Abstract
Although volumetric and activation changes in the cerebellum have frequently been reported in studies on major depression, its role in the neural mechanism of depression remains unclear. To understand how the cerebellum may relate to affective and cognitive dysfunction in depression, we investigated the resting-state functional connectivity between cerebellar regions and the cerebral cortex in samples of patients with geriatric depression (n = 11) and healthy controls (n = 18). Seed-based connectivity analyses were conducted using seeds from cerebellum regions previously identified as being involved in the executive, default-mode, affective-limbic, and motor networks. The results revealed that, compared with controls, individuals with depression show reduced functional connectivity between several cerebellum seed regions, specifically those in the executive and affective-limbic networks with the ventromedial prefrontal cortex (vmPFC) and increased functional connectivity between the motor-related cerebellum seed regions with the putamen and motor cortex. We further investigated whether the altered functional connectivity in depressed patients was associated with cognitive function and severity of depression. A positive correlation was found between the Crus II–vmPFC connectivity and performance on the Hopkins Verbal Learning Test-Revised delayed memory recall. Additionally, the vermis–posterior cinglate cortex (PCC) connectivity was positively correlated with depression severity. Our results suggest that cerebellum–vmPFC coupling may be related to cognitive function whereas cerebellum–PCC coupling may be related to emotion processing in geriatric depression.
Introduction
Depression has been modeled as a failure of the coordination between the dorsal cognitive control system and the ventral emotional system [1]. While numerous neuroimaging studies support the essential role of the prefrontal-striato-limbic circuits in depression [1], [2], [3], there are also a number of reports indicating an altered neural response in the cerebellum [4], such as increased cerebellar-vermal blood flow in depressed patients with cognitive impairment [5], reduced cerebellar volume during depressed state [6], [7], and progressively reduced cerebellar volume over time [8]. Although there is clear evidence of the involvement of the cerebellum in depression [4], [9], the functional role of the cerebellum in depression remains unclear.
There is ample evidence that the cerebellum not only subserves motor function, planning, and coordination of movement, but also plays an important role in emotion and cognition [10]. Recent reviews have paid much attention to the involvement of the cerebellum in emotion and cognition [11], [12]. Anatomically, regions of the cerebellum, such as the vermis, fastigial nucleus, and flocculonodular lobe, have reciprocal connections with brainstem reticular nuclei [13], [14] and regions in the limbic and autonomic system, including hypothalamus [15], [16], [17], ventral tegmental area, periaqueductal gray [18], hippocampus, and amygdala [19]. The cerebellum also receives projections from the rostral and caudal anterior cingulate through the pons [20]. These connections may provide an anatomical basis for the cerebellum's role in emotion. Connections between the prefrontal cortex and the cerebellum have also been found, which include descending projections from the dorsolateral and dorsomedial prefrontal cortex to the cerebellum through the medial pons and ascending projects from cerebellum through thalamus to prefrontal cortex. These connections are hypothesized to be the neural substrates for the cognitive function of the cerebellum [9], [10], [21]. In animal studies, electrical stimulation of the vermis area of the cerebellum evoked responses in the orbitofrontal cortex, anterior cingulate, amygdala, and hippocampus [19], [20], [22]. In human studies, stimulation of the surface of cerebellum through the implantation of electrodes revealed alleviation of depression [19]. The cognitive affective syndrome in patients with cerebeller lesions reported by Schmahmann and Sherman [23] provides strong evidence of the involvement of the cerebellum in emotion and cognition. In addition, response to emotional stimuli in the cerebellum, particularly in the vermis, has been found in a number of neuroimaging studies [24], [25]. These studies provide empirical evidence supporting an essential contribution of the cerebellum to the affective and cognitive dysfunction in depression.
Recent use of intrinsic resting-state functional connectivity enables us to understand the functional connectivity between the cerebellum and the cerebrum [26]. Using an independent component analysis (ICA) approach, Habas and colleagues [27] identified regions in the cerebellum that ‘belong’ to the dorsal executive, salience, default-mode, and sensorimotor networks, separately. Meanwhile, using seeds from these four neural networks in the cerebral cortex, Krienen and Buckner [26] also investigated the regions in the cerebellum that are functionally connected with the dorsal executive, default-mode, affective, and motor networks of the cerebrum. They found that the lateral hemisphere of the cerebellum was functionally connected with the dorsolateral prefrontal cortex (dlPFC), suggesting its potential involvement in executive function. Additionally, the Crus I of the cerebellum was functionally connected with the medial prefrontal cortex and anterior cingulate indicating its involvement in default-mode activity and emotional processing [26]. These studies of functional connectivity between the cerebellum and cerebrum provide a topographic functional map of the cerebellum, which could explain the role of cerebellar volumetric [6], [28], [29], [30] and activation changes [5], [31], [32] in depression. However, to our knowledge, there is no direct evidence showing how the relationship of the frontal-cerebellar connectivity with mood or cognitive function is altered in depression.
Using seeds in the cerebellum that were suggested to be involved in emotional and cognitive function by Krienen and Buckner [26], we compared the intrinsic functional connectivity between the cerebellum and the cerebrum in the executive, default-mode, affective-limbic, and motor networks in depressed patients and healthy controls. Given that cognitive impairment is frequently seen in geriatric depression, this study focused on older individuals to investigate the association of altered connectivity with severity of depression, memory, and executive functions. We also examined the cerebellum-cerebrum functional connectivity in older healthy individuals to validate previous network findings in older adults.
We hypothesized that depressed patients would have decreased functional connectivity between the executive network-related regions in the cerebellum and the prefrontal cortex, and they would have increased connectivity between the affective-limbic network-related regions in the cerebellum and affective regions including the amygdala or ventral striatum. We also hypothesized that the alterations in the connectivity from the affective-limbic network-related regions of the cerebellum to the cerebrum would be associated with severity of depression.
Materials and Methods
Participants
Twenty-nine individuals participated in this study (11 depressed, 18 non-depressed controls). Participants were recruited from the Conte Center for the Neuroscience of Depression in Late Life at Duke University Medical Center. All depressed patients met DSM-IV criteria for major depression. They were either in an active major unipolar depressive episode or at least had some depressed symptoms, with a Montgomery-Åsberg Depression Rating Scale (MADRS) [33] score of 8 or more at the time of participation in the study. The exclusion criteria for depressed subjects included: (1) another major psychiatric illness, including bipolar disorder, schizophrenia, or dementia; (2) alcohol or drug abuse or dependence; (3) neurological illness, including dementia, stroke, and epilepsy; (4) medical illness, medication use, or disability that would prevent the participant from completing neuropsychological testing; and (5) contraindications to MRI. All non-depressed subjects were cognitively intact, had no history or clinical evidence of dementia, and all scored 28 or more on the Mini-Mental State Examination. Among the 11 depressed participants, 6 were receiving antidepressant monotherapy (3 on an SSRI, 2 on venlafaxine, and 1 on amitriptyline) and 5 were receiving combination treatment (4 on SSRI combined with either SNRI, SARI, or DNRI and 1 on SSRI, NDRI, and SNRI).
Prior to the fMRI, subjects' cognitive function was assessed using a short, 30-minute battery of neuropsychological tests. The neuropsychological tests included the Mini-Mental State Exam (MMSE), Category Fluency (Vegetable Naming), Hopkins Verbal Learning Test-Revised (HVLT-R), Immediate and Delayed Story Recall from the Rivermead Behavioral Memory Test, Trail Making Test (Trail A and Trail B), WAIS-III Digit Span, WAIS-III Digit–Symbol Substitution Test (DSST), and Stroop Color and Word Test. The study received ethics committee approval by Duke School of Medicine Institutional Review Board and, after being explained the purpose and procedures to be used in the study, all subjects gave verbal and written consent.
Neuroimaging Acquisition
We obtained a 5-minute resting fMRI scan for each participant. Participants were instructed to rest without moving, keep their eyes open, and focus on a fixation cross presented in the center of the screen inside the scanner. All participants were scanned using a research-dedicated 3.0 T GE EXCITE HD scanner (GE Medical Systems, Milwaukee, Wisconsin). Oblique spoiled gradient-recalled acquisition images (three-dimensional, whole-brain) were acquired parallel to the anterior commissure (AC) - posterior commissure (PC) plane for high-resolution T1-weighted structural images with a matrix of 256×256×169, slice thickness of 1 mm. Inward spiral sequence functional images were acquired with the following parameters: TR = 2000 ms, TE = 31 ms, FOV = 24 cm, flip angle = 90°, matrix = 64×64×34, slice thickness = 3.75 mm with 3.75 mm3 isotropic voxels.
Data Analyses
FEAT (FMRI Expert Analysis Tool) Version 5.98, part of the FSL analysis package (fMRIB's Software Library, www.fmrib.ox.ac.uk/fsl), was used to conduct the standard image pre-processing procedures including slice-timing alignment, motion correction, coregistration, non-brain voxel extraction, normalization, and smoothness (6 mm3 kernel). Temporal filtering settings were applied using a high-pass filter (Gaussian-weighted least-squares straight line fitting, with sigma = 100.0 s) and a low-pass filter (Gaussian low-pass temporal filtering: HWHM 2.8 s) following Biswal and colleagues [34].
To identify the functionally-connected networks between the cerebellum and cerebrum, seed-based correlation analyses were carried out by extracting the time series from regions of interest (ROI) using FSL's FLIRT. Seeds that were shown to have a fronto-cerebellar connection from Krienen and Buckner [26] and Stoodley and Schmahmann [35] were used to identify executive, default-mode, affective-limbic, and motor networks in the cerebellum. For the executive network, three pairs of bilateral seeds were chosen: Crus IExec1, Crus IIExec2 and Lobule VIantExec. Both Crus IExec1 and Crus IIExec2 have been shown to be functionally connected with the posterior region of the dlPFC by Krienen and Buckner [26]. Lobule VIantExec was shown to be functionally coupled with the anterior portion of the dlPFC [26]. For the default-mode network, bilateral Crus IDMN seeds were selected, which were found to have functional connections to default-mode network (DMN) regions [26]. We used bilateral Lobule VILimbic and the left VermisLimbic for the affective-limbic network. These regions of the cerebellum were found to be activated during emotional processing [35]. For the motor-network regions, bilateral Lobule VMotor seeds were used and previously found to be functionally connected to the motor cortex [35]. In Table 1, these seed regions are grouped by network with their center coordinates listed. A 5-mm radius sphere was drawn from each center point as an ROI. The timecourse during the 5-minute resting scan within the sphere was extracted. The timecourse of each ROI was then entered as a regressor into the first-level (within-subject) general linear model (GLM) using FEAT. Nuisance regressors (global signal, white matter, and motion parameters) were also entered into the model.
Table 1. Cerebellar Regions of Interest (seeds) and Coordinates Grouped By Network.
| Cerebellar Network | MNI (x,y,z) |
| Executive Network | |
| L Crus IExec1 | −12, −78, −28 |
| R Crus IExec1 | 12,−78,−28 |
| L Crus IIExec2 | −36,−70,−46 |
| R Crus IIExec2 | 36, −68, −44 |
| L Lobule VIantExec | −36, −52, −34 |
| R Lobule VIantExec | 36, −52, −34 |
| Default-Mode Network | |
| L Crus IDMN | −32, −76, −34 |
| R Crus IDMN | 34, −80, −36 |
| Affective-Limbic Network | |
| R Lobule VILimbic | 26, −64, −34 |
| L Lobule VILimbic | −26, −64, −34 |
| L VermisLimbic | −4, −80, −34 |
| Motor Network | |
| R Lobule VMotor | 22 −52 −22 |
| L Lobule VMotor | −20 −50 −24 |
To examine group differences for each region's seed-based functional connectivity, we used a mixed-effects (FLAME 1) two-sample t-test analysis on each seed-based connectivity map. To test the relationship between the functional connectivity and individual variation in severity of depression, we conducted a regression analysis in the depressed group using participants' MADRS scores as a regressor. To test the relationship between the functional connectivity and individual variation in memory performance and executive function, we conducted a regression analyses using HVLT-R delay scores and Stroop Color and Word test scores from all participants, including both patients and controls, as covariates in separate third-level models. Given the age difference between the two groups, we used age as a regressor to remove any age effect from the third level analyses. Statistical results used a voxel significance threshold of z>2.3 and a whole-brain-corrected cluster-significance threshold of p<0.05 [36]. Significant clusters were selected as ROIs for the regression plots to double confirm the voxel-based whole-brain analysis.
Results
Clinical Profile of the Participants
Demographic details of the participants and performance on the memory and executive function-related tests from the neuropsychological battery are listed in Table 2. In summary, there were 11 females and 7 males in the control group whereas there were 10 females and 1 male in the depressed group (chi square = 3.04, df = 1, p = 0.08). The depressed group was significantly younger in age than the control group (two-sample t-test, t26 = 3.05, p = 0.005). Therefore, age was used as a covariate in data analyses to remove any age effect.
Table 2. Participant Demographic and Neuropsychological Testing Data.
| Control N = 18 | Depressed N = 11 | P values | |
| Age (SD) | 71.2 (6.6) | 64.9 (4.5) | 0.005 |
| No. of Female/Male | 11/7 | 10/1 | |
| Education (SD) years | 15.8 (2.3) | 14.4 (3.1) | 0.192 |
| MADRS (SD) | 0.4 (0.8) | 17.5 (8.2) | <0.001 |
| No. with early/late onset depression | N/A | 7/4 | |
| Age of onset (SD) | N/A | 33.5 (18.5) | |
| Duration of illness in years (SD) | N/A | 31.3 (17.9) | |
| No. with multiple depressive episodes | N/A | 8 | |
| No. taking antidepressants | N/A | 11 | |
| MMSE (SD) | 29.2 (1.2) | 28.9 (1.7) | 0.605 |
| Memory function | |||
| Rivermead Delay(SD) | 7.5 (3.0) | 3.9 (3.1) | 0.011 |
| HVLT Delay(SD) | 10.1 (1.3) | 6.0 (4.3) | 0.021 |
| Executive function | |||
| Digit span (SD) | 14.1 (4.5) | 11.6 (3.0) | 0.095 |
| Symbol-Digit Modality (SD) | 70.1 (11.3) | 53.8 (16.7) | 0.021 |
| Stroop Color-Word task (SD) | 34.1 (4.6) | 30.7 (8.2) | 0.267 |
The mean (SD) depression severity score measured by the MADRS for the depressed group was 17.5 (8.2), which was significantly greater than the control group (two-sample t-test, t26 = 6.85, p<0.001). Among the 11 depressed patients, all were on multiple or mono- antidepressant therapy. Although the MMSE was not significantly different between the depressed and the control group (two-sample t-test, t26 = 0.44, p = 0.605), the depressed group revealed significantly poorer performance on the neuropsychological tests, including the two memory tests—the HVLT-R delay (two-sample t-test, t26 = 2.49, p = 0.021) and Delayed Story Recall from the Rivermead Behavioral Memory Test (two-sample t-test, t26 = 2.86, p<0.011), and one of the executive tests—the DSST(two-sample t-test, t26 = 2.64, p = 0.021).
The Cerebellar-Cerebral Functional Connectivity in Healthy Older Subjects
Our study on cerebellar-cerebral functional connectivity in healthy elderly subjects largely replicated the findings of Krienen and Buckner in younger subjects [26]. The executive network region within the cerebellum (right Crus IIExec2 and right Lobule VIantExec) did show connectivity with the executive network in the cerebral cortex, specifically the contra-lateral dlPFC and the inferior parietal cortex (Figure 1). Interestingly, the significant functional coupling between the cerebellum and dlPFC was only found in the right cerebellar seed and left dlPFC, but not with the cerebellar seeds in the left hemisphere. In addition, the executive network regions within the cerebellum (specifically right Crus IIExec2) also showed functional connectivity with the putamen and default-mode network regions including the posterior cingulate cortex (PCC) and the ventral medial prefrontal cortex (vmPFC).
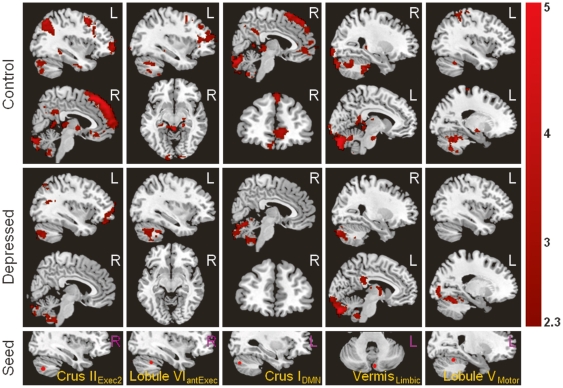
Figure 1. Resting-state functional connectivity of cerebellum seed regions (row five) with cerebral regions from the healthy control group (rows one and two) and depression group (rows three and four).
The significant functional connectivity maps shown in each column were computed from the cerebellar seed region shown in the bottom row. The seed regions shown here include: Crus IIExec2, the Crus II region found to be functionally connected with a cerebral executive region (the posterior dlPFC) in previous studies; Lobule VIantExec, the Lobule VI region found to be functionally connected with a cerebral executive region (the anterior dlPFC) in previous studies; Crus IDMN, the Crus I region found to be functionally connected with the default-mode network (mPFC) in previous studies; VermisLimbic, the vermis region found to be functionally connected with a limbic region (ACC); Lobule VMotor, the Lobule V region found to be functionally connected with the motor cortex.
The seed within the default-mode network (DMN) in the cerebellum, left Crus IDMN, showed functional connectivity with default-mode network regions in the cerebrum including the ventromedial prefrontal cortex (vmPFC), dorsomedial prefrontal cortex (dmPFC), and the PCC (Figure 1). The right Crus IDMN, however, did not show significant functional connectivity with the default-mode network regions. Instead, it showed functional connectivity with the caudate, thalamus, and fusiform gyrus when using a relatively lenient threshold on cluster correction—z>2 rather than z>2.3—and a cluster corrected significance threshold of p = 0.05.
The affective-limbic network of the cerebellum, specifically bilateral Lobule VILimbic and left VermisLimbic, demonstrated functional connectivity with the PCC, inferior parietal cortex, brainstem, thalamus, and hypothalamus in addition to regions within the cerebellum (Figure 1).
The cerebellar motor network seed regions demonstrated functional connectivity with the sensorimotor regions including the cerebellum, medial lemnis fasiculus in the pons, bilateral red nucleus in the brainstem, right putamen, right thalamus, bilateral sensory cortex, and anterior cingulate cortex (ACC) (Figure 1).
Decreased Cerebellar-Cerebral Functional Connectivity in Depressed Patients Relative to Healthy Control Subjects
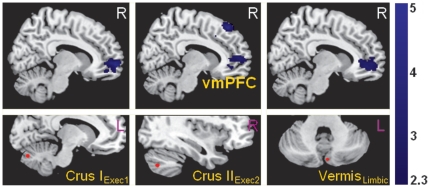
Compared with healthy control subjects, the depressed group showed significantly reduced functional connectivity between the vmPFC and several cerebellar seed regions from the executive and affective-limbic networks, specifically bilateral Crus IExec1 and right Crus IIExec2 seeds, as well as left VermisLimbic (Figure 2).
Figure 2. Significantly reduced functional connectivity in depressed patients between cerebellar executive and affective-limbic seed regions (shown in the lower row) with the ventromedial prefrontal cortex (vmPFC).
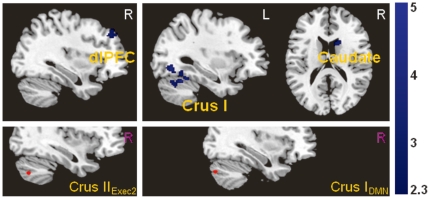
In addition, the depressed group showed reduced functional coupling relative to controls between the executive region in the cerebellum and the executive regions in the cerebral cortex, specifically between the right Crus IIExec2 seed and the right dlPFC and dmPFC (BA8) (Figure 3). The executive seed regions left Crus IIExec2 and Lobule VIantExec also showed reduced connectivity with other regions within the cerebellum.
Figure 3. Significantly reduced functional connectivity in depressed patients between cerebellar executive and default-mode seed regions (shown in the lower row) with cerebral areas.
The default-mode network seed in the cerebellum, specifically right Crus IDMN, showed decreased connectivity with the right head of the caudate, right insula/putamen, left fusiform gyrus, and left Lobule VI in depressed patients relative to controls (Figure 3).
For the affective-limbic network in the cerebellum, depressed patients had reduced functional connectivity relative to controls between the left Lobule VILimbic with the right inferior parietal cortex (BA39) and the PCC and the left VermisLimbic with the left ventrolateral prefrontal cortex (vlPFC, BA47).
Interestingly, the left Lobule VMotor seed showed decreased connectivity with the dorsal executive regions in the cerebral cortex—the left dlPFC and vlPFC—in patients.
All regions showing decreased connectivity with cerebellar seeds in depressed patients relative to controls are listed in Table 3.
Table 3. Brain Regions Showing Decreased Cerebeller-Cerebral Connectivity in Geriatric Depression Compared With the Control Group.
| Cerebellar Seed Region | Regions showing decreased connectivity in geriatric depression | BA | Side | Voxel size | MNI Coordinates (x,y,z) | Z value |
| Executive Network | ||||||
| R Crus IExec1 | mPFC | BA10 | R | 765 | 10, 50, 0 | 3.75 |
| vmPFC/rACC | BA32 | R | 14, 42, 2 | 3.63 | ||
| L Crus IExec1 | vmPFC | BA10 | R | 500 | 12, 60, −2 | 4.51 |
| R CrusIIExec2 | vmPFC/rACC | BA32 | R | 485 | 14, 44, 6 | 4.27 |
| dmPFC | BA8 | R | 691 | 6, 38, 52 | 4.59 | |
| dlPFC | BA9 | R | 490 | 28, 40, 38 | 4.44 | |
| L Crus IIExec2 | Lobule VI | R | 910 | 30, −56, −24 | 3.64 | |
| Vermis | L | −2, −46, 10 | 3.96 | |||
| Brainstem (red nuclus) | 0, −24, −8 | 3.61 | ||||
| L LobuleVIantExec | Lobule VI | R | 67 | 0,−72,−14 | 3.58 | |
| Crus I | R | 82 | 24,−78,−26 | 3.25 | ||
| Crus I | L | 86 | −8,−74,−24 | 3.48 | ||
| Default-Mode Network | ||||||
| R Crus IDMN | Caudate | R | 83 | 16, 12, 16 | 3.55 | |
| Putamen | R | 72 | 30, 10, −6 | 4.13 | ||
| Fusiform Gyrus | BA19 | L | 84 | −22, −54, −14 | 4.27 | |
| Lobule VI | L | 539 | −32, −56, −24 | 4.38 | ||
| Affective-Limbic Network | ||||||
| L VermisLimbic | vmPFC/mPFC | BA10 | R | 517 | 16, 46, 6 | 3.62 |
| vlPFC | BA47 | L | 474 | −44, 26, 4 | 4.09 | |
| L Lobule VILimbic | mPFC | BA10 | R | 660 | 8, 62, 30 | 3.99 |
| Precuneus/PCC | BA23 | R | 1096 | 8, −50, 24 | 3.76 | |
| Inferior parietal cortex | BA39 | R | 632 | 50, −58, 22 | 4.32 | |
| Motor Network | ||||||
| L Lobule VMotor | vlPFC | BA47 | L | 1301 | −50 ,28, 2 | 3.84 |
| dlPFC | BA10 | L | −40, 58, 2 | 3.48 |
Increased Cerebellar-Cerebral Functional Connectivity in Depressed Patients Relative to Healthy Control Subjects
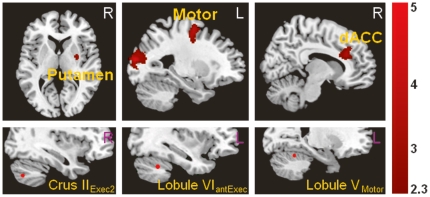
Within the executive network in the cerebellum, a number of seeds showed increased connectivity with other networks in depressed patients, predominantly with the self-referential processing-related region, the dmPFC, or interoceptive cortex, the insula [37]. Specifically, the right Crus IIExec1 displayed increased connectivity with the dmPFC (BA10) in patients relative to controls. A similar pattern of increased connectivity in patients was seen in the right Crus IIExec1 with the insula and the right Crus IIExec2 also with the insula. The Crus IIExec2 also showed increased connectivity with the motor network, specifically the putamen; and the left Lobule VIantExec showed increased connectivity with the supplementary motor area as well as the middle temporal cortex (Figure 4).
Figure 4. Significantly increased functional connectivity in depressed patients between cerebellar executive and motor seed regions (shown in the lower row) with cerebral areas.
The left Lobule VMotor showed increased functional connectivity with the right dorsal ACC/dmPFC (Figure 4). All regions showing increased connectivity with cerebellar seeds in depressed patients relative to controls are listed in Table 4.
Table 4. Brain Regions Showing Increased Cerebeller-Cerebral Connectivity in Geriatric Depression Compared With the Control Group.
| Cerebellar Seed Region | Regions showing increased connectivity in geriatric depression | BA | Side | Voxel size | MNI Coordinates (x,y,z) | Z value |
| Executive Network | ||||||
| R Crus IExec1 | dmPFC | BA10 | L | 481 | −18,60,18 | 4.51 |
| Insula | BA13 | L | 3497 | −44, −12,18 | 5.14 | |
| R Crus IIExec2 | Insula | BA13 | R | 695 | 44, −34, 22 | 3.66 |
| Putamen | R | 30, −2, 6 | 3.14 | |||
| L Lobule VIantExec | Precentral cortex | BA6 | L | 446 | −42, 0, 46 | 4.42 |
| Middle Occipital | BA19 | L | 685 | −26, −90, 16 | 4.5 | |
| Affective-Limbic Network | ||||||
| L Lobule VILimbic | Middle temporal | BA39 | L | 1029 | −34, −50, 6 | 3.67 |
| Motor Cortex | BA2 | L | 2056 | −42, −36, 62 | 6.96 | |
| Motor Network | ||||||
| L Lobule VMotor | dmPFC/dACC | R | 762 | 20, 28, 36 | 3.75 |
Correlation with Cognition and Affect
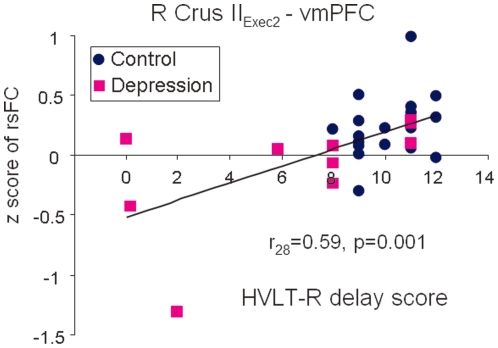
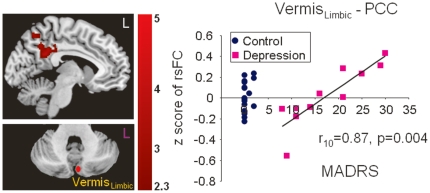
We further investigated whether any of the significant differences in the resting state functional connectivity between depressed patients and controls were correlated with scores on memory and executive function tasks or with severity of depression. Using scores for each participant on the HVLT-R delay test and the Stroop Color and Word test as regressors in separate third-level group analysis models, we found a positive correlation between the right Crus IIExec2–vmPFC connectivity and performance on the HVLT-R delay (r27 = 0.59, p = 0.001) across all subjects (Figure 5). Given that the majority of control subjects had a MADRS score of 0, the correlation between the functional connectivity with severity of depression was conducted only in the depressed group. Within the depressed group, the VermisLimbic–PCC coupling was positively correlated with severity of depression measured by the MADRS (r10 = 0.87, p = 0.004) (Figure 6).
Figure 5. Significant correlation of decreased right Crus II Exec2-vmPFC connectivity with poorer performance on the HVLT-R delay across both depressed (purple squares) and control subjects (blue dots).
Data from all participants were included in this regression analysis.
Figure 6. Significant correlation of increased VermisLimbic- PCC connectivity with severity of depression symptoms in the depressed group (purple squares).
Data from the depressed patients only were included in this regression analysis.
Discussion
We found aberrant functional connectivity in geriatric depression patients between the cerebellum and the cerebral cortex in several neural networks. The cerebellar seed regions in both the executive and affective-limbic networks revealed decreased functional connectivity with the vmPFC. Furthermore, the decreased Crus II–vmPFC connectivity was correlated with poorer memory performance (HVLT-R delay) confirming a role of the Crus II–vmPFC connectivity in cognition. In addition, there was a significant correlation between the Vermis–PCC connectivity and severity of depression, which supports the involvement of the cerebellum in emotional processing. Therefore, our results provide strong support for the contribution of the cerebellum to both cognitive and affective dysfunction in depression.
The vmPFC has wide connections with both affective-limbic regions—such as the amygdala, hippocampus, and hypothalamus—and with executive control and emotional regulation regions—such as the lateral orbital frontal cortex (OFC), dlPFC, vlPFC, and dorsal ACC. It possibly connects to the cerebellum through the cingulate-pontine-cerebellar pathway [38]. Several studies have reported the important role of the vmPFC in emotional regulation [39], extinction memory [40], and self-referential processing [35]. Together with the dmPFC, PCC, and hippocampus, the vmPFC has also been proposed as a node of the affective appraisal network [41] and the vmPFC showed a preference for positive emotional experiences [42]. Consistent with its key role in both cognition and emotion, we found decreased functional coupling between both cerebellar regions related to executive and affective-limbic networks with the vmPFC in depression. Furthermore, across both controls and depressed patients, we found the Crus IIExec2–vmPFC connectivity was positively correlated with the performance on the HVLT-R delay test, which highlights the importance of the functional coupling between the two regions in memory.
In addition to the decreased connectivity with the vmPFC, cerebeller seeds showed decreased connectivity within the executive network—the Crus IIExec2–dlPFC coupling—and decreased connectivity within the affective-limbic network—the VermisLimbic–vlPFC coupling. The vlPFC has consistently been found to be involved in emotional regulation and activated during emotional reappraisal [43], [44], supporting the notion that the VermisLimbic indeed could be related to affective regulation. Consistently, a similar result was found in the study of Frodl and colleagues who reported reduced orbitofrontal cortex (OFC) and cerebellum coupling during negative emotional processing in younger patients with depression [45]. Interestingly, higher OFC-cerebellum connectivity was found in antidepressant non-responders [46]. Meanwhile, the VermisLimbic–PCC coupling was positively correlated with severity of depression. The PCC is one of the key nodes of the default-mode network and the affective appraisal network. Consistent with previous findings of hyper default activity in the subgenual cingulate and thalamus area, here we also showed increased VermisLimbic–PCC coupling, which could possibly represent heightened rumination during resting state, and decreased VermisLimbic–vlPFC coupling as possible weakened emotion regulation in depressed patients.
The default-mode region of the cerebellum showed reduced connectivity with the caudate and ventral putamen. This result is in alignment with a recent study that showed reduced functional connectivity between the default-mode network and the caudate [47]. The caudate is part of the reward system and typically activated by motivation or reward tasks [48]. Lack of motivation and reduced response to reward is one of the core deficits in depression [49]. Reduced functional coupling between the default-mode network and reward system might reflect anhedonia, a state in depressed patients marked by a habitual lack of happiness or motivation.
The motor network region of the cerebellum, specifically the left Lobule VMotor, had reduced connectivity with the attention-executive network, the left dlPFC, which might be related to the psychomotor retardation in geriatric depression. However, the left Lobule VMotor also showed increased connectivity with another attention-executive region, the right dorsal ACC. Interestingly, we also found increased connectivity between other cerebellar seeds and the motor network including the left Lobule VIantExec with the supplementary motor cortex and Lobule VILimbic with the motor cortex. The increased connectivity between these regions could represent compensatory pathways related to the reduced coupling of Lobule VMotor–dlPFC, right Crus IIExec2–vmPFC, and Lobule VILimbic–vmPFC.
Overall, by replicating the findings of Krienen and Buckner [26] in younger, healthy individuals, our study in a healthy older population confirmed the involvement of the cerebellum in executive, affective-limbic, default-mode, and motor networks of the cerebrum. Limitations of our study include the small sample size of the geriatric depression group and potential antidepressant effects. Future studies in a larger sample of geriatric depression patients free of medication to further investigate the causal relationship among the networks would further enhance our understanding of the role of the cerebellum in depression.
In summary, we found abnormal cerebellum-cerebral couplings in cognitive, default-mode, affective-limbic, and motor networks in geriatric depression patients. Therefore, by extending previous findings, our study has provided possible neural mechanisms for the involvement of the cerebellum in depression. Adding the cerebellum into the model of depression can stimulate the development of alternative interventions in depression, such as exercise or transcranial magnetic stimulation (TMS) of the cerebellum to modulate the altered cerebellar-cerebral pathways. Exercise has been shown to improve mood [50], expedite the recovery of depressed patients [51], and improve motor coordination skills and cognition [52]. While exercise can have many effects such as improving vascular endothelial function and blood flow [53], elevating the concentration of neurotransmitters such as serotonin and norepinephrine [54], increasing generation of BDNF (Brain-Derived Neurotrophic Factor), and promoting neurogenesis [55], [56], [57] and increasing the number of dendrite connections between neurons, it is possible that frequent exercise can also improve the efficiency of neural functional connectivity across all networks including the cerebellar-cerebral connectivity. Therefore, the altered functional cerebellar-cerebral connectivity found in our study can, potentially, be a target for monitoring the effect of exercise in geriatric depression. Future studies in depression to investigate the association between improved cognition and reduced depression symptoms with changes in the functional connectivity of the cerebellum would further enhance our understanding of the affective and cognitive function of the cerebellum.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Paul B. Beeson Career Developmental Awards (K23-AG028982), a National Alliance for Research in Schizophrenia and Depression Young Investigator Award (LW), and the Duke Silvio O. Conte Center for the Neuroscience of Depression (P50-MH60451). DC is supported by a NIMH Mid-Career Development Award (K24 MH70027). GP is supported by NIMH Career Development Award (K23 MH087741). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 2.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolan RJ, Bench CJ, Brown RG, Scott LC, Friston KJ, et al. Regional cerebral blood flow abnormalities in depressed patients with cognitive impairment. J Neurol Neurosurg Psychiatry. 1992;55:768–773. doi: 10.1136/jnnp.55.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escalona PR, Early B, McDonal WM. Reduction of cerebellar volume in major depression: a controlled magnetic resonance imaging study. Depression. 1993;1:156–158. [Google Scholar]
- 7.Peng J, Liu J, Nie B, Li Y, Shan B, et al. Eur J Radiol.; 2010. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: A voxel-based morphometry study. doi: 10.1016/j.ejrad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 9.Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- 10.Schmahmann J. The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics. 2000;13:189–214. [Google Scholar]
- 11.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 12.Stoodley CJ. Cerebellum.; 2011. The Cerebellum and Cognition: Evidence from Functional Imaging Studies. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- 13.Andrezik JA, Dormer KJ, Foreman RD, Person RJ. Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neuroscience. 1984;11:497–507. doi: 10.1016/0306-4522(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 14.Qvist H. The cerebellar nuclear afferent and efferent connections with the lateral reticular nucleus in the cat as studied with retrograde transport of WGA-HRP. Anat Embryol (Berl) 1989;179:471–483. doi: 10.1007/BF00319590. [DOI] [PubMed] [Google Scholar]
- 15.Dietrichs E, Haines DE. Demonstration of hypothalamo-cerebellar and cerebello-hypothalamic fibres in a prosimian primate (Galago crassicaudatus). Anat Embryol (Berl) 1984;170:313–318. doi: 10.1007/BF00318735. [DOI] [PubMed] [Google Scholar]
- 16.Dietrichs E, Zheng ZH. Are hypothalamo-cerebellar fibers collaterals from the hypothalamo-spinal projection? Brain Res. 1984;296:225–231. doi: 10.1016/0006-8993(84)90060-x. [DOI] [PubMed] [Google Scholar]
- 17.Haines DE, Dietrichs E, Sowa TE. Hypothalamo-cerebellar and cerebello-hypothalamic pathways: a review and hypothesis concerning cerebellar circuits which may influence autonomic centers affective behavior. Brain Behav Evol. 1984;24:198–220. doi: 10.1159/000121317. [DOI] [PubMed] [Google Scholar]
- 18.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 19.Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45:268–287. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- 20.Vilensky JA, van Hoesen GW. Corticopontine projections from the cingulate cortex in the rhesus monkey. Brain Res. 1981;205:391–395. doi: 10.1016/0006-8993(81)90348-6. [DOI] [PubMed] [Google Scholar]
- 21.Brodal P. The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4:193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- 22.Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatry. 1978;13:501–529. [PubMed] [Google Scholar]
- 23.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 121 ( Pt. 1998;4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 24.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 25.Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 26.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 29.Pillay SS, Yurgelun-Todd DA, Bonello CM, Lafer B, Fava M, et al. A quantitative magnetic resonance imaging study of cerebral and cerebellar gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Biol Psychiatry. 1997;42:79–84. doi: 10.1016/S0006-3223(96)00335-6. [DOI] [PubMed] [Google Scholar]
- 30.Shah SA, Doraiswamy PM, Husain MM, Escalona PR, Na C, et al. Posterior fossa abnormalities in major depression: a controlled magnetic resonance imaging study. Acta Psychiatr Scand. 1992;85:474–479. doi: 10.1111/j.1600-0447.1992.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 31.Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 32.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 35.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews, PM, Smith, SM, editors. Functional MRI: An Introduction to Methods. Oxford Oxford University Press; 2001. [Google Scholar]
- 37.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 38.Schmahmann JD, Pandya DN. Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett. 1995;199:175–178. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- 39.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 41.Etkin A, Wager, TD . Brain systems underlying anxiety disorders: a view from neuroimaging. In: Simpson BH, Schneier F, NeriaY, Lewis-Fernandez R, editors. Anxiety Disorders: Theory, Research, and Clinical Perspectives. Cambridge: Cambridge University Press; 2010. pp. 192–202. [Google Scholar]
- 42.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 44.Phelps EA, Cannistraci CJ, Cunningham WA. Intact performance on an indirect measure of race bias following amygdala damage. Neuropsychologia. 2003;41:203–208. doi: 10.1016/s0028-3932(02)00150-1. [DOI] [PubMed] [Google Scholar]
- 45.Frodl T, Bokde AL, Scheuerecker J, Lisiecka D, Schoepf V, et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry. 2010;67:161–167. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 46.Lisiecka D, Meisenzahl E, Scheuerecker J, Schoepf V, Whitty P, et al. Int J Neuropsychopharmacol; 2010. Neural correlates of treatment outcome in major depression. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 47.Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 48.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 49.Layne C. Motivational deficit in depression: people's expectations x outcomes' impacts. J Clin Psychol. 1980;36:647–652. doi: 10.1002/1097-4679(198007)36:3<647::aid-jclp2270360306>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 51.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, et al. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J Gerontol. 1989;44:M147–157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 53.Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009;39:797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Ma Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24:265–270. doi: 10.1007/s12264-008-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 56.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf SA, Melnik A, Kempermann G. Brain Behav Immun; 2010. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. doi: 10.1016/j.bbi.2010.10.014. [DOI] [PubMed] [Google Scholar]