Summary
Deregulation of the G1/G0 phase of the cell cycle can lead to cancer. During G1, most cells commit alternatively to DNA replication and division, or to cell cycle exit and differentiation. The anaphase-promoting complex or cyclosome (APC/C) activated by Cdh1 coordinately eliminates positive cell cycle regulators and also inhibitors of differentiation, coupling cell cycle exit and differentiation. Misregulation of Cdh1 thus has the potential to promote both cell cycle re-entry and either perturbed differentiation or dedifferentiation. Additionally, APC/CCdh1 is required to maintain genomic stability. As a result, loss of Cdh1 can contribute to tumorigenesis in the form of proliferation of poorly differentiated and genetically unstable cells.
Keywords: anaphase-promoting complex, ubiquitin, proteasome, differentiation, genomic instability, tumor suppression
Introduction
The regulated degradation of specific proteins is critical for cell cycle control and progression. Ubiquitin ligase complexes perform this function by targeting substrates for proteasomal destruction by the covalent attachment of ubiquitin chains, with the APC/C acting from the onset of anaphase through the ensuing G1. Substrates of the APC/C are often misregulated in various tumors, and both APC/CCdh1 substrates including Polo-like- and Aurora-Kinases (Barr & Gergely, 2007; Archambault & Glover, 2009), as well as the proteasome itself are targets of emerging cancer therapies (Adams, 2004; Strebhardt & Ullrich, 2006; Taylor & Peters, 2008; Lapenna & Giordano, 2009). Recent research establishes the APC/C activator Cdh1 as a novel tumor suppressor. Mounting evidence demonstrates that Cdh1, by timely and coordinated substrate degradation, plays a critical role in the control of proliferation, differentiation and maintenance of genomic integrity (Figure 1a, b). These processes are frequently dysregulated in cancer cells and can result in unrestrained cell proliferation, genomic instability, and blocks in differentiation. Here we review how APC/CCdh1 has emerged as a conserved cell cycle regulator from yeast to man and the various mechanisms by which APC/CCdh1 may be involved in tumorigenesis.
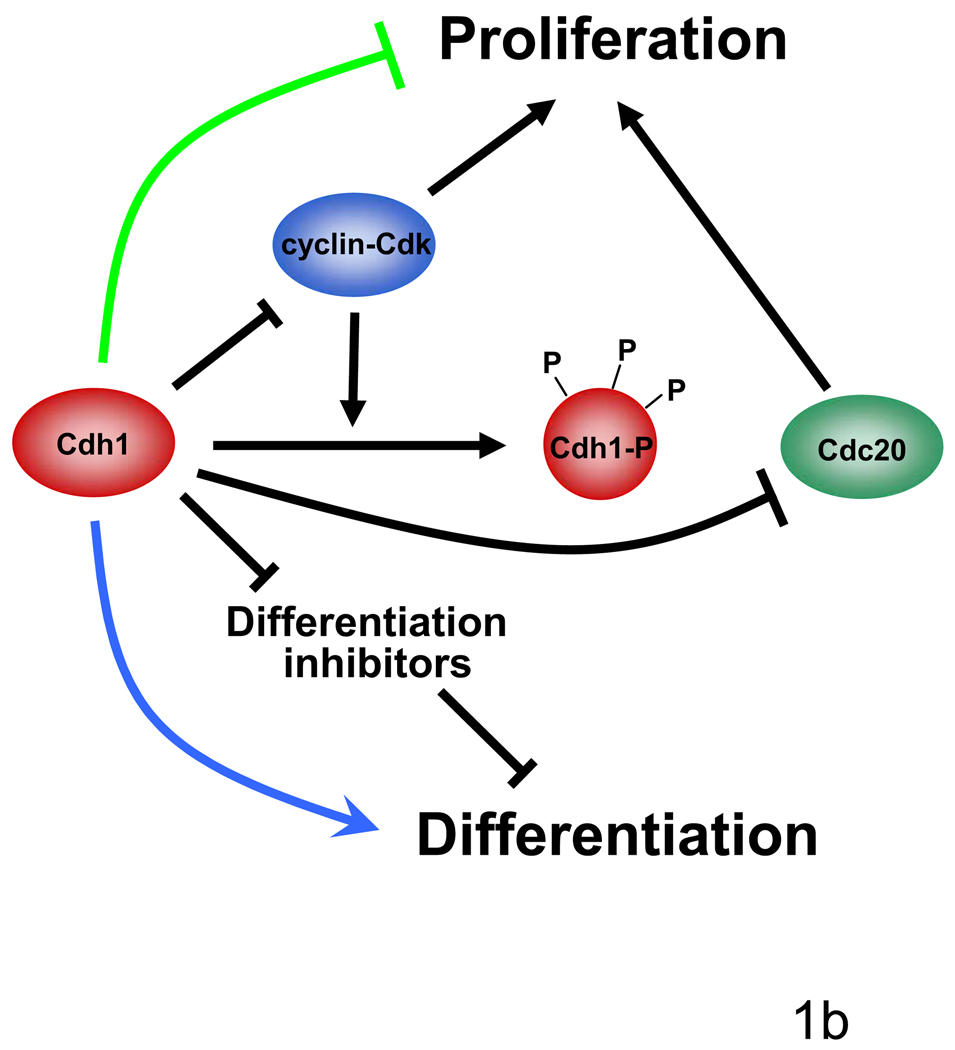
Figure 1. APC/CCdh1-dependent control of the cell cycle.
a, The anaphase-promoting complex/cyclosome (APC/C) is activated by Cdh1 from the end of mitosis through G1 (APC/CCdh1). APC/CCdh1 controls G1 to either allow differentiation or to prepare for a new round of cell division. In G2 APC/CCdh1 can be activated in response to DNA damage to block mitotic entry and to initiate DNA repair. In mitosis APC/C activated by Cdc20 (APC/CCdc20) mediates chromosome separation and initiates mitotic exit. At the end of mitosis APC/CCdh1 inactivates APC/CCdc20 and modulates anaphase spindle dynamics and cytokinesis. Inactivation of Cdh1 can contribute to tumorigenesis by deregulation of these cell cycle transitions.
b, APC/CCdh1-dependent degradation of positive cell cycle regulators (such as cyclin-Cdk) and inhibitors of differentiation keeps the balance in G1/G0 between proliferation and differentiation. When APC/CCdh1 remains active in G1 cells can exit the cell cycle and differentiate, or Cdh1 is switched off in late G1 and the cell enters a new cell cycle. APC/CCdc20 maintains the proliferative state by controlling progression through mitosis before it is inactivated by APC/CCdh1.
Cdh1 in G0/G1 regulation from yeast to human
The ordered and unidirectional progression of the cell cycle requires the oscillatory activity of cyclins and other crucial regulators (Bloom & Cross, 2007; Malumbres & Barbacid, 2009). Inactivation of mitotic cyclin-dependent kinases (Cdks) is essential for the transition from mitosis to G1; this is primarily performed by the APC/C (Wäsch & Engelbert, 2005; Peters, 2006; Pesin & Orr-Weaver, 2008; Li & Zhang, 2009). The regulatory circuits are best characterized in budding yeast, and appear to be well conserved from yeast to man. The APC/C acts in coordination with two homologous coactivators, Cdc20 and Cdh1. APC/CCdh1 mediates the degradation of the major mitotic cyclin Clb2 during exit from mitosis (Schwab et al., 1997; Visintin et al., 1997). A genetic screen in Saccharomyces cerevisiae identified degradation of the chromosome separation control protein securin and the major S-phase cyclin Clb5 by APC/CCdc20 being essential for mitotic exit. It was proposed that degradation of securin and Clb5 by APC/CCdc20 leads to activation of APC/CCdh1 and the Cdk-inhibtor Sic1, thus inactivating the mitotic cyclin Clb2 and resulting in mitotic exit (Shirayama et al., 1999).
Further research demonstrated that Clb2 is degraded in two phases sequentially regulated by Cdc20 and Cdh1 (Yeong et al., 2000). Contributing to this is the APC/C’s ability to recognize different motifs in target proteins (Table 1). Recognition of these target motifs by the coactivators Cdc20 and Cdh1 leads to binding and ubiquitination by the APC/C. In addition, direct interaction of the APC/C core with substrates via the degron motifs has been shown (Yamano et al., 2004). The core subunit Doc1/Apc10 is a candidate for such a direct interaction (Carroll & Morgan, 2002; Passmore et al., 2003; Hayes et al., 2006).
Table 1.
Degron motifs recognized by the APC/C. Most prominent among the motifs involved in targeting APC/C substrates for degradation are the destruction (D) and KEN boxes. While the D box is recognized by both APC/CCdc20 and Cdh1, the KEN box is preferentially recognized by APC/CCdh1 Several other sequences have also been identified in APC/C substrates. While it is well established that interaction of substrates with the coactivators Cdc20 or Cdh1 leads to substrate recruitment to the APC/C, Cdc20/Cdh1 independent interaction of the APC/C with substrates has also been demonstrated.
| Name | Sequence | Substrate | APC/C activator | Reference |
|---|---|---|---|---|
| D box | RxxL(xxxxN) | Cyclin B | Cdc20, Cdh1 | (Glotzer et al., 1991) |
| KEN box | KENxxxN | Cdc20 | Cdh1 | (Pfleger & Kirschner, 2000) |
| A box or DAD (D box activating domain) | RxLxPSN | Aurora A | Cdh1 | (Littlepage & Ruderman, 2002) (Castro et al., 2002) |
| CRY box | CRYxPS | Cdc20 | Cdh1 | (Reis et al., 2006) |
| GxEN box | GxEN | Xkid | Cdc20, Cdh1 | (Castro et al., 2003) |
| Spo13 D box | LxExxxN | Spo13 | Cdc20? | (Sullivan & Morgan, 2007) |
| O box | unknown sequences in the N-terminal domain | ORC1 | Cdh1 | (Araki et al., 2003) |
The destruction (D) box (RxxL motif) and the KEN box are the most commonly found degron motifs in APC/C substrates. While the D box is recognized by APC/CCdc20 and APC/CCdh1, APC/CCdh1 also recognizes targets with a KEN box motif (Glotzer et al., 1991; Pfleger & Kirschner, 2000). Mutating D and KEN box sequences in the genomic CLB2 to prevent APC/C-dependent Clb2 proteolysis at endogenous expression levels (Wäsch & Cross, 2002) revealed that the degradation of Clb2 is essential for mitotic exit and failure to degrade Clb5 may rather lead to replication problems and genomic instability. It further demonstrated that the first phase of Clb2 degradation executed by APC/CCdc20 is sufficient for most aspects of mitotic exit. Moreover, combined deletion of Cdh1 and Sic1 does not block exit from mitosis. Thus, APC/CCdc20 dependent degradation of Clb2 is essential for mitotic exit, while Cdh1 contributes to further Clb2-Cdk1 inactivation during mitotic exit and is particularly important in keeping mitotic cyclin activity low in G1. This regulates cell growth and the length of G1, which may be necessary for cell differentiation and/or correct assembly of pre-replication complexes at origins and a subsequent ordered DNA replication (Wäsch & Cross, 2002). This is also supported by the fact that the embryonic cell cycle, which lack a distinct G1 phase is governed by a Cdc20-dependent oscillatory mechanism and that the Cdh1-dependent oscillator is required to establish a G1 phase in somatic cells (Sigrist & Lehner, 1997; Lorca et al., 1998). Since Cdc20 and Cdh1 are conserved these two oscillators may function similarly throughout all eukaryotes (Cross, 2003).
To ensure a strict alternation of APC/CCdc20 and APC/CCdh1 activity, activation of the APC/C by Cdc20 and Cdh1 is regulated oppositely by phosphorylation events. Cyclin-Cdk complexes phosphorylate APC/C subunits, promoting binding of Cdc20 to the APC/C. In contrast, cyclin-Cdk complexes phosphorylate Cdh1, inhibiting its binding to the APC/C. In mitosis Cdc20 binds preferentially to phosphorylated APC/C, while at the end of mitosis and during G1, Cdh1 has to be dephosphorylated to activate the APC/C (Zachariae et al., 1998; Kramer et al., 2000; Rudner & Murray, 2000). Cdh1 is responsible for the degradation of Cdc20, preventing simultaneous activation of both APC/C coactivators (Pfleger & Kirschner, 2000). In addition, Cdh1 is regulated by changes in its abundance with higher levels in mitosis and a gradual decline in G1 (Kramer et al., 2000). This may be due to Cdh1-dependent ubiquitination of itself (Listovsky et al., 2004) and directly or indirectly maintained during S-phase by the SCF complex (Benmaamar & Pagano, 2005). The subcellular localization of Cdh1 is also cell cycle regulated with Cdh1 beeing nuclear in G1 and cytoplasmic from S phase until the end of mitosis, providing an element of spatial regulation to its activity (Jaquenoud et al., 2002; Zhou et al., 2003); nuclear export is promoted by cyclin-Cdk phosphorylation, and may sequester Cdh1 from either the APC/C or nuclear targets. Since cyclins are major biological targets of APC/CCdh1-promoted ubiquitination and degradation, and at the same time cyclin-Cdk phosphorylation blocks Cdh1 binding to the APC/C and removes it from the nucleus where its most important targets are located, this situation can create a double-negative feedback loop equivalent to positive feedback (Ferrell, 2002). Such circuitry could result in hysteresis with stability of both the G1 low cyclin/high Cdh1 state and the S/G2/M high cyclin/low Cdh1 state.
Thus, for cell cycle re-entry, APC/CCdh1 must be turned off to allow for the re-accumulation of mitotic cyclins, thus closing the circuit of Cdh1 activity. To ensure this, external stimulation by growth factors at the G1/S transition may lead to E2F-dependent cyclin A accumulation resulting in the phosphorylation and inactivation of Cdh1 (Lukas et al., 1999). In yeast, this job is probably carried out by G1 cyclins and by S-phase B-type cyclins acting in concert (Zachariae et al., 1998). In both animal cells and yeast, inhibitors of APC/CCdh1 (Emi1 (Hsu et al., 2002) and Acm1 (Enquist-Newman et al., 2008)) also accumulate. As with cyclin A, both are also under transcriptional control of G1/S-specific activators (for Emi1, E2F, activated by cyclin-Cdk phosphorylation of Rb (Hsu et al., 2002); for Acm1, most likely SBF/MBF, activated by G1 cyclin-Cdk (Spellman et al., 1998). These inhibitors may contribute to the double-negative feedback loop stabilizing the Cdh1 on and off states.
A striking demonstration of this potential bistable switch is found in studies of fly embryos with lowered Cdh1 (fzr) activity (Jacobs et al., 2002). Individual cells within embryos with weakened fzr alleles were either fully committed to degradation of mitotic cyclins and entry into G1 arrest, or failed to degrade mitotic cyclins and thus maintained mitotic proliferation.
In human cells APC/CCdh1 also targets the ubiquitin ligase SCFSkp2, which mediates degradation of the Cdk inhibitors p21 and p27, still further amplifying the stability of G1, since these inhibitors block cyclin-Cdk phosphorylation of Cdh1 and Rb. Depletion of Cdh1 by RNA interference (RNAi) stabilizes Skp2, resulting in p21 and p27 proteolysis in G1 and premature entry into S phase (Bashir et al., 2004; Wei et al., 2004). This coordinated interaction between APC/C and SCF is a further important mechanism by which APC/CCdh1 maintains a stable G1.
APC/CCdh1 targets are sequentially degraded in vivo, and it has been shown in vitro that this ordered substrate degradation is in part determined by different kinetics of APC/C-dependent ubiquitination for the different substrates. Moreover, the D box motif may be involved in these differences of ubiquitination kinetics and subsequent destruction (Rape et al., 2006). Interestingly, perhaps after ‘running out’ of other targets in G1, the APC/CCdh1 (E3) mediates the proteasomal degradation of its own ubiquitin-conjugating enzyme (E2) UbcH10. Since cyclin A is ubiquitinated by APC/CCdh1 (Sorensen et al., 2001), this would allow cyclin A accumulation in late G1 by a mechanism intrinsic to the cell cycle machinery, which in turn further inactivates APC/CCdh1 (Rape & Kirschner, 2004). Cdh1-dependent Cdh1 ubiquitination in G1 (Listovsky et al., 2004) could contribute towards the same end. Such a mechanism could provide the switch that destabilizes the otherwise highly stable G1 state. In yeast, no such mechanism is known, and the G1 state is most likely destabilized by synthesis of cyclins (G1 cyclins and S-phase B-type cyclins) that are immune to Cdh1-mediated degradation, but that can phosphorylate and inactivate Cdh1. Interestingly, these cyclins as well as ACM1 are all expressed in a burst of transcription at the G1/S border, driven by a positive feedback loop (Spellman et al., 1998; Skotheim et al., 2008), which may serve to rapidly and decisively inactivate Cdh1.
Control of differentiation by Cdh1
While Cdh1 activity at the end of mitosis is important for origin licensing to ensure accurate DNA replication in the next S phase, the inactivation of Cdh1 during G1 is important to enter a new cell cycle (Diffley, 2004). Several lines of experimentation suggest that continuous Cdh1 activity in contrast may promote cell cycle exit and differentiation in at least certain cell types. It is intriguing that a key component of a bistable switch controlling G1 residence has apparently been recruited to control differentiation components, resulting in coordinate G1 arrest and differentiation (Figure 1a, b). Notably, there is a common theme for different regulators: like inactivation of the tumor suppressor Cdh1, activation of the oncogene ras can block differentiation and promote proliferation by inhibiting the expression of the differentiation promoting transcription factor MyoD (Lassar et al., 1989).
Support for the role of Cdh1 in differentiation in the literature is extensive. In yeast, Cdh1 is required for proper pheromone-induced G1 arrest (Schwab et al., 1997). In mammalian cells several observations indicate a role of APC/CCdh1 in cell cycle exit and differentiation. The conditional knockdown of the subunit Apc2 in resting hepatocytes in mice caused re-entry into the cell cycle and unscheduled proliferation of these cells, which may be caused by the lack of APC/CCdh1 activity (Wirth et al., 2004). Other work demonstrates a putative role for Cdh1 in lens and muscle cell differentiation (Li et al., 2007; Wu et al., 2007). APC/CCdh1 is expressed in postmitotic neurons and suppresses axonal growth in the mammalian brain in vitro and in vivo (Gieffers et al., 1999; Konishi et al., 2004). Axonal growth is required to establish neuronal connectivity in the brain. Heterozygous Cdh1-knockout mice show defects in neuromuscular coordination, learning and memory, validating that a compromised APC/CCdh1 function in neurons has physiological consequences (Garcia-Higuera et al., 2008; Li et al., 2008).
An important mechanism regulating axonal growth may be modulation of the antiproliferative TGFβ signalling pathway by APC/CCdh1. In response to TGFβ stimulation Smad3 and Smad2 translocate into the nucleus and induce degradation of the transcriptional corepressor SnoN leading to activation of TGFβ target genes and growth inhibition in cultured cells. Smad3 and Smad2 interact with both the APC/C and SnoN resulting in the recruitment of the APC/CCdh1 to SnoN and the subsequent ubiquitination and degradation of SnoN and thus blocking axonal growth (Stroschein et al., 2001; Wan et al., 2001; Stegmüller et al., 2006; Stegmüller et al., 2008). Another mechanism of cell cycle arrest by TGFβ signalling may be via APC/CCdh1-dependent Skp2 degradation and subsequent stabilization of the Cdk inhibitor p27, thus blocking S phase entry (Liu et al., 2007).
Stimulation of neuroblastoma cells by retinoic acid also induces cell cycle arrest and differentiation through modulation of APC/CCdh1, Skp2 degradation and p27 accumulation (Cuende et al., 2008), suggesting that APC/CCdh1 may be activated by different inducers of differentiation. Id (inhibitor of differentiation/DNA binding) proteins modulate cellular proliferation and differentiation of various cell types, such as neural or hematopoietic cells, and their deregulation may play a role in tumorigenesis (Perk et al., 2005). While Id proteins are downregulated during cell cycle exit, overexpression of Id proteins in terminally differentiated cells can induce re-entry into the cell cycle under certain conditions (Chaudhary et al., 2005). It has been suggested that Id1, 2 and 4 may be targets of APC/CCdh1 in primary neurons and APC/CCdh1-dependent degradation of Id2 can also inhibit axonal growth (Lasorella et al., 2006). Expression of Id1 and Id2 may contribute to enhanced proliferation and inhibited differentiation of myeloid progenitors and the development of myeloid malignancies (Suh et al., 2008). Knockout and knockdown of Id2 in mice induces lymphoid differentiation and impairs erythroid development, while overexpression of Id2 promotes erythroid commitment and inhibits lymphoid and myeloid differentiation of hematopoietic progenitors (Ji et al., 2008). These data imply that APC/CCdh1 could be involved in hematopoietic stem cell differentiation and lineage commitment via regulation of Id-proteins and most likely other targets.
The role of Cdh1 in differentiation may be as important as that of p21, p27 or the retinoblastoma protein (pRb) as cell cycle exit is also promoted by an interaction of pRb with APC/CCdh1, controlling p27 stability by targeting Skp2 for degradation. It has also been suggested that pRb directs APC/CCdh1 to additional targets during differentiation (Binne et al., 2007). This process may further contribute to maintaining a differentiated state. Comparable to Cdh1, ras also interacts with pRB to affect differentiation, contributing to tumorigenesis and perhaps even metastatic disease (Takahashi & Ewen, 2006), and raising the potential for overlapping pathways to transformation. Cell cycle exit and differentiation is controlled by various mechanisms. Although the importance of many of the described mechanisms has to be confirmed by in vivo models, it is apparent that APC/CCdh1 plays a critical role to coordinate proliferation and differentiation in several ways (Figure 2)(Table 2).
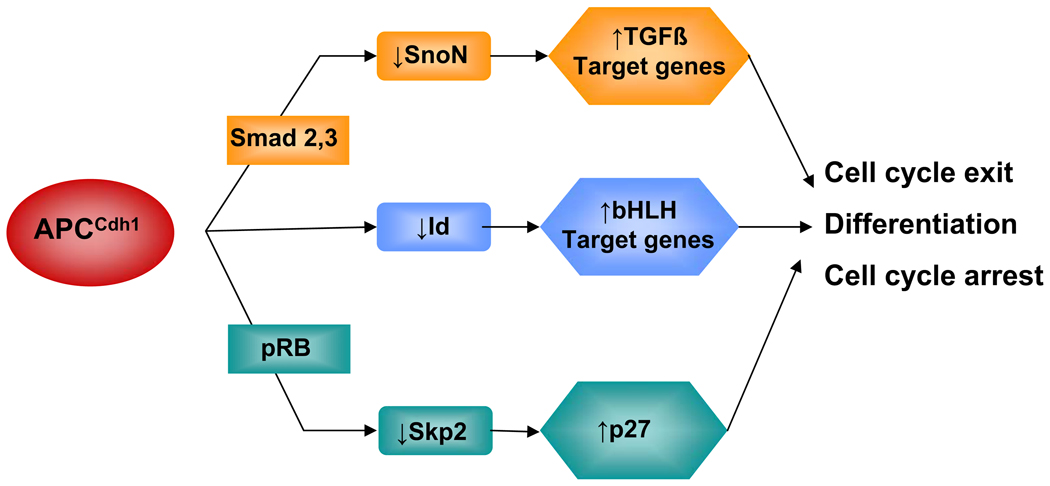
Figure 2. Mechanisms of differentiation control by APC/CCdh1.
In response to TGFβ stimulation Smad3 and Smad2 can recruit APC/CCdh1 to ubiquitinate SnoN leading to its degradation and activation of TGF-beta target genes and growth inhibition. Similarly, the retinoblastoma protein (pRB) can recruit APC/CCdh1 to target Skp2 resulting in p27 stabilization and cell cycle arrest. APC/CCdh1 also targets Id (inhibitor of differentiation/DNA binding) proteins. This leads to activation of basic helix-loop-helix (bHLH) transcription factors and target gene expression mediating differentiation in various cell types.
Table 2.
Cdh1 target proteins with a role in differentiation or maintenance of genomic stability as discussed in the text.
| Cdh1 target | Physiological roles of APC/CCdh1- dependent proteolysis |
Reference |
|---|---|---|
| SnoN | TGFβ signalling | (Stroschein et al., 2001) (Wan et al., 2001) |
| Id2 | inhibition of axonal growth in primary neurons | (Lasorella et al., 2006) |
| Cdc20 | contributes to sequential activity of the APC/C coactivators | (Pfleger & Kirschner, 2000) |
| Cdh1 | APC/CCdh1 inactivation by self ubiquitination to allow G1-S transition | (Listovsky et al., 2004) |
| Skp2 | G1 stability cell cycle exit | (Bashir et al., 2004) (Wei et al., 2004) (Binne et al., 2007) |
| UbcH10 | APC/CCdh1 inactivation by cyclin A accumulation to allow G1-S transition | (Rape & Kirschner, 2004) |
| Cdc6 | prevention of re-replication | (Vaziri et al., 2003) |
| Geminin | origin licensing | (McGarry & Kirschner, 1998) (Zielke et al., 2008) |
| Clb2 | mitotic exit, G1 stability | (Schwab et al., 1997) (Visintin et al., 1997) (Wäsch & Cross, 2002) |
| Cyclin A, B | G1 stability, origin licensing | (Sudo et al., 2001) (Engelbert et al., 2008) (Garcia-Higuera et al., 2008) |
| Aurora A, B | spindle dynamics, cytokinesis | (Meraldi et al., 2002) (Engelbert et al., 2008) (Garcia-Higuera et al., 2008) (Floyd et al., 2008) |
| Plk1 | G2 DNA damage checkpoint Cytokinesis | (Sudo et al., 2001) (Meraldi et al., 2002) (Lindon & Pines, 2004) (Engelbert et al., 2008) (Garcia-Higuera et al., 2008) (Bassermann et al., 2008) |
| Cin8/Eg5, Kip1 | spindle dynamics | (Hildebrandt & Hoyt, 2001; Crasta et al., 2006; Crasta et al., 2008) |
| Ase1/Prc1 | spindle dynamics, cytokinesis | (Juang et al., 1997; Visintin et al., 1997) |
| anillin | Cytokinesis | (Zhao & Fang, 2005) |
Cdh1 maintains genomic integrity
High fidelity DNA replication and chromosome separation is required to maintain genomic integrity and prevent carcinogenesis. Downregulation of APC/CCdh1 during mitotic exit and G1 results in the unscheduled accumulation of target proteins including cyclins A and B, and the mitotic kinases Aurora A and Polo-like kinase 1 (Plk1). This has the clear potential to promote aberrant DNA replication and chromosome separation in yeast, mouse and human cells (Wäsch & Cross, 2002; Ross & Cohen-Fix, 2003; Wäsch & Engelbert, 2005; Engelbert et al., 2008; Garcia-Higuera et al., 2008) (Figure 3). Activation of p53/p21 in human cells and p16 expression in mice in Cdh1-depleted cells are suggestive of a DNA damage response and premature senescence (Engelbert et al., 2008; Garcia-Higuera et al., 2008; Li et al., 2008). Cdh1 downregulation in human and murine cells leads to genomic instability as evidenced by centrosome aberrations, multipolar mitosis, anaphase bridges and micronuclei (Engelbert et al., 2008; Garcia-Higuera et al., 2008). Consistent with these observations, cultured Cdh1-null mouse embryonic fibroblasts (MEFs) show substantial numerical and structural chromosomal aberrations (Garcia-Higuera et al., 2008).
Figure 3. Dysregulation of APC/CCdh1 can cause genomic instability.
Inactivation of Cdh1 leads to stabilization and unscheduled expression of several target proteins. This results in deregulation of DNA replication and mitosis leading to chromosomal aberrations. See text for details.
Stabilization of the licensing factor Cdc6 may contribute to genetic instability by inducing re-replication (Vaziri et al., 2003). The untimely activity of cyclin A and B during mitotic exit and in G1 seen in Cdh1-depleted mammalian cells on the other hand may interfere with loading of replication origins. (Sudo et al., 2001; Engelbert et al., 2008; Garcia-Higuera et al., 2008). Stabilization of the licensing inhibitor geminin may contribute to the same end (McGarry & Kirschner, 1998). In fact, slightly reduced levels of Mcm4 and Mcm5 on chromatin suggest a defect in the formation of pre-replication complexes in Cdh1-deficient mice (Garcia-Higuera et al., 2008). As a consequence, DNA replication initiates prematurely from a smaller number of origins resulting in slowed DNA replication and potentially replication errors. Additionally, if cells with stalled replication forks and underreplicated DNA enter mitosis, chromosome breakage and breakage-fusion-bridge cycles can occur (Lengronne & Schwob, 2002; Ekholm-Reed et al., 2004). This may explain the chromosomal translocations observed in Cdh1-null MEFs (Garcia-Higuera et al., 2008).
Cdh1 is required for a G2 DNA-damage checkpoint (Figure 1a), as initially observed in chicken cells (Sudo et al., 2001) and later described in human cells (Bassermann et al., 2008). Cdh1 is activated in G2 upon genotoxic stress leading to Plk1 degradation and prevention of mitotic entry in order to repair damaged DNA (Bassermann et al., 2008). Although mitotic entry is still delayed in Cdh1-depleted cells (Engelbert et al., 2008) since there are other G2 DNA damage checkpoints, the Cdh1-dependent checkpoint should nevertheless not be functional in these cells. Therefore, DNA-damaged cells may enter mitosis more easily, albeit somewhat slowly, leading to genetic lesions.
Cdh1 inactivation also stabilizes mitotic kinases such as Aurora A and Plk1. This may be involved in the observed cytokinesis defect, which can result in polyploidization, supernumerary centrosomes and multipolar mitosis in the subsequent cell cycle, thus leading to aneuploidy (Engelbert et al., 2008; Garcia-Higuera et al., 2008). Similar phenotypes have been observed with Aurora A or Plk1 overexpression (Meraldi et al., 2002; Anand et al., 2003). In fact, by looking closer at mitosis it has been demonstrated that a failure to degrade Aurora kinases in human Cdh1-knockdown cells causes defects in anaphase spindle organization and premature cytokinesis (Floyd et al., 2008), which may contribute to the failure to complete cytokinesis in some of these cells (Engelbert et al., 2008; Garcia-Higuera et al., 2008) (Figure 1a). Finally, failed degradation of additional targets may contribute to spindle and cytokinesis defects, such as the motor proteins Cin8/Eg5 and Kip1 (Hildebrandt & Hoyt, 2001; Crasta et al., 2006; Crasta et al., 2008) or Ase1/Prc1 (Juang et al., 1997; Visintin et al., 1997) and anillin (Zhao & Fang, 2005) (Table 2).
Tumor suppression by Cdh1
The multiple roles of APC/CCdh1 in cellular proliferation and differentiation make Cdh1 a prime candidate for tumor suppression. A mouse model has recently been developed, allowing for this hypothesis to be formally tested. Cdh1 knockout is embryonic lethal due to placental defects. However, in a conditional knockout, where Cdh1 is expressed in the placenta but not in the embryo, the homozygous mice died shortly after birth for unknown reasons. Heterozygous mice primarily developed epithelial tumors; plasmacytosis and myelodysplastic syndrome were also found. Some of these tumors were analyzed for loss of heterozygosity by PCR, and there was no gross allelic loss or change evident, suggesting that Cdh1 may be a haploinsufficient tumor suppressor. (Garcia-Higuera et al., 2008). However, inactivation of the remaining allele by small deletions or point mutations is still possible; this ought to be addressed by future experiments. Notably, downregulation of Cdh1 has been detected in several cell lines from both hematological neoplasias and solid tumors (Engelbert et al., 2008). Reduced Cdh1 expression has also been described during the malignant progression of a murine B-cell lymphoma cell line (Wang et al., 2000).
An important role of APC/CCdh1 has been reported in mantle cell lymphoma (MCL) and other human non-Hodgkin lymphomas (NHLs) (Lwin et al., 2007). This study shows how the bone marrow stroma regulates cell cycle progression of these tumor cells. Cell adhesion leads to a reversible G1 arrest through upregulation of Cdh1, degradation of Skp2 and a resulting stabilization of p21 and p27. This observation provides an explanation of how Cdh1 downregulation during malignant progression (Wang et al., 2000) may lead to increased proliferation of lymphoma cells in the bone marrow. The interaction of APC/CCdh1 and SCFSkp2 is also deregulated in colorectal and breast cancer (Fujita et al., 2008a; Fujita et al., 2008b). Interestingly, various APC/CCdh1 targets, such as cyclin B, aurora A and B, TPX2, Cdc6 and Cdc20, are among the most frequently overexpressed genes in cancers with chromosomal instability (Carter et al., 2006). APC/CCdh1 targets are also overexpressed at the protein level in malignant tumors (Lehman et al., 2007) and a database search revealed that Cdh1 is downregulated in many common tumors, including those of the prostate, ovary, liver, and brain (Bassermann et al., 2008). Thus, inactivation of APC/CCdh1 in these tumors may lead to stabilization of target proteins and their unscheduled activity may contribute to the development of genetically unstable cancers.
Future directions
Recent studies have demonstrated both strong mechanistic and correlative ties between APC/CCdh1 and carcinogenesis. Reduced activity of Cdh1 leads to cell cycle deregulation and genomic instability. Some of the experimental data offer insights as to how this genomic instability might occur. However, further work is still needed to elucidate the underlying mechanisms. Evaluating the fate of single cells by live cell imaging will be useful to further characterize the phenotype of Cdh1-deficient mammalian cells, allowing for the dysregulation of spindle assembly, elongation, disassembly, chromosome separation and perhaps origin licensing to be characterized in vivo. Fluorescence labeling of intracellular structures and APC/CCdh1 target proteins will allow direct tracking of these proteins (Keppler et al., 2003; Steigemann et al., 2009). Thereby spindles, chromosomes and proteins regulating DNA replication and chromosome separation become visible. Such an approach has already elucidated the influence of APC/CCdh1 on spindle dynamics and cytokinesis by targeting Aurora kinases (Floyd et al., 2008). Further work in this direction may illustrate in much greater detail than currently available, precisely how deregulation of APC/CCdh1 and its targets leads to genomic instability.
Although, in contrast to human cells (Bashir et al., 2004; Engelbert et al., 2008), changes of p21 or p53 expression were not detected in Cdh1 knockout MEFs (Li et al., 2008), additional inactivation of p53 in Cdh1 knockout mice should nevertheless aggravate genomic instability similar as in human cells, which may reduce latency of tumor development in heterozygous mice and lead to a better understanding of the tumor suppressor function of Cdh1.
Another important step will be to better characterize the apparent role of Cdh1 in G2 control, especially to dissect the involvement of the newly discovered Cdh1-dependent G2 DNA damage checkpoint (Figure 1a) in the development of genomic instability. According to this checkpoint, APC/CCdh1 activation in G2 leads to degradation of Plk1 and prevents mitotic entry before DNA damage is repaired (Bassermann et al., 2008). In Cdh1-depleted cells Plk1 levels may be high in G2. Reduction of Plk1 activity e.g. by a small molecule inhibitor in G2 may restore the checkpoint in these cells, which would lead again to a sufficient G2 arrest allowing for DNA repair. Therefore, restoration of this checkpoint should potentially eliminate genomic instability resulting from DNA damage in the previous S phase. This might allow for the uncoupling of the DNA damage sequellae from the other consequences of Cdh1 misregulation.
APC/CCdh1 has an important role in neuronal differentiation and accumulating evidence suggests that this is also true for various other cells. Further investigations should focus on the potential role of Cdh1 in the differentiation of other cell types as well as in stem cells. For example, it will be exciting to see whether Cdh1 is involved in the regulation of self-renewal and differentiation in hematopoietic stem cells, a well-studied system amenable to genetic manipulation. In this context it will also be important to further investigate the homozygous Cdh1 knockout mice. The bone marrow stroma acts upon lymphoma cells (and possibly other hematopoietic cells) so as to control proliferation and effect a cell cycle arrest through APC/CCdh1 activation (Lwin et al., 2007). It is possible that Cdh1 null mice have as of yet uncharacterized defects in hematopoiesis. Although the heterozygous Cdh1 knockout mice mainly develop epithelial tumors, there are suggestions that abnormal Cdh1 function may also be involved in the development of hematopoietic malignancies. If true, Cdh1 inactivation in hematopoietic stem cells could on one hand interfere with proliferation, self-renewal and differentiation, and on the other hand lead to genomic instability, which is a characteristic of several hematological malignancies including myelodysplasias and leukemias. Finally, there are most likely unidentified APC/CCdh1 substrates involved in cell cycle exit and differentiation awaiting recognition and characterization.
The twelve years of research on Cdh1 since its discovery have revealed surprisingly conserved mechanisms of APC/CCdh1-dependent cell cycle regulation from yeast to man. A better understanding of these mechanisms in tumorigenesis should allow for both a better understanding of cancer itself and the development of more sophisticated targeted cancer therapeutics.
Acknowledgements
We thank Andrea Schmidts for help with Figure 2 and Monika Engelhardt for critical reading of the manuscript. RW thanks Roland Mertelsmann for continuous support.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Araki M, Wharton RP, Tang Z, Yu H, Asano M. Degradation of origin recognition complex large subunit by the anaphase-promoting complex in Drosophila. Embo J. 2003;22:6115–6126. doi: 10.1093/emboj/cdg573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–2996. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:56–67. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle. 2005;4:1230–1232. doi: 10.4161/cc.4.9.2048. [DOI] [PubMed] [Google Scholar]
- Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG, Jr, et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol. 2007;9:225–232. doi: 10.1038/ncb1532. [DOI] [PubMed] [Google Scholar]
- Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Morgan DO. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat Cell Biol. 2002;4:880–887. doi: 10.1038/ncb871. [DOI] [PubMed] [Google Scholar]
- Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- Castro A, Vigneron S, Bernis C, Labbe JC, Lorca T. Xkid is degraded in a D-box, KEN-box, and A-box-independent pathway. Mol Cell Biol. 2003;23:4126–4138. doi: 10.1128/MCB.23.12.4126-4138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A, Vigneron S, Bernis C, Labbe JC, Prigent C, Lorca T. The D-Box-activating domain (DAD) is a new proteolysis signal that stimulates the silent D-Box sequence of Aurora-A. EMBO Rep. 2002;3:1209–1214. doi: 10.1093/embo-reports/kvf241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK. The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate. Biol Reprod. 2005;72:1205–1217. doi: 10.1095/biolreprod.104.035717. [DOI] [PubMed] [Google Scholar]
- Crasta K, Huang P, Morgan G, Winey M, Surana U. Cdk1 regulates centrosome separation by restraining proteolysis of microtubule-associated proteins. Embo J. 2006;25:2551–2563. doi: 10.1038/sj.emboj.7601136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasta K, Lim HH, Giddings TH, Jr, Winey M, Surana U. Inactivation of Cdh1 by synergistic action of Cdk1 and polo kinase is necessary for proper assembly of the mitotic spindle. Nat Cell Biol. 2008;10:665–675. doi: 10.1038/ncb1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross FR. Two redundant oscillatory mechanisms in the yeast cell cycle. Dev Cell. 2003;4:741–752. doi: 10.1016/s1534-5807(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Cuende J, Moreno S, Bolanos JP, Almeida A. Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene. 2008;27:3339–3344. doi: 10.1038/sj.onc.1210987. [DOI] [PubMed] [Google Scholar]
- Diffley JF. Regulation of early events in chromosome replication. Curr Biol. 2004;14:R778–R786. doi: 10.1016/j.cub.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol. 2004;165:789–800. doi: 10.1083/jcb.200404092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbert D, Schnerch D, Baumgarten A, Wäsch R. The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells. Oncogene. 2008;27:907–917. doi: 10.1038/sj.onc.1210703. [DOI] [PubMed] [Google Scholar]
- Enquist-Newman M, Sullivan M, Morgan DO. Modulation of the mitotic regulatory network by APC-dependent destruction of the Cdh1 inhibitor Acm1. Mol Cell. 2008;30:437–446. doi: 10.1016/j.molcel.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Floyd S, Pines J, Lindon C. APC/C Cdh1 targets aurora kinase to control reorganization of the mitotic spindle at anaphase. Curr Biol. 2008;18:1649–1658. doi: 10.1016/j.cub.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Fujita T, Liu W, Doihara H, Date H, Wan Y. Dissection of the APCCdh1-Skp2 cascade in breast cancer. Clin Cancer Res. 2008a;14:1966–1975. doi: 10.1158/1078-0432.CCR-07-1585. [DOI] [PubMed] [Google Scholar]
- Fujita T, Liu W, Doihara H, Wan Y. Regulation of Skp2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am J Pathol. 2008b;173:217–228. doi: 10.2353/ajpath.2008.070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Higuera I, Manchado E, Dubus P, Canamero M, Mendez J, Moreno S, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802–811. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci U S A. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- Hildebrandt ER, Hoyt MA. Cell cycle-dependent degradation of the Saccharomyces cerevisiae spindle motor Cin8p requires APC(Cdh1) and a bipartite destruction sequence. Mol Biol Cell. 2001;12:3402–3416. doi: 10.1091/mbc.12.11.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Jacobs H, Richter D, Venkatesh T, Lehner C. Completion of mitosis requires neither fzr/rap nor fzr2, a male germline-specific Drosophila Cdh1 homolog. Curr Biol. 2002;12:1435–1441. doi: 10.1016/s0960-9822(02)01074-6. [DOI] [PubMed] [Google Scholar]
- Jaquenoud M, van Drogen F, Peter M. Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/C(Cdh1) Embo J. 2002;21:6515–6526. doi: 10.1093/emboj/cdf634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M, Li H, Suh HC, Klarmann KD, Yokota Y, Keller JR. Id2 intrinsically regulates lymphoid and erythroid development via interaction with different target proteins. Blood. 2008;112:1068–1077. doi: 10.1182/blood-2008-01-133504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang YL, Huang J, Peters JM, McLaughlin ME, Tai CY, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- Kramer E, Scheuringer NAV, Mann M, Peters J. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, et al. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- Lassar AB, Thayer MJ, Overell RW, Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989;58:659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Lehman NL, Tibshirani R, Hsu JY, Natkunam Y, Harris BT, West RB, et al. Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors. Am J Pathol. 2007;170:1793–1805. doi: 10.2353/ajpath.2007.060767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengronne A, Schwob E. The Yeast CDK Inhibitor Sic1 Prevents Genomic Instability by Promoting Replication Origin Licensing in Late G1. Molecular Cell. 2002;9:1067–1078. doi: 10.1016/s1097-2765(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Li M, Shin YH, Hou L, Huang X, Wei Z, Klann E, et al. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008;10:1083–1089. doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009;4:2. doi: 10.1186/1747-1028-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu G, Wan Y. The dual effects of Cdh1/APC in myogenesis. Faseb J. 2007;21:3606–3617. doi: 10.1096/fj.07-8159com. [DOI] [PubMed] [Google Scholar]
- Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listovsky T, Oren YS, Yudkovsky Y, Mahbubani HM, Weiss AM, Lebendiker M, et al. Mammalian Cdh1/Fzr mediates its own degradation. Embo J. 2004;23:1619–1626. doi: 10.1038/sj.emboj.7600149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlepage LE, Ruderman JV. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 2002;16:2274–2285. doi: 10.1101/gad.1007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wu G, Li W, Lobur D, Wan Y. Cdh1-anaphase-promoting complex targets Skp2 for destruction in transforming growth factor beta-induced growth inhibition. Mol Cell Biol. 2007;27:2967–2979. doi: 10.1128/MCB.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, et al. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. Embo J. 1998;17:3565–3575. doi: 10.1093/emboj/17.13.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- Lwin T, Hazlehurst LA, Dessureault S, Lai R, Bai W, Sotomayor E, et al. Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin B-cell lymphomas. Blood. 2007;110:1631–1638. doi: 10.1182/blood-2006-11-060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore LA, McCormack EA, Au SW, Paul A, Willison KR, Harper JW, et al. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. Embo J. 2003;22:786–796. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Rape M, Kirschner M. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature. 2004;432:588–595. doi: 10.1038/nature03023. [DOI] [PubMed] [Google Scholar]
- Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Reis A, Levasseur M, Chang HY, Elliott DJ, Jones KT. The CRY box: a second APCcdh1-dependent degron in mammalian cdc20. EMBO Rep. 2006;7:1040–1045. doi: 10.1038/sj.embor.7400772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KE, Cohen-Fix O. The role of Cdh1p in maintaining genomic stability in budding yeast. Genetics. 2003;165:489–503. doi: 10.1093/genetics/165.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner A, Murray A. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J Cell Biol. 2000;149:1377–1390. doi: 10.1083/jcb.149.7.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Toth A, Galova M, Nasmyth K. APCCdc20 promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature. 1999;402:203–207. doi: 10.1038/46080. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Skotheim JM, Di Talia S, Siggia ED, Cross FR. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature. 2008;454:291–296. doi: 10.1038/nature07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression. Mol Cell Biol. 2001;21:3692–3703. doi: 10.1128/MCB.21.11.3692-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, et al. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmüller J, Huynh MA, Yuan Z, Konishi Y, Bonni A. TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J Neurosci. 2008;28:1961–1969. doi: 10.1523/JNEUROSCI.3061-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmüller J, Konishi Y, Huynh MA, Yuan Z, Dibacco S, Bonni A. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron. 2006;50:389–400. doi: 10.1016/j.neuron.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473–484. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- Stroschein SL, Bonni S, Wrana JL, Luo K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 2001;15:2822–2836. doi: 10.1101/gad.912901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Ota Y, Kotani S, Nakao M, Takami Y, Takeda S, et al. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. Embo J. 2001;20:6499–6508. doi: 10.1093/emboj/20.22.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HC, Leeanansaksiri W, Ji M, Klarmann KD, Renn K, Gooya J, et al. Id1 immortalizes hematopoietic progenitors in vitro and promotes a myeloproliferative disease in vivo. Oncogene. 2008 doi: 10.1038/onc.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M, Morgan DO. A novel destruction sequence targets the meiotic regulator Spo13 for anaphase-promoting complex-dependent degradation in anaphase I. J Biol Chem. 2007;282:19710–19715. doi: 10.1074/jbc.M701507200. [DOI] [PubMed] [Google Scholar]
- Takahashi C, Ewen ME. Genetic interaction between Rb and N-ras: differentiation control and metastasis. Cancer Res. 2006;66:9345–9348. doi: 10.1158/0008-5472.CAN-06-1250. [DOI] [PubMed] [Google Scholar]
- Taylor S, Peters JM. Polo and Aurora kinases: lessons derived from chemical biology. Curr Opin Cell Biol. 2008;20:77–84. doi: 10.1016/j.ceb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Vaziri C, Saxena S, Jeon Y, Lee C, Murata K, Machida Y, et al. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell. 2003;11:997–1008. doi: 10.1016/s1097-2765(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol Cell. 2001;8:1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- Wang CX, Fisk BC, Wadehraq M, Su H, Braun J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood. 2000;96:259–263. [PubMed] [Google Scholar]
- Wäsch R, Cross FR. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature. 2002;418:556–562. doi: 10.1038/nature00856. [DOI] [PubMed] [Google Scholar]
- Wäsch R, Engelbert D. Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene. 2005;24:1–10. doi: 10.1038/sj.onc.1208017. [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- Wirth KG, Ricci R, Gimenez-Abian JF, Taghybeeglu S, Kudo NR, Jochum W, et al. Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes Dev. 2004;18:88–98. doi: 10.1101/gad.285404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Glickstein S, Liu W, Fujita T, Li W, Yang Q, et al. The anaphase-promoting complex coordinates initiation of lens differentiation. Mol Biol Cell. 2007;18:1018–1029. doi: 10.1091/mbc.E06-09-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Mahbubani H, Hunt T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol Cell. 2004;13:137–147. doi: 10.1016/s1097-2765(03)00480-5. [DOI] [PubMed] [Google Scholar]
- Yeong FM, Lim HH, Padmashree CG, Surana U. Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol Cell. 2000;5:501–511. doi: 10.1016/s1097-2765(00)80444-x. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- Zhao WM, Fang G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem. 2005;280:33516–33524. doi: 10.1074/jbc.M504657200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ching YP, Chun AC, Jin DY. Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J Biol Chem. 2003;278:12530–12536. doi: 10.1074/jbc.M212853200. [DOI] [PubMed] [Google Scholar]
- Zielke N, Querings S, Rottig C, Lehner C, Sprenger F. The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes Dev. 2008;22:1690–1703. doi: 10.1101/gad.469108. [DOI] [PMC free article] [PubMed] [Google Scholar]