The complex retinal pigment epithelium/choroid in the old normal mouse becomes an immunologically active tissue, recruits leukocytes from the circulation, and activates the complement cascade. On this basis, extrapolating to the human retina, it is supposed that the existence of an error in regulation of immunological activity causes AMD in an elderly individual.3 Although the pathogenesis of the disease is unknown, oxidative stress might have a meaningful role. Treatment with antioxidant vitamins and zinc can reduce the risk of developing advanced AMD by about a quarter in patients at moderate risk.4
Rod outer segment phagocytotic activity in immortalized human retinal pigment epithelium/Bruch’s membrane is significantly higher on younger than on older Bruch’s membrane. This effect can be mimicked by nonenzymatic nitration of the extracellular matrix in vitro. Such changes due to aging of the retinal pigment epithelium/Bruch’s membrane seem to have a role in the pathogenesis of AMD.5 Retrospective observational case series have shown a relative new feature of AMD, ie, reticular pseudodrusen, considered to be a potential risk factor for late age-related maculopathy. Spectral-domain optical coherence tomography (OCT) shows granular hyper-reflective material located primarily between the retinal pigment epithelium and the inner and outer segments of the photoreceptors. This unexpected location suggests that potential pathophysiologic mechanisms on both sides of the retinal pigment epithelium need to be taken into account in theories related to the development of AMD.6–8
Family and twin studies have shown that susceptibility to AMD is genetically influenced. Genetic investigations have shown that complement factor H, a regulator of the alternative complement pathway, and LOC387715/ARMS2 and HTRA1 genes, are the most consistent genetic risk factors for AMD. The Y402H coding variant (rs1061170 according to the Single Nucleotide Polymorphism Database nomenclature) in the complement factor H gene on chromosome 1, area q31, has been extensively studied via genetic and molecular approaches which provide strong statistical evidence for a disease association and a plausible biological context supporting this variant as an attractive candidate for a causal polymorphism leading to the development of AMD. However, the compelling association between Y402H and AMD observed in European cohorts is not as relevant to the disease risk in populations of Asian ancestry. The tyrosine-to-histidine polymorphism (rs1061170) at amino acid 402 of complement factor H may be a primary pathogenic variation increasing the risk of developing AMD. Single nucleotide polymorphisms in chromosome 10q26 are associated with the risk of having AMD in several populations. Because several 10q26 single nucleotide polymorphisms are associated with disease risk, it has been difficult to determine if the T allele of rs10490924 located in the coding region of the LOC387715/ARMS2 (age-related maculopathy susceptibility 2) gene, or the A allele of rs11200638 located in the promoter region of high-temperature requirement factor A1 (HTRA1) has a role in the pathogenesis of the disease. The HTRA1 gene encodes a heat shock serine protease which is expressed in the retina and can regulate transforming growth factor beta signaling. The LOC387715/ARMS2 encodes a mitochondrial outer membrane protein that is also expressed in the retina.9–12 However, the identification of genetic factors has not resulted in therapeutic strategies to modify the disease so far, and additional genetic and environmental factors are yet to be discovered to influence the onset and the progression of AMD.13
Conclusions
Alterations in macular structure that occur with advancing age, retinal blood flow modification, activation of inflammation pathways, and genetics can interact in the onset and development of AMD (Figure 1).
Figure 1.
Main factors that may interact in the onset and development of age-related macular degeneration.
Are new therapies for AMD effective?
Systematic reviews: 5
Meta-analyses: 1
Randomized controlled trials: 19
Case series: 1
Longitudinal prospective studies: 4
Longitudinal retrospective study: 1
Preclinical studies: 2
Concerning neovascular AMD, systematic reviews conclude that the prognosis is improved by clinical use of antivascular endothelial growth factor (anti-VEGF) drugs that target all isotypes of VEGF and maintain vision in over 90% and substantial improvement in 25%–40% of patients (Table 1).14,15
Table 1.
Results of principal anti-vascular endothelial growth factor studies in age-related macular degeneration.
| Name of study | Type of study | Patients (n) | Treatment modality | Choroidal neovascularization type | Losing fewer than 15 letters at year 11 | Losing fewer than 15 letters at 2 years1 | Improvements of 1 or more lines in vision at year 11 | Improvements of 1 or more lines in vision at year 21 | Duration |
|---|---|---|---|---|---|---|---|---|---|
| VISION | RCT | 1186 | Monthly intravitreal injections of pegaptanib or sham treatment | All subfoveal angiographic lesion types | yes | 2 years | |||
| MARINA | RCT | 716 | Monthly intravitreal injections of 0.3 or 0.5 mg of Ranibizumab or sham treatment | Minimally classic or purely occult choroidal neovascularization | yes | yes | yes | yes | 2 years |
| ANCHOR | RCT | 423 | Monthly intravitreal injections of 0.3 or 0.5 mg of Ranibizumab or standard PDT indicated at 3 month intervals | Predominantly classic subfoveal choroidal neovascularization because of AMD | yes | yes | 2 years | ||
| PIER | RCT | 182 | Ranibizumab intravitreal administrations monthly for three doses followed by dosing 0.3 or 0.5 mg every 3 months or sham treatment | All types of choroidal neovascularization | yes | yes | yes | 2 years | |
| SUSTAIN | RCT | 531 | Whether treatment naïve or completed treatment with ranibizumab or verteporfin photodynamic therapy | All subfoveal angiographic lesion types | yes | 1 year | |||
| EXCITE | RCT | 353 | 0.3 mg quarterly, 0.5 mg quarterly or 0.3 mg monthly doses of ranibizumab. After a loading phase of 3 consecutive monthly injections | All subfoveal angiographic lesion types | yes | 1 year | |||
| PRONTO | Prospective, uncontrolled | 40 | Loading phase of 3 consecutive monthly intravitreal injections of ranibizumab 0.5 mg followed by retreatment in case of OCT evidence | All subfoveal angiographic lesion types | yes | yes | 2 years |
Statistically significant difference between the sham/no ranibizumab treatment and doses of ranibizumab administered.
Abbreviations: RCT, randomized controlled trial; VISION, VEGF inhibition study in ocular neovascularization; MARINA, Minimally classic/occult trial of the Anti-VEGF antibody ranibizumab in the treatment of neovascular AMD; ANCHOR, Antivascular endothelial growth factor (VEGF) antibody for the treatment of predominantly classic choroidal neovascularization (CNV) in age-related macular degeneration; PIER, Phase IIIb, multicenter, randomized, double-masked, sham injection-controlled study of efficacy and safety of ranibizumab in subjects with subfoveal choroidal neovascularization with or without classic CNV secondary to age-related macular degeneration; SUSTAIN, Study of ranibizumab in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration; EXCITE, Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration; PRONTO, Prospective optical coherence tomography (OCT) imaging of patients with neovascular age-related macular degeneration (AMD) treated with intra-ocular Lucentis™ (ranibizumab)
Clinical trials have established the efficacy of treating patients with choroidal neovascularization in AMD using antiangiogenic therapies in a regimented continuous dosing schedule. Other trials have investigated the use of these agents on an as-needed dosing schedule. Monthly intravitreal injection of ranibizumab was demonstrated to be superior to placebo and also to photodynamic therapy in minimally classic and occult subtypes of neovascular AMD.16,17
Long-term regular follow-up is necessary for patients treated with ranibizumab to obtain and preserve any significant visual acuity gain, and not only to achieve visual stabilization.18 Ranibizumab given monthly for three months was superior to placebo for treatment of neovascular AMD.19 However, visual gain and overall results were less favorable compared with monthly intravitreal injection of ranibizumab. There is evidence that after the first three monthly injections of ranibizumab, retreatment should be performed when typical signs of active lesion occur, such as deterioration of visual acuity and retinal thickening.20 It was clearly demonstrated that quarterly maintenance dosing of ranibizumab was inferior to monthly dosing in the treatment of all types of neovascular AMD.21
An OCT-guided variable dosing regimen with intravitreal ranibizumab resulted in visual acuity outcomes comparable with those of previous Phase III studies over 12 and 24 months, but with fewer injections.22–24 Uncertainty remains regarding the relative benefits of pegaptanib compared with ranibizumab and other unlicensed drugs (eg, bevacizumab), due to the nature of the evidence. Head-to-head randomized controlled trials and economic evaluations comparing these alternatives are needed.24–26 Combination therapy is likely to play an increasing role in treating neovascular AMD, and may improve visual acuity outcomes, reduce retreatment rates, and consequently extend treatment-free intervals.27 Combination with photodynamic therapy seems to reduce the number of injections and to maintain the effects of treatment for longer.28 This may lead to a lower treatment burden for many patients and potentially to a reduction of overall treatment costs. Numerous new drugs under investigation suggest that new combinations will follow.
New antiangiogenic approaches acting in various steps of the angiogenic cascade are being studied and may soon be available.29 Randomized controlled clinical trials are necessary to support the use of these molecules, the production of which is growing. Among the factors involved in angiogenesis, the most attention has been given to VEGF. Many modalities other than VEGF blockers are under investigation for their potential to inhibit VEGF, including VEGF Trap-Eye (Regeneron Pharmaceuticals Inc, Tarrytown, NY) and small interfering RNA therapies. Other investigational strategies have focused on blocking the downstream effects of VEGF and targeting other factors involved in angiogenesis. VEGF Trap-Eye is a recombinant protein consisting of the binding regions of VEGF receptors 1 and 2 and the constant region of human immunoglobulin, G1. The binding of VEGF Trap-Eye to VEGF forms an inert stable complex, thereby blocking proangiogenic effects.30
RNA interference has a catalytic mechanism that enables one small interfering RNA (siRNA) molecule to facilitate the cleavage of thousands of mRNA molecules. Two siRNAs are under investigation as potential treatments for neovascular AMD, one targeting VEGF-A (bevasiranib, OPKO Health, Miami, FL)31 and the other targeting the VEGF receptor 1 (AGN211745 or SIRNA-027, Allergan, Irvine, CA).32 VEGF binds to two receptor tyrosine kinases, ie. VEGF R1 and VEGF R2. Several tyrosine kinase inhibitors may provide a means to target these receptors, including vatalanib (previously known as PTK787, Novartis AG, Basel, Switzerland).33 Vatalanib blocks the phosphorylation of VEGF and platelet-derived growth factor receptors, and has been shown to prevent retinal neovascularization induced by ischemia in mice. Vatalanib is currently under investigation in clinical trials for patients with choroidal neovascularization due to AMD.
Multikinase inhibitors, such as TG100572 (TargeGen, San Diego, CA), are also under investigation for neovascular AMD.34 Delivery of TG100572 to the retina and choroid seems to be enhanced by administration in the form of a prodrug, TG100801. Topical use of TG100801 (TargeGen)35 suppressed laser-induced choroidal neovascularization in mice and reduces fluorescein leakage and retinal thickening in a rat model of retinal vein occlusion. Other multikinase inhibitors under investigation include pazopanib (GW786034, GlaxoSmithKline, Philadelphia, PA),36 which inhibits VEGF receptors 1, 2, and 3, platelet-derived growth factor receptors α and β, and c-kit. Neovascular AMD is more than simply a vascular disease, and includes not only angiogenic but also vascular and inflammatory components. The important role of complement in the pathogenesis of neovascular AMD has raised interesting possibilities for the development of new treatments. A number of agents are under investigation as potential complement inhibitors, including TA106 (a monoclonal antibody against complement 3, Taligen Therapeutics, Aurora, CO), POT-4 (a peptide that binds to complement 3, Potential Pharmaceuticals, Louisville, KY),37 and ARC1905 (an anticomplement 5 aptamer, Ophthotech, Princeton, NJ).38
There is also evidence for the involvement of a wide variety of inflammatory cells in neovascular AMD pathogenesis. Targeting leucocytes or adhesion molecules involved in their recruitment may provide the potential for new treatments. For example, blockade of vascular adhesion protein-1, an endothelial cell adhesion molecule involved in leucocyte recruitment, has been shown to reduce choroidal neovascularization size, fluorescein leakage, and macrophage accumulation, while reducing the expression of inflammatory mediators, such as tumor necrosis factor-α, monocyte chemoattractant protein-1, and intercellular adhesion molecule-1.
Platelet-derived growth factor may also play a role in choroidal neovascularization, and acts as a mitogen for pericytes, smooth muscle cells, fibroblasts, and other mesenchymal cells. Pericytes may be critical for the maintenance of established blood vessels, and may be important in the maturation process in choroidal neovascularization. There is evidence that, in newly formed vessels, there is a period during which endothelial cells depend on VEGF and are responsive to the withdrawal of platelet-derived growth factor. In contrast, established mature vessels may exhibit less dependence on VEGF and may be associated with resistance to anti-VEGF, or with rapid recurrence of choroidal neovascularization due to AMD. These observations are consistent with evidence that blockade of both VEGF-A and platelet-derived growth factor is more effective at suppressing neovascularization in experimental models than blockade of VEGF-A alone. Platelet-derived growth factor has been shown to stimulate angiogenesis and pericyte recruitment. The loss of pericytes from retinal vessels may be associated with vascular abnormalities and instability, including the formation of microaneurysms and vascular permeability. Blocking platelet-derived growth factor appears to result in loss of pericytes and possible regression of maturing neovascularization. Continued production of VEGF-A by pericytes under certain conditions may protect endothelial cells from the effects of VEGF-A blockade by anti-VEGF agents. Anti-platelet-derived growth factor, Aptamer E10030 (Ophthotech), targets platelet-derived growth factor which regulates neovascular pericytes.39 In preclinical models, E10030 successfully induced neovascular regression when administered in combination with anti-VEGF agents. This effect is further supported by published studies in which inhibition of binding of platelet-derived growth factor to its receptor, PDGFR-β, plus an anti-VEGF agent, causes neovascular regression in ocular angiogenesis models. A Phase II study of E10030 in combination with ranibizumab is assessing the efficacy and safety of this combination treatment regimen.
Integrins are cell adhesion molecules involved in the mediation of cell-cell, cell-extracellular matrix, and cell-pathogen interactions. Structurally, integrins consist of a heterodimer with a 1α and a 1β subunit. There is growing evidence that β1 integrins are involved in angiogenesis, especially with regard to the maturation and organization of neovascularization. Blockade of integrins has been shown to inhibit endothelial cell proliferation and induce apoptosis, while several inhibitors of angiogenesis, such as endostatin, can mediate their effects by binding to integrins. Integrins have also been shown to play a role in VEGF-mediated cell adhesion and endothelial cell migration, which can be suppressed by blocking integrin α5β1 with antibodies. There has been interest in the role of integrins in neovascular AMD, based on evidence that integrin receptors play important roles in tumor angiogenesis. Blockade of integrin α5β1, the most important of the fibronectin receptors, has been shown to result in suppression of tube formation stimulated by VEGF and in apoptosis of proliferating endothelial cells. An anti-α5β1 antibody inhibited VEGF-dependent and VEGF-independent angiogenesis in an experimental model, while anti-VEGF inhibited only VEGF-dependent angiogenesis. Volociximab (M200, Ophthotech), a chimeric monoclonal antibody targeting integrin α5β1 to block its ligation of fibronectin, has robustly inhibited human umbilical vein endothelial cell tube formation in laboratory tests. It does so regardless of initial growth factor stimulation. It has also inhibited neovascularization in primate choroid tissue and tumor angiogenesis in rabbits.40
Pigment epithelium-derived factor is an antiangiogenic factor that counters the effects of VEGF. Therefore, administering pigment epithelium-derived factor could have potential antiangiogenic effects, although high doses appear to have the opposite effect and lead to increased neovascularization. It may also be possible to increase local production of this factor by delivering the pigment epithelium-derived factor gene to the retina using an adenovector.
Evidence is gathering concerning the role of an activated immune system in AMD. Accordingly, overexpression of the pleiotropic cytokine, tumor necrosis factor, which is mainly produced by T cells and monocytes, has been found in neovascular membranes of eyes with AMD. Interactions between tumor necrosis factor and its receptor(s) are important for the regulation of the activities of the retinal pigment epithelium, including cell attachment, spreading, chemotaxis, migration, and proliferation. Expression of various apoptotic factors in retinal pigment epithelium cells in diseases such as AMD is upregulated by tumor necrosis factor. A drug that interferes with tumor necrosis factor/tumor necrosis factor receptor interactions may represent a legitimate therapeutic approach for patients with neovascular AMD.41
A variety of other potential antiangiogenic treatments could merit further investigation in neovascular AMD, including microtubule inhibitors, nucleic acid therapies, peroxisome proliferator-activated receptor agonists, and rapamycin. Further investigation is needed to determine whether these agents have the potential to target angiogenesis and improve outcomes in neovascular AMD.
Systematic reviews suggest that, unlike neovascular AMD, there is no treatment for geographic atrophy presently approved by health authorities.
In one study, people at high risk of developing advanced AMD lowered their risk by about 25% when treated with a high-dose combination of vitamin C, vitamin E, beta-carotene, and zinc. In the same high-risk group, these nutrients reduced the risk of vision loss caused by advanced AMD by about 19%. For those study participants who had either no AMD or early AMD, the nutrients did not provide an apparent benefit.42 Observational studies have also suggested benefits from increased dietary intake of macular xanthophylls and omega-3 fatty acids.43
Fenretinide, a hydrophobic drug structurally derived from vitamin A, reduces lipofuscin deposition interfering with vitamin A transport proteins, known as fluorophores, that seem to contribute directly to the pathogenesis of AMD.44 Fenretinide has shown positive results for slowing the progression of dry macular degeneration.45
Many publications have linked both oxidative stress and inflammation to the progression of geographic atrophy. OT-551 is a topically-dosed, patented small molecule that acts on oxidative stress and disease-induced inflammation.46 Topical OT-551 has been demonstrated to be well tolerated by patients with geographic atrophy and not associated with any serious adverse effects. However, the absence of significant effects on other outcomes suggests that OT-551, with the current concentration and mode of delivery, may have limited or no benefit as a treatment for geographic atrophy.47
Conclusions
There are still many unanswered questions to be resolved by future research. Regarding exudative AMD treatment, what regimen of anti-VEGF administration is better, ie. monthly dosing or OCT-guided dosing? How many patients need retreatment, and how many injections are needed during the second and third year? Do any situations alter the pharmacogenetics of anti-VEGF drugs (eg, vitrectomy surgery or glaucoma medications)?
Quarterly injections of ranibizumab seems to result in inferior visual outcomes compared with monthly dosing. An OCT-guided regimen appeared to achieve visual results similar to those from monthly dosing, with fewer injections, but patients still require monthly visits, examinations, and OCT assessment. As regards geographic atrophy, it may be possible to reduce the progression of dry AMD with a multi-vitamin integration, and many molecules are being tested.
Are new therapies for AMD safe?
Systematic review: 1
Randomized controlled trials: 2
Case series: 1
Longitudinal retrospective study: 1
Recent retrospective cohort studies have included patients receiving photodynamic therapy, intravitreal pegaptanib, bevacizumab, or ranibizumab. The mortality risk was significantly lower with ranibizumab therapy than with photodynamic therapy or pegaptanib use, and the risk of myocardial infarction was significantly lower with ranibizumab than with photodynamic therapy. There were no significant differences in the mortality or myocardial infarction risk between bevacizumab use and the other therapies. No significant relationship was found between treatment group and bleeding events or stroke.48
A review of the current literature suggests that anti-VEGF is generally a safe and effective treatment for neovascular AMD for up to 2–3 years. Presently, there is Level 1 evidence to substantiate this conclusion for pegaptanib and ranibizumab, but not for bevacizumab.49 Multiple intravitreal ranibizumab injections are safe over the long term for treating choroidal neovascularization in AMD. Ranibizumab is well tolerated, and the incidence of serious adverse events is low and unrelated to dose.50,51 Regarding local complications, intravitreal injection may generate subconjunctival hemorrhage, followed by a transient increase in intraocular pressure, tractional retinal detachments, bacterial endophthalmitis, and uveitis. More rarely observed are rhegmatogenous detachment and vitreous hemorrhage.52
Conclusions
There is no significant clinical evidence that use of anti-VEGF drugs is associated with systemic adverse events. Some ocular complications may occur.
The practice
Potential pitfalls
While monthly injections of intravitreal anti-VEGF therapy for neovascular AMD have produced visual outcomes superior to prior therapies, the frequency of office visits and injections can place a tremendous burden on patients and the health care system. Unfortunately, large and potentially devastating submacular hemorrhages may occur almost immediately after a high-quality OCT examination showing an absence of fluid. Theoretically, eyes treated with an OCT-guided “as needed” regimen, in which patients may go for long intervals without VEGF suppression, could be at greater risk for sight-threatening submacular hemorrhages compared with eyes receiving more frequent and regular anti-VEGF treatments. There are no approved treatments for geographic atrophy.
Management
Even if anti-VEGF agents become cheaper and more available, an increasing number of patients will have to rely on them and, as a result, the burden on the health care system will increase. It may be more cost-effective for AMD prevention to perform:
Automonitoring
Periodic eye examination
Angiography with fluorescein and indocyanine green, OCT
In case of neovascular AMD, it is important to follow the patient with strict controls in order to detect recurrences as soon as possible using:
Patient information (increasing of visual distortion)
Worsening of visual acuity
Ophthalmoscopic appearance of macular hemorrhages
OCT features
Treatment
For prevention of AMD:
Use nutritional supplements, such as the Age Related Eye Disease Study formula, lutein and zeaxanthin, omega-3 fatty acid, and berry extracts
Modify lifestyle, including cessation of smoking and reduction of body mass index
Filter sunlight, ie, with sunglasses and blue-blocking intraocular lenses
For geographic atrophy:
No treatment is commercially available at present For choroidal neovascularization:
Intravitreal injections of anti-VEGF drugs Indications for specialist referral:
A familial history of AMD
Progressive or sudden worsening of visual acuity
Occurrence of visual distortion or visual perception of central dark spot
Footnotes
Date of preparation: March 3, 2011
Conflict of interest: None declared
References
- 1.Ehrlich R, Harris A, Kheradiya NS, Winston D, Ciulla TA, Wirostko B. Age-related macular degeneration and the aging eye. Clin Interv Aging. 2008;3:473–482. doi: 10.2147/cia.s2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickla DL, Wallman J. The multifunctional choroid. Prog Ret Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Liu B, Lukas TJ, Neufeld AH. The Aged Retinal Pigment Epithelium/Choroid: A potential substratum for the pathogenesis of age-related macular degeneration. PloS One. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman HR, Chan CC, Ferris FL, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun K, Cai H, Tezel TH, Paik D, Gaillard ER, Del Priore LV. Bruch’s membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol Vis. 2007;13:2310–2319. [PubMed] [Google Scholar]
- 6.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Querques G, Querques L, Martinelli D, et al. Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina. 2011;31:518–526. doi: 10.1097/IAE.0b013e3181f04974. [DOI] [PubMed] [Google Scholar]
- 8.Yehoshua Z, Rosenfeld PJ, Gregori G, et al. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology. 2011;118:679–686. doi: 10.1016/j.ophtha.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein RJ, Zeiss C, Emily Y, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 11.Fritsche LG, Loenhardt T, Janssen A, et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40:892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- 12.Leveziel N, Zerbib J, Richard F, et al. Genotype-phenotype correlations for exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;14:2263–2271. doi: 10.1167/iovs.07-1540. [DOI] [PubMed] [Google Scholar]
- 13.Scholl HP, Fleckenstein M, Issa PC, Keilhauer C, Holz FG, Weber BHF. An update on the genetics of age-related macular degeneration. Mol Vis. 2007;13:196–205. [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda AL, Colquitt J, Clegg AJ, Jones J. Pegaptanib and ranibizumab for neovascular age related macular degeneration: A systematic review. Br J Ophthalmol. 2007;91:1177–1182. doi: 10.1136/bjo.2007.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt-Erfurth UM, Richard G, Augustin A, et al. Guidance for the treatment of neovascular age-related macular degeneration. Acta Ophthalmol Scand. 2007;85:486–494. doi: 10.1111/j.1600-0420.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, David M, Brown DM, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 17.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy or neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SY, Dubois L, Tadayoni R, et al. Results of one-year’s treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2009;148:409–413. doi: 10.1016/j.ajo.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Regillo CD, David M, Brown DM, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Meyer CH, Eter N, Holz FG. Ranibizumab in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Interim results from the SUSTAIN trial. Invest Ophthalmol Vis Sci. 2008;49E Abstr 273. [Google Scholar]
- 21.Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: The EXCITE study. Ophthalmology. 2010 Dec 9; doi: 10.1016/j.ophtha.2010.09.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Querques G, Azrya S, Martinelli D, et al. Ranibizumab for exudative age-related macular degeneration: 24-month outcomes from a single-centre institutional setting. Br J Ophthalmol. 2010;94:292–296. doi: 10.1136/bjo.2009.170670. [DOI] [PubMed] [Google Scholar]
- 25.Gower EW, Cassard SD, Bass EB, Schein OD, Bressler NM. A cost-effectiveness analysis of three treatments for age-related macular degeneration. Retina. 2010;30:212–221. doi: 10.1097/IAE.0b013e3181babd8e. [DOI] [PubMed] [Google Scholar]
- 26.Bashschur ZF, Haddad ZA, Schakal AR, Jaafar RF, Saad A, Noureddin B. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: The second year of a prospective study. Am J Ophthalmol. 2009;148:59–65. doi: 10.1016/j.ajo.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Patel S. Combination therapy for age-related macular degeneration. Retina. 2009;29(6 Suppl):S45–48. doi: 10.1097/IAE.0b013e3181ad22d5. [DOI] [PubMed] [Google Scholar]
- 28.Chen E, Brown DM, Wong TP, et al. Lucentis using Visudyne study: Determining the threshold-dose fluence of verteporfin photodynamic therapy combined with intravitreal ranibizumab for exudative macular degeneration. Clin Ophthalmol. 2010;4:1073–1079. doi: 10.2147/OPTH.S13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni Z, Hui P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica. 2009;223:401–410. doi: 10.1159/000228926. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen QD, Shah SM, Browning DJ, et al. A phase I study of intravitreal vascular endothelial growth factor trap-eye in patients with neovascular age-related macular degeneration. Ophthalmology. 2009;116:2141–2148. doi: 10.1016/j.ophtha.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health Safety and efficacy study of small interfering ribonucleic acid (RNA) molecule (C and 5) to treat wet age-related macular degeneration. Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 32.National Institutes of Health A dose escalation trial of an intravitreal injection of Sirna-027 in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration (AMD) Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 33.National Institutes of Health Safety and efficacy of oral PTK787 in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration (AMD) Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 34.Doukas J, Mahesh S, Umeda N, et al. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovascularization and retinal edema. J Cell Physiol. 2008;216:29–37. doi: 10.1002/jcp.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institutes of Health Open-label, pilot study of TG100801 in patients with choroidal neovascularization due to AMD. Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 36.National Institutes of Health A study to evaluate pazopanib tablets in patients who have neovascular age-related macular degeneration. Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 37.Deal watch: Alcon licenses complement pathway inhibitor for macular degeneration. Nat Rev Drug Discov. 2009;8:922. doi: 10.1038/nrd3063. [DOI] [PubMed] [Google Scholar]
- 38.National Institutes of Health A study of ARC1905 (anti-C5 aptamer) in subjects with dry age-related macular degeneration. Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 39.National Institutes of Health A Phase 1, safety, tolerability and pharmacokinetic profile of intravitreous injections of E10030 (anti-PDGF pegylated aptamer) in subjects with neovascular age-related macular degeneration. Available at: www.clinicaltrials.gov. Accessed April 14, 2011.
- 40.Kuwada SK. Drug evaluation: Volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr Opin Mol Ther. 2007;9:92–98. [PubMed] [Google Scholar]
- 41.Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;147:825–830. doi: 10.1016/j.ajo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang LL, Coleman HR, Kim J, et al. Oral supplementation of lutein/zeaxanthin and omega-3 long chain polyunsaturated fatty acids in persons aged 60 years or older, with or without AMD. Invest Ophthalmol Vis Sci. 2008;49:3864–3869. doi: 10.1167/iovs.07-1420. [DOI] [PubMed] [Google Scholar]
- 44.Radu RA, Han Y, Bui TV, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: A potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 45.National Institutes of Health Study of fenretinide in the treatment of geographic atrophy associated with dry age-related macular degeneration. 2007. Jan, http://clinicaltrials.gov/ct2/show/NCT00429936?term=fenretinide&rank=10 Accessed 19 April 2011.
- 46.Tanito M, Li F, Anderson RE. Protection of retinal pigment epithelium by OT-551 and its metabolite TEMPOL-H against light-induced damage in rats. Exp Eye Res. 2010;91:111–114. doi: 10.1016/j.exer.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong WT, Kam W, Cunningham D, et al. Treatment of geographic atrophy by the topical administration of OT-551: Results of a Phase II clinical trial. Invest Ophthalmol Vis Sci. 2010;51:6131–6139. doi: 10.1167/iovs.10-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–1279. doi: 10.1001/archophthalmol.2010.223. [DOI] [PubMed] [Google Scholar]
- 49.Jeganathan VS, Verma S. Safety and efficacy of intravitreal anti-VEGF injections for age-related macular degeneration. Curr Opin Ophthalmol. 2009;20:223–225. doi: 10.1097/ICU.0b013e328329b656. [DOI] [PubMed] [Google Scholar]
- 50.Singer M. Horizon extension trial of ranibizumab (Lucentis®) for neovascular age-related macular degeneration (AMD): Two-year safety and efficacy results. Invest Ophthalmol Vis Sci. 2009 E-Abstr 3093. [Google Scholar]
- 51.Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116:1731–1739. doi: 10.1016/j.ophtha.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 52.Lihteh Wu, Martínez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin®): Results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246:81–87. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]