Abstract
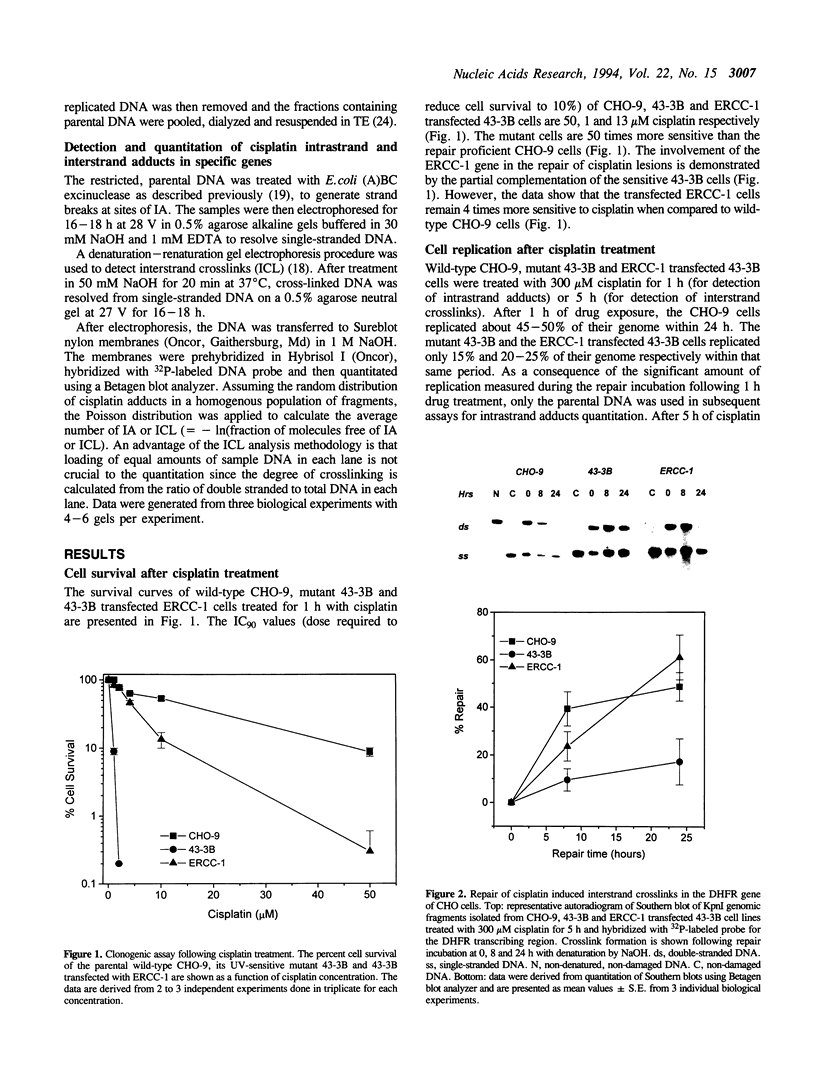
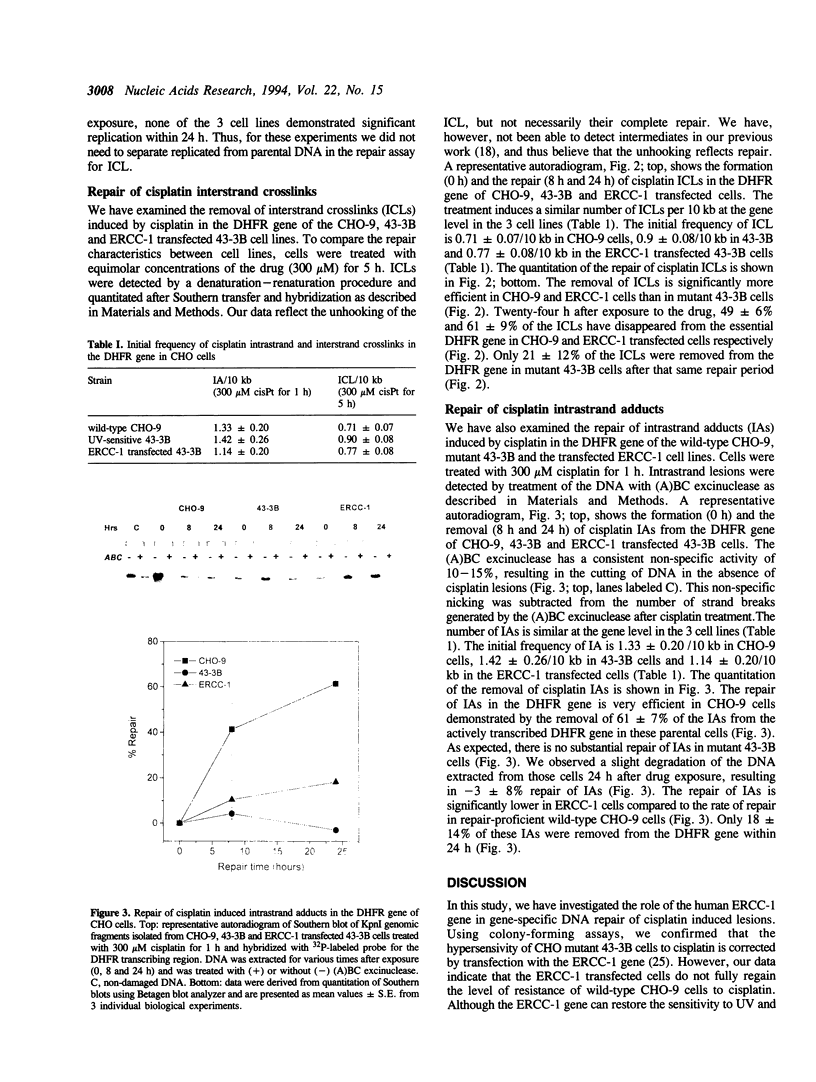
The human excision repair gene ERCC-1 gene restores normal resistance to UV and mitomycin C in excision repair deficient chinese hamster ovary cells of complementation group 1. To investigate the involvement of the ERCC-1 gene in gene-specific repair of bulky lesions, we have studied the removal of damage induced by the antitumor agent cisplatin in CHO mutant 43-3B cells of group 1, with or without transfection with the ERCC-1 gene. Firstly, we determined the contribution of the ERCC-1 gene to the repair of interstrand crosslinks (ICL) induced by cisplatin and found efficient removal of ICLs from the dihydrofolate reductase (DHFR) gene in the ERCC-1 transfected 43-3B cells. We then assessed the contribution of ERCC-1 to the repair of intrastrand adducts (IA) induced by cisplatin. Compared to the wild-type parental cell line, the ERCC-1 transfected 43-3B cells repaired the IAs in the DHFR gene inefficiently. Thus, our data suggest that the ERCC-1 gene is more involved in the repair of interstrand crosslinks than in the removal of intrastrand adducts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belt P. B., van Oosterwijk M. F., Odijk H., Hoeijmakers J. H., Backendorf C. Induction of a mutant phenotype in human repair proficient cells after overexpression of a mutated human DNA repair gene. Nucleic Acids Res. 1991 Oct 25;19(20):5633–5637. doi: 10.1093/nar/19.20.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff M., Szymkowski D. E., Wood R. D. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J. 1993 Sep;12(9):3685–3692. doi: 10.1002/j.1460-2075.1993.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff M., Wood R. D. Requirement for ERCC-1 and ERCC-3 gene products in DNA excision repair in vitro. Complementation using rodent and human cell extracts. J Biol Chem. 1992 Apr 5;267(10):6879–6885. [PubMed] [Google Scholar]
- Bohr V. A., Chu E. H., van Duin M., Hanawalt P. C., Okumoto D. S. Human repair gene restores normal pattern of preferential DNA repair in repair defective CHO cells. Nucleic Acids Res. 1988 Aug 11;16(15):7397–7403. doi: 10.1093/nar/16.15.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Ho L., Hanawalt P. C. Characterization of a DNA repair domain containing the dihydrofolate reductase gene in Chinese hamster ovary cells. J Biol Chem. 1986 Dec 15;261(35):16666–16672. [PubMed] [Google Scholar]
- Bootsma D. The genetic defect in DNA repair deficiency syndromes. EACR--Mühlbock Memorial Lecture, 1993. Eur J Cancer. 1993;29A(10):1482–1488. doi: 10.1016/0959-8049(93)90026-c. [DOI] [PubMed] [Google Scholar]
- Bramson J., Panasci L. C. Effect of ERCC-1 overexpression on sensitivity of Chinese hamster ovary cells to DNA damaging agents. Cancer Res. 1993 Jul 15;53(14):3237–3240. [PubMed] [Google Scholar]
- Burki H. J., Lam C. K., Wood R. D. UV-light-induced mutations in synchronous CHO cells. Mutat Res. 1980 Feb;69(2):347–356. doi: 10.1016/0027-5107(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Calsou P., Frit P., Salles B. Repair synthesis by human cell extracts in cisplatin-damaged DNA is preferentially determined by minor adducts. Nucleic Acids Res. 1992 Dec 11;20(23):6363–6368. doi: 10.1093/nar/20.23.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabholkar M., Bostick-Bruton F., Weber C., Bohr V. A., Egwuagu C., Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992 Oct 7;84(19):1512–1517. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- Darroudi F., Westerveld A., Natarajan A. T. Cytogenetical characterisation of Chinese hamster 43-3B transferants with the amplified or non-amplified human DNA repair gene ERCC-1. Mutat Res. 1989 Jun;212(2):113–122. doi: 10.1016/0027-5107(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Husain I., Chaney S. G., Sancar A. Repair of cis-platinum-DNA adducts by ABC excinuclease in vivo and in vitro. J Bacteriol. 1985 Sep;163(3):817–823. doi: 10.1128/jb.163.3.817-823.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. C., Zhen W. P., Reed E., Parker R. J., Sancar A., Bohr V. A. Gene-specific formation and repair of cisplatin intrastrand adducts and interstrand cross-links in Chinese hamster ovary cells. J Biol Chem. 1991 Apr 15;266(11):7101–7107. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Szymkowski D. E., Yarema K., Essigmann J. M., Lippard S. J., Wood R. D. An intrastrand d(GpG) platinum crosslink in duplex M13 DNA is refractory to repair by human cell extracts. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10772–10776. doi: 10.1073/pnas.89.22.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Thomas D. C., Morton A. G., Bohr V. A., Sancar A. General method for quantifying base adducts in specific mammalian genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3723–3727. doi: 10.1073/pnas.85.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A. E., Bardwell A. J., Bardwell L., Tappe N. J., Friedberg E. C. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature. 1993 Apr 29;362(6423):860–862. doi: 10.1038/362860a0. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Construction of DNA substrates modified with psoralen at a unique site and study of the action mechanism of ABC excinuclease on these uniformly modified substrates. J Biol Chem. 1986 Oct 25;261(30):14135–14141. [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Weeda G., Hoeijmakers J. H., Bootsma D. Genes controlling nucleotide excision repair in eukaryotic cells. Bioessays. 1993 Apr;15(4):249–258. doi: 10.1002/bies.950150405. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Burki H. J. Repair capability and the cellular age response for killing and mutation induction after UV. Mutat Res. 1982 Aug;95(2-3):505–514. doi: 10.1016/0027-5107(82)90281-0. [DOI] [PubMed] [Google Scholar]
- Zhen W., Link C. J., Jr, O'Connor P. M., Reed E., Parker R., Howell S. B., Bohr V. A. Increased gene-specific repair of cisplatin interstrand cross-links in cisplatin-resistant human ovarian cancer cell lines. Mol Cell Biol. 1992 Sep;12(9):3689–3698. doi: 10.1128/mcb.12.9.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., Vredeveldt G., Mayne L. V., Odijk H., Vermeulen W., Klein B., Weeda G., Hoeijmakers J. H., Bootsma D., Westerveld A. The cloned human DNA excision repair gene ERCC-1 fails to correct xeroderma pigmentosum complementation groups A through I. Mutat Res. 1989 Mar;217(2):83–92. doi: 10.1016/0921-8777(89)90059-1. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- van Vuuren A. J., Appeldoorn E., Odijk H., Yasui A., Jaspers N. G., Bootsma D., Hoeijmakers J. H. Evidence for a repair enzyme complex involving ERCC1 and complementing activities of ERCC4, ERCC11 and xeroderma pigmentosum group F. EMBO J. 1993 Sep;12(9):3693–3701. doi: 10.1002/j.1460-2075.1993.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





