Abstract
The aim of the present study was to test the antigenicity of alpha-deoxyribonucleotides in order to develop a new tool for the detection of nucleic acid sequences for use in diagnostic applications. We describe four monoclonal antibodies (Mabs) which recognize alpha-deoxyribonucleotides. Two were raised against a poly(alpha-dT) sequence and specifically recognized the alpha-dT nucleotide. Two were raised against a sequence containing all four common nucleotides as alpha-nucleotides and, surprisingly, only recognized the alpha-dG nucleotide. For all four Mabs, no cross reactivity was observed with beta-oligonucleotides. These Mabs were reactive with alpha-oligonucleotide sequences whether these sequences were single-stranded or hybridized to DNA or RNA. The four Mabs were tested in a sandwich hybridization assay that consisted of an alpha-oligonucleotide (for target sequence recognition), one of the four Mabs (for recognition of the hybridized alpha-oligonucleotide), and goat anti-mouse antibody conjugated to horse radish peroxidase (HRP) (for detection). One of the monoclonal antibodies, Mab 2E11D7, was directly conjugated to HRP and used in sandwich hybridization to detect PCR fragments of HPV 18 DNA. The sensitivity of this reaction was 1 pg of plasmid DNA containing the HPV 18 fragment. The specificity of the detection was demonstrated using HPV 6/11 and 16 DNA sequences.
Full text
PDF

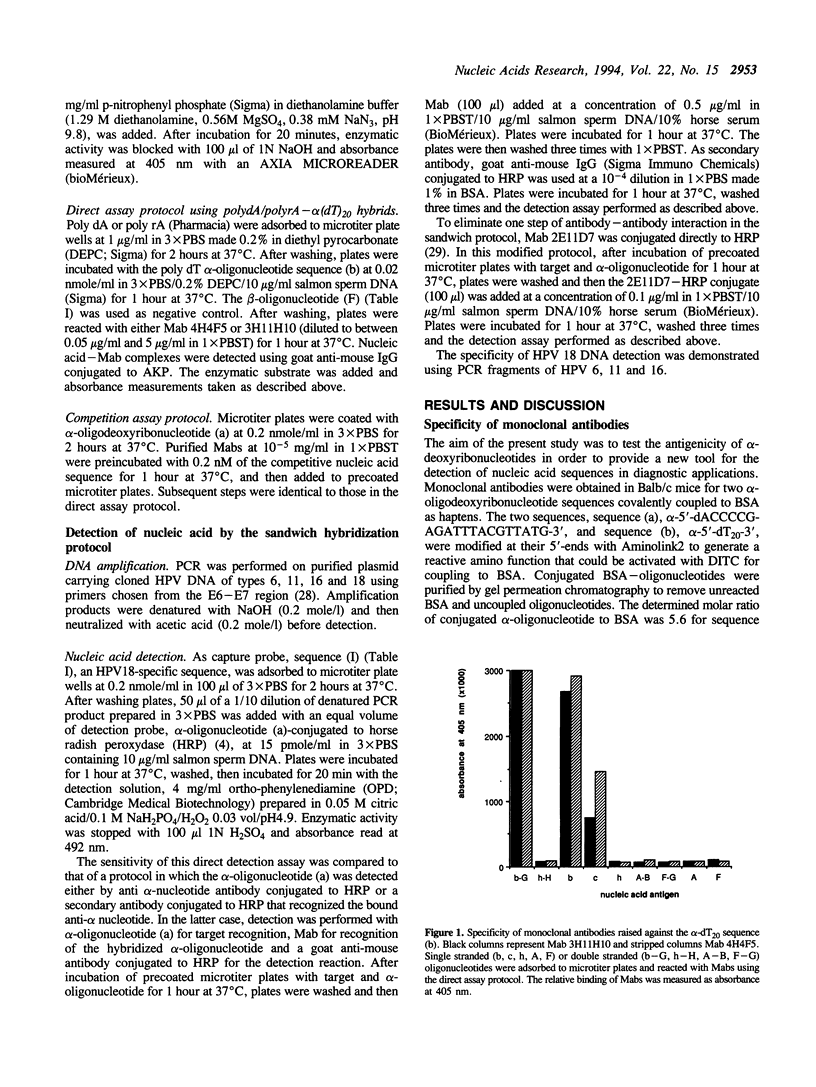
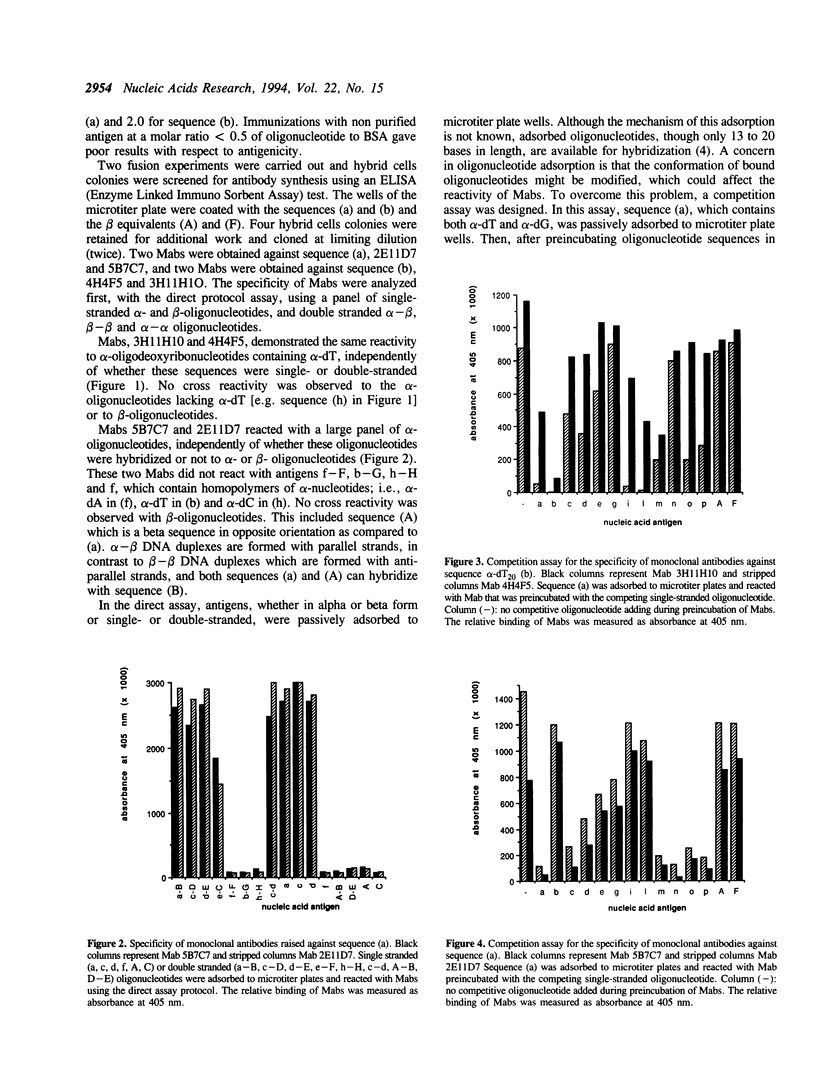
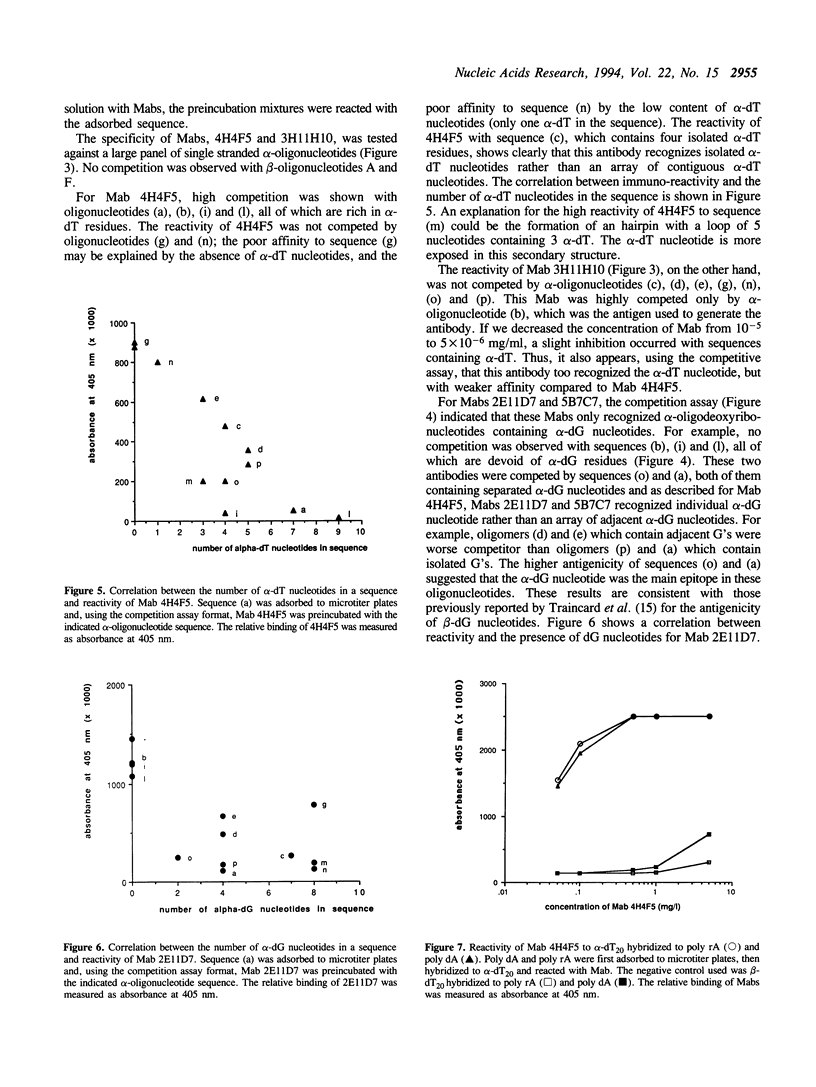
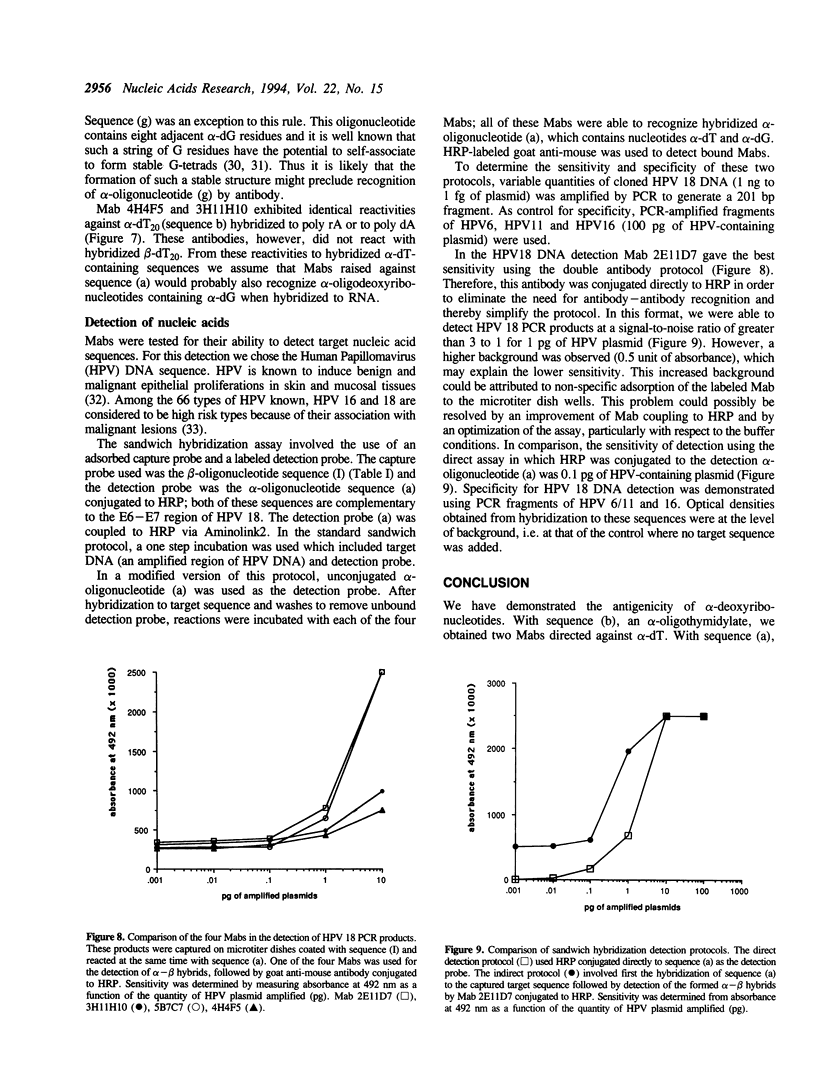

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Cygler M., Braun R. P., Lee J. S. Antibodies to DNA. Bioessays. 1988 Feb-Mar;8(2):69–74. doi: 10.1002/bies.950080206. [DOI] [PubMed] [Google Scholar]
- Boiziau C., Kurfurst R., Cazenave C., Roig V., Thuong N. T., Toulmé J. J. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 1991 Mar 11;19(5):1113–1119. doi: 10.1093/nar/19.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Abbass H., Dalabetta G., Hook N. E., Shah K., Viscidi R. P. Detection of HPV-16 in cell lines and cervical lavage specimens by a polymerase chain reaction-enzyme immunoassay assay. J Med Virol. 1992 May;37(1):22–29. doi: 10.1002/jmv.1890370105. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Mayur K., Yolken R. H., Viscidi R. P. Immunodetection of DNA with biotinylated RNA probes: a study of reactivity of a monoclonal antibody to DNA-RNA hybrids. Anal Biochem. 1989 Aug 15;181(1):96–105. doi: 10.1016/0003-2697(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Cros P., Allibert P., Mandrand B., Tiercy J. M., Mach B. Oligonucleotide genotyping of HLA polymorphism on microtitre plates. Lancet. 1992 Oct 10;340(8824):870–873. doi: 10.1016/0140-6736(92)93284-t. [DOI] [PubMed] [Google Scholar]
- Fliss I., St Laurent M., Emond E., Lemieux R., Simard R. E., Ettriki A., Pandian S. Production and characterization of anti-DNA-RNA monoclonal antibodies and their application in Listeria detection. Appl Environ Microbiol. 1993 Aug;59(8):2698–2705. doi: 10.1128/aem.59.8.2698-2705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L. Immunoenzymatic techniques applied to the specific detection of nucleic acids. A review. J Immunol Methods. 1992 Jun 24;150(1-2):33–49. doi: 10.1016/0022-1759(92)90063-y. [DOI] [PubMed] [Google Scholar]
- Gupta G., Garcia A. E., Guo Q., Lu M., Kallenbach N. R. Structure of a parallel-stranded tetramer of the Oxytricha telomeric DNA sequence dT4G4. Biochemistry. 1993 Jul 20;32(28):7098–7103. doi: 10.1021/bi00079a005. [DOI] [PubMed] [Google Scholar]
- Kinney J. S., Viscidi R. P., Vonderfecht S. L., Eiden J. J., Yolken R. H. Monoclonal antibody assay for detection of double-stranded RNA and application for detection of group A and non-group A rotaviruses. J Clin Microbiol. 1989 Jan;27(1):6–12. doi: 10.1128/jcm.27.1.6-12.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J. Functional groups on 'Z' DNA recognized by monoclonal antibodies. FEBS Lett. 1984 Mar 26;168(2):303–306. doi: 10.1016/0014-5793(84)80267-7. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Rousseau N., Leng M. Interaction between antibodies to Z-form deoxyribonucleic acid and double-stranded polynucleotides. Biochemistry. 1982 Oct 26;21(22):5463–5467. doi: 10.1021/bi00265a013. [DOI] [PubMed] [Google Scholar]
- Mallet F., Hebrard C., Brand D., Chapuis E., Cros P., Allibert P., Besnier J. M., Barin F., Mandrand B. Enzyme-linked oligosorbent assay for detection of polymerase chain reaction-amplified human immunodeficiency virus type 1. J Clin Microbiol. 1993 Jun;31(6):1444–1449. doi: 10.1128/jcm.31.6.1444-1449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty D., Bansal M. Conformational polymorphism in G-tetraplex structures: strand reversal by base flipover or sugar flipover. Nucleic Acids Res. 1993 Apr 25;21(8):1767–1774. doi: 10.1093/nar/21.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Lee M., Hartley J. A., Chang D. K., Lown J. W. alpha-DNA-V. Parallel annealing, handedness and conformation of the duplex of the unnatural alpha-hexadeoxyribonucleotide alpha-[d(CpApTpGpCpG)] with its beta-complement beta-[d(GpTpApCpGpC)] deduced from high field 1H-NMR. Nucleic Acids Res. 1987 Sep 11;15(17):7027–7044. doi: 10.1093/nar/15.17.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Human papillomaviruses and genital cancer. Adv Cancer Res. 1987;48:113–147. doi: 10.1016/s0065-230x(08)60691-0. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Chassignol M., Takasugi M., Le Doan T., Thuong N. T., Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J Mol Biol. 1987 Aug 20;196(4):939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Schönborn J., Oberstrass J., Breyel E., Tittgen J., Schumacher J., Lukacs N. Monoclonal antibodies to double-stranded RNA as probes of RNA structure in crude nucleic acid extracts. Nucleic Acids Res. 1991 Jun 11;19(11):2993–3000. doi: 10.1093/nar/19.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C., Allibert P., Chardonnet Y., Cros P., Mandrand B., Thivolet J. Detection of human papillomavirus types 6, 11, 16 and 18 in mucosal and cutaneous lesions by the multiplex polymerase chain reaction. J Virol Methods. 1991 Nov-Dec;35(2):143–157. doi: 10.1016/0166-0934(91)90130-r. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. The experimental induction of antibodies to nucleic acids. Methods Enzymol. 1980;70(A):70–85. doi: 10.1016/s0076-6879(80)70042-3. [DOI] [PubMed] [Google Scholar]
- Sun J. S., Giovannangeli C., François J. C., Kurfurst R., Montenay-Garestier T., Asseline U., Saison-Behmoaras T., Thuong N. T., Hélène C. Triple-helix formation by alpha oligodeoxynucleotides and alpha oligodeoxynucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6023–6027. doi: 10.1073/pnas.88.14.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuong N. T., Asseline U., Roig V., Takasugi M., Hélène C. Oligo(alpha-deoxynucleotide)s covalently linked to intercalating agents: differential binding to ribo- and deoxyribopolynucleotides and stability towards nuclease digestion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5129–5133. doi: 10.1073/pnas.84.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traincard F., Chevrier D., Mazie J. C., Guesdon J. L. Monoclonal anti-nucleoside antibodies. Characterization and application in an enzyme immunoassay of single-stranded DNA. J Immunol Methods. 1989 Sep 29;123(1):83–91. doi: 10.1016/0022-1759(89)90032-x. [DOI] [PubMed] [Google Scholar]
- Traincard F., Sakamoto H., Rouyre S., Mazie J. C., Guesdon J. L. Calibration of target amounts of DNA in hybridization experiments using monoclonal anti-nucleoside antibodies. Mol Cell Probes. 1989 Mar;3(1):27–38. doi: 10.1016/0890-8508(89)90034-0. [DOI] [PubMed] [Google Scholar]
- Walker J., Dougan G. DNA probes: a new role in diagnostic microbiology. J Appl Bacteriol. 1989 Sep;67(3):229–238. doi: 10.1111/j.1365-2672.1989.tb02490.x. [DOI] [PubMed] [Google Scholar]
- Whetsell A. J., Drew J. B., Milman G., Hoff R., Dragon E. A., Adler K., Hui J., Otto P., Gupta P., Farzadegan H. Comparison of three nonradioisotopic polymerase chain reaction-based methods for detection of human immunodeficiency virus type 1. J Clin Microbiol. 1992 Apr;30(4):845–853. doi: 10.1128/jcm.30.4.845-853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott M. J. Advances in nucleic acid-based detection methods. Clin Microbiol Rev. 1992 Oct;5(4):370–386. doi: 10.1128/cmr.5.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



