Abstract
The primary goal was to test the hypothesis that agonist-induced corticotropin-releasing factor type 1 (CRF1) receptor phosphorylation is required for β-arrestins to translocate from cytosol to the cell membrane. We also sought to determine the relative importance to β-arrestin recruitment of motifs in the CRF1 receptor carboxyl terminus and third intracellular loop. β-Arrestin-2 translocated significantly more rapidly than β-arrestin-1 to agonist-activated membrane CRF1 receptors in multiple cell lines. Although CRF1 receptors internalized with agonist treatment, neither arrestin isoform trafficked with the receptor inside the cell, indicating that CRF1 receptor-arrestin complexes dissociate at or near the cell membrane. Both arrestin and clathrin-dependent mechanisms were involved in CRF1 receptor internalization. To investigate molecular determinants mediating the robust β-arrestin-2-CRF1 receptor interaction, mutagenesis was performed to remove potential G protein-coupled receptor kinase phosphorylation sites. Truncating the CRF1 receptor carboxyl terminus at serine-386 greatly reduced agonist-dependent phosphorylation but only partially impaired β-arrestin-2 recruitment. Removal of a serine/threonine cluster in the third intracellular loop also significantly reduced CRF1 receptor phosphorylation but did not alter β-arrestin-2 recruitment. Phosphorylation was abolished in a CRF1 receptor possessing both mutations. Surprisingly, this mutant still recruited β-arrestin-2. These mutations did not alter membrane expression or cAMP signaling of CRF1 receptors. Our data reveal the involvement of at least the following two distinct receptor regions in β-arrestin-2 recruitment: 1) a carboxyl-terminal motif in which serine/threonine residues must be phosphorylated and 2) an intracellular loop motif configured by agonist-induced changes in CRF1 receptor conformation. Deficient β-arrestin-2-CRF1 receptor interactions could contribute to the pathophysiology of affective disorders by inducing excessive CRF1 receptor signaling.
Keywords: corticotropin-releasing factor, G protein-coupled receptor kinase, receptor phosphorylation, internalization, stress adaptation
The magnitude and duration of cellular signals transduced by G protein-coupled receptors (GPCRs) depend upon stringent regulation to prevent the deleterious effects of unrestrained receptor activation (12, 26, 27, 31, 45, 50, 55). Many studies have shown that agonist-induced GPCR signaling is rapidly attenuated by a mechanism termed “homologous desensitization.” According to the classic model of homologous desensitization, agonist-activated receptors are phosphorylated by a family of G protein-coupled receptor kinases (GRKs) on specific serine/threonine residues in the receptor's third intracellular loop (IC3) and/or carboxyl terminus (26, 27, 31, 45, 50). GRK-mediated phosphorylation of receptor proteins is believed to play a major role in the recruitment of arrestin proteins to the cell surface where they bind to activated GPCRs. The affinity of certain GPCRs for arrestin has been found to increase as much as 30-fold following GRK-catalyzed phosphorylation (45, 50). Recent studies, however, have shown that phosphorylation-independent determinants exposed in the active conformation of receptors can also contribute to arrestin binding to GPCRs (33). Binding of a single arrestin sterically uncouples the receptor from its cognate G protein, resulting in the “arrest” or termination of agonist-mediated signal transduction (12, 26, 27, 31, 45, 50).
The nonvisual arrestins, β-arrestin-1 and β-arrestin-2, target desensitized receptors to clathrin-coated pits for endocytosis by functioning as adaptor proteins that link the GPCR to components of the endocytic machinery, such as adaptor protein-2 and clathrin (12, 14, 26-29, 31, 33, 45, 50, 55). Internalized receptors are either dephosphorylated and recycled back to the plasma membrane to respond again to agonist (resensitization) or degraded inside the cell (downregulation; see Refs. 12, 26, 27, 31, 45, 50, 55). The stability of the receptor-arrestin interaction during the internalization process distinguishes two classes of GPCRs, termed class A and class B (31, 39, 40). Class A GPCRs, such as the β2-adrenergic receptor, form transient complexes with arrestins that dissociate at or near the plasma membrane. Class B GPCRs, such as the vasopressin V2 receptor, form stable complexes with arrestins that internalize as a unit into endocytic vesicles. Differences in the stability of the receptor-arrestin interaction have been shown to regulate the rate of receptor resensitization. In addition to their multi-faceted role regulating G protein-dependent receptor transduction, arrestins have also been shown to initiate G protein-independent signaling events, including activation of the extracellular signal-regulated kinase (ERK)-mitogen-activated protein (MAP) kinase cascade, by bringing various signaling molecules into a complex with the activated GPCR (26, 31).
Corticotropin-releasing factor (CRF) and the related peptides urocortin 1 (UCN1), urocortin 2 (UCN2), and urocortin 3 (UCN3) regulate a wide variety of central and peripheral functions by acting at one or both high-affinity CRF receptors, CRF1 and CRF2 (5, 15, 16, 46). CRF released from the paraventricular nucleus of the hypothalamus binds to CRF1 receptors on ACTH-secreting pituitary corticotropes to regulate the neuroendocrine response to stress. Importantly, CRF centrally modulates autonomic, immune, behavioral, emotional, and cognitive responses to stress by acting at one or both CRF receptors in cortical and limbic brain regions (5, 15, 16, 46). Although much less is known about the recently discovered urocortins, evidence suggests that they too modulate stress responses. Although UCN1 binds to both CRF receptor subtypes, UCN2 and UCN3 are specific CRF2 receptor agonists (5, 15, 16, 46). Hypersecretion of CRF and dysregulation of CRF receptor signal transduction in the central nervous system have been hypothesized to contribute importantly to anxiety, stress, and depressive disorders (5, 15, 16, 46).
Exposure to high concentrations of CRF or UCN1, a setting favoring strong GRK action, rapidly desensitizes CRF1 receptors endogenously expressed in human retinoblastoma Y79, neuroblastoma IMR-32, mouse pituitary AtT-20, rat anterior pituitary, and human myometrial cells and recombinantly expressed in fibroblast Ltk- and HEK293 cell (6, 7, 10, 17, 18, 20, 22, 49, 51, 53). Of the five members of the GRK family expressed outside the visual system, GRK3 has been implicated by several groups as important for homologous desensitization of CRF1 receptors. We originally demonstrated that uptake of a GRK3 antisense oligonucleotide or transfection with a GRK3 antisense cDNA construct decreased GRK3 expression ~55% and inhibited homologous CRF1 receptor desensitization ~65% in Y79 cells (6). Teli et al. (53) recently found that acutely stimulating transfected HEK293 cells with CRF triggered a rapid translocation of GRK3 and GRK6 from cytosol to cell membrane. This group also demonstrated that antibodies targeting GRK3 or GRK6 suppressed CRF-stimulated CRF1 receptor desensitization (53).
Following CRF1 receptor activation, both β-arrestin-1 and β-arrestin-2 can translocate to and bind the receptor at the plasma membrane (22, 47, 48). Consistent with the importance of receptor-β-arrestin interactions, inhibition of arrestin function with a dominant-negative mutant reduced agonist-dependent CRF1 receptor desensitization by ~60% in transfected HEK293 cells (53). In addition, overexpression of GRK3 or GRK6 increased the association of β-arrestin with CRF-activated membrane CRF1 receptors (22), suggesting that phosphorylation of CRF1 receptors may be necessary for arrestin binding. CRF1 receptors are internalized subsequently in a clathrin-dependent fashion (18, 47, 48). Clathrin-mediated endocytosis normally requires receptors to be phosphorylated by GRKs and bound by arrestins. In the case of CRF1 receptors, however, a recent report has suggested that the internalization pathway is both phosphorylation and arrestin independent (48).
Although both GRKs and arrestins appear to play important roles in CRF1 receptor desensitization, no studies have assessed directly whether recruitment of β-arrestin to the agonist-activated CRF1 receptor is regulated by GRK-mediated phosphorylation or mechanisms independent of GRK action. Thus the primary goal of this study was to test the hypothesis that agonist-induced phosphorylation of CRF1 receptors is required for β-arrestins to translocate from the cytosol to the cell membrane. Furthermore, we sought to determine the relative importance of the CRF1 receptor carboxyl terminus and the IC3 in the recruitment of β-arrestin to membrane CRF1 receptors activated by agonist binding. Results from our study reveal the presence of phosphorylation-dependent and phosphorylation-independent β-arrestin-2-binding motifs in the carboxyl terminus and intracellular loops of the activated CRF1 receptor, respectively, that contribute importantly to CRF1 receptor regulation.
MATERIALS AND METHODS
Reagents and peptides
Reagent purchases were as follows: 1) BSA (fraction V), isobutyl methylxanthine, and other highly pure chemicals (Sigma, St. Louis, MO); 2) aprotinin (Trasylol; Calbiochem, San Diego, CA); 3) defined FBS serum (Hyclone, Logan, UT). Ovine CRF (oCRF), human/rat CRF (h/rCRF), UCN1, or sauvagine (SVG, purity >98%; Bachem, Torrance, CA or Phoenix Pharmaceuticals, Belmont, CA) were used to activate CRF1 receptors and stimulate cAMP accumulation. All SDS-PAGE reagents were purchased from Invitrogen-NOVEX (Carlsbad, CA). The following other reagents were also used: 1) protein A-Sepharose (Oncogene Research Products, Cambridge, MA) and 2) mouse monoclonal anti-hemagglutinin (HA) antibodies [HA.11 from BabCo (Berkeley, CA) or no. 12CA5 from Roche Applied Science (Indianapolis, IN)].
HA epitope tagging and mutagenesis of human CRF1 receptor
The human CRF1 receptor was previously amplified from a human retinoblastoma Y79 cell cDNA library by PCR and subcloned into pcDNA3 (Invitrogen, Carlsbad, CA) using KpnI and XbaI sites (8). The influenza HA epitope tag (YPYDVPDYA) was inserted between residues Cys30 and Glu31, which is an inert region of the NH2 terminus, using oligo-directed mutagenesis (Quick Change kit; Strata-gene, La Jolla, CA; see Ref. 19). cAMP signaling experiments confirmed that the sensitivity (EC50) and maximum for agonist-stimulated cAMP accumulation were not altered by insertion of the HA epitope tag in the CRF1 receptor NH2 terminus. The EC50 (0.54 ± 0.06 nM) for stimulation of cAMP accumulation by oCRF (0–100 nM) in HEK293 transiently transfected with the HA-tagged CRF1 receptor (see Table 1 and Fig. 7) was equivalent to the EC50 (1.00 ± 0.20 nM) for oCRF-stimulated cAMP accumulation in HEK293 cells transiently transfected with the wild-type human CRF1 receptor without an epitope tag. Furthermore, Scatchard binding experiments indicated that the dissociation constant (Kd) and maximal binding (Bmax) values of the HA-tagged CRF1 receptor were 0.88 ± 0.34 nM and 2.20 ± 0.34 pmol/mg, respectively, which are similar to the Kd and Bmax values we have measured for the nontagged wild-type human CRF1 receptor transiently expressed in HEK293 cells (8). Finally, internalization properties of the HA-tagged CRF1 receptor resembled those of the nontagged CRF1 receptor (data not shown). The HA-tagged wild-type CRF1 receptor was truncated in the following positions: 1) Ser412, which removed the last four amino acids (i.e., STAV) of the carboxyl terminus (Δ412) and 2) Ser386, which removed the last 30 amino acids (i.e.,SIRARVARAMSIPTSPTRVSFHSIKQSTAV) of the carboxyl terminus (Δ386), a segment containing all putative GRK and protein kinase C phosphorylation sites (Fig. 1). These two truncated CRF1 receptors were reinserted in the pcDNA3 vector at restriction enzyme sites generated by PCR. A serine/threonine cluster (Ser301Thr302Thr303Ser304Glu305Thr306) contained within the IC3 appears to conform to a GRK phosphorylation site because of the presence of an acidic residue (13, 23). These six amino acids were mutagenized to alanines in both HA-tagged full-length wild-type and Δ386 CRF1 receptors. The resulting mutants were designated IC3-5ST/A and Δ386-IC3-5ST/A, respectively. Sequences of cDNA constructs were confirmed using single-stranded DNA sequencing.
Table 1.
Summary of cAMP signaling and agonist-binding properties of full-length wild-type and mutated HA-tagged human CRF1 receptors
| cAMP Accumulation | Membrane Agonist Binding |
||||
|---|---|---|---|---|---|
| CRF1 Receptor Construct |
oCRF EC50, nM |
UCN1 EC50, nM |
SVG EC50, nM |
Kd, nM | Bmax, pmol/mg |
| WT | 0.54±0.06* | 1.07±0.08 | 0.38±0.03† | 0.88±0.34 | 2.20±0.34 |
| Δ412 | 0.88±0.06 | 1.08±0.18 | 0.62±0.06 | 0.97±0.18 | 1.87±0.35 |
| Δ386 | 0.61±0.05 | 0.93±0.08 | 0.43±0.03‡ | 1.46±0.28 | 1.80±0.06 |
Values are means ± SE. HA, hemagglutinin; CRF1, corticotropin-releasing factor type 1; oCRF, ovine CRF; UCN1, urocortin 1; SVG, sauvagine; Kd, dissociation constant; Bmax, maximal binding; WT, wild type. The only post hoc differences found to be statistically significant between agonist groups were the following:
P < 0.01 vs. UCN1
P < 0.001 vs. UCN1
P < 0.05 vs. UCN1.
No significant differences were detected by ANOVA across receptor construct groups for EC50, Kd, or Bmax values.
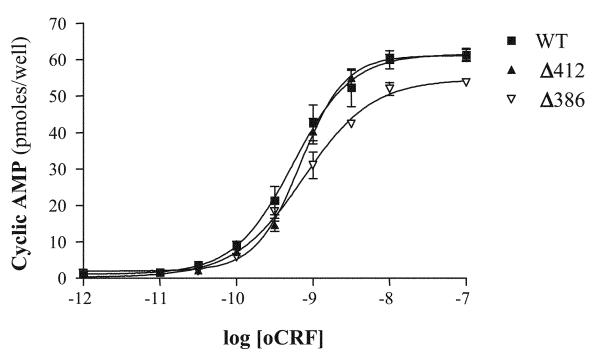
Fig. 7.
Concentration-response curves for the stimulation of intracellular cAMP accumulation by CRF in HEK293 cells expressing full-length wild-type and mutant CRF1 receptors. cAMP levels (pmol/well) were measured in quadruplicate in HEK293 cells transfected with cDNAs for full-length wild-type (WT), Δ412, or Δ386 CRF1 receptors and then stimulated with CRF (0–1,000 nM). See Table 1 for EC50 and maximum response values for cAMP signaling in HEK293 cells expressing the three CRF1 receptor constructs.
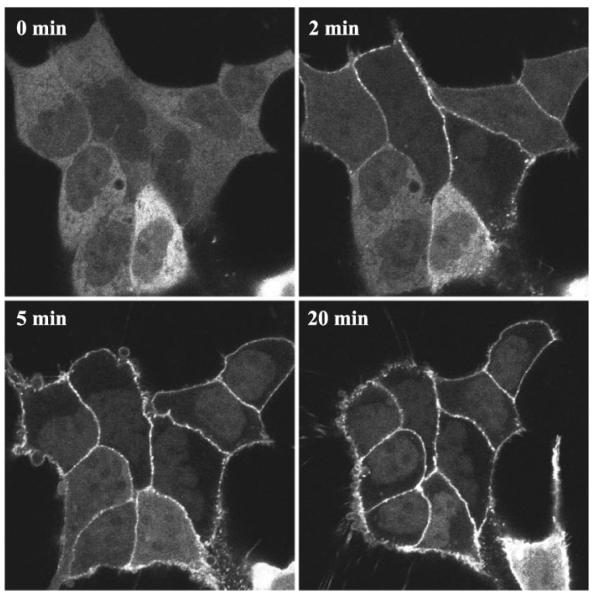
Fig. 1.
Recruitment of β-arrestin-1 by the agonist-activated full-length wild-type corticotropin-releasing factor type 1 (CRF1) receptor in transfected HEK293 cells. Confocal microscopy was used to evaluate the interaction of β-arrestin-1-green fluorescent protein (GFP) with full-length CRF1 receptors transiently expressed in HEK293 cells. This representative experiment shows the distribution of β-arrestin-1-GFP in HEK293 cells before (0 min) and after (2, 5, and 20 min) treatment with 200 nM CRF.
Other plasmid cDNAs
Construction of the β-arrestin-1-green fluorescent protein (GFP), β-arrestin-2-GFP, and dynamin I dominant-negative mutant K44A expression vectors has been described previously (39, 41, 42). Sequences of cDNA constructs were confirmed using single-stranded DNA sequencing.
Cell culture and transfection
All cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). For confocal microscopy and flow cytometry experiments, HEK293 and COS-7 cells were cultured in DMEM supplemented with 10% (vol/vol) heat-inactivated FCS and gentamicin (100 μg/ml). CHO-K1 cells were cultured in DMEM-F-12 supplemented with 10% (vol/vol) heat-inactivated FCS and gentamicin (100 μg/ml). Transient transfections were performed using LipofectAMINE (Life Technologies, Gaithersburg, MD) or a modified calcium phosphate coprecipitation method as described previously (39). For CRF1 receptor phosphorylation experiments, HEK293 cells were seeded at 6 × 105 cells/10-cm dish in DMEM containing 10% (vol/vol) FBS, 100 μg/ml streptomycin, and 100 IU/ml penicillin and then transiently transfected using 5 ml of OptiMEM containing 10 μg/ml LipofectAMINE (Life Technologies) and 5 μg of HA-tagged CRF1 receptor cDNA for 6 h at 37°C, as previously described (17, 19). For quantification of β-arrestin-GFP translocation, human osteosarcoma U-2 OS cells were cultured in minimal essential medium (MEM) supplemented with 10% (vol/vol) heat-inactivated FBS, 10 mM HEPES, 2 mM l-glutamine, and 10 μg/ml gentamicin, and transient transfections were performed using FuGene 6 (Roche Applied Science).
cAMP assay
Following extensive cell washing 48 h after transfection, intracellular cAMP levels were measured in ether-extracted lysates using a double-antibody RIA kit ([125I]cAMP assay system, RPA 509; Amersham Biosciences, Little Chalfont, UK), as previously described (6, 8, 18).
Confocal microscopy
HEK293 and CHO-K1 cells transiently expressing β-arrestin-1-GFP or β-arrestin-2-GFP and either the wild-type CRF1 receptor, Δ412, Δ386, IC3-5ST/A, or Δ386-IC3-5ST/A mutant were plated on 35-mm glass-bottom culture dishes (MatTek, Ashland, MA) and cultured overnight. Before the experiment (1 h), the medium was removed and replaced with serum- and phenol red-free medium supplemented with 10 mM HEPES. The β-arrestin translocation response was assessed in cells treated with vehicle or a range of CRF concentrations (100, 200, and 400 nM). Identical results were found for recruitment of β-arrestins by CRF-activated CRF1 receptors independent of the three different saturating concentrations (100, 200, or 400 nM) of CRF used. Confocal microscopy was performed on a Zeiss laser scanning microscope (LSM 5 Pascal). Images were acquired from live cells in real time before and after CRF treatment using single excitation (488 nm). For colocalization studies, HEK293 cells expressing β-arrestin-2-GFP and wild-type CRF1 receptor were incubated with a mouse monoclonal anti-HA antibody (no. 12CA5) at 4°C for 1 h. Following washes at 4°C, cells were incubated in the absence or presence of 200 nM CRF at 37°C for 30 min. Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and incubated with a goat anti-mouse antibody conjugated to Texas Red (Invitrogen-Molecular Probes, Carlsbad, CA) at room temperature for 30 min. Fluorescence was visualized on a Zeiss LSM 5 Pascal using dual excitation (488 nm for GFP; 543 nm for Texas Red) and emission (band pass 505–530 nm for GFP; long pass 560 for Texas Red) filter sets.
Quantification of β-arrestin-2-GFP translocation
U-2 OS cells transiently expressing CRF1 receptor and β-arrestin-2-GFP were seeded 24 h posttransfection at 15,000 cells/well in a black 96-well ViewPlate (Packard Instruments, Meriden, CT). U-2 OS cells were employed over HEK293 cells for their superior adherence properties and image quality (43). After an overnight incubation at 37°C, the media was removed and replaced with 100 μl of phenol red-free MEM with 10 mM HEPES and 2 mM l-glutamine for a 45-min serum starvation at 37°C. Cells were stimulated for 30 min at 37°C with various concentrations of CRF or vehicle. The cells were then fixed, and nuclei were stained by addition of 120 μl of 4% (vol/vol) formaldehyde in PBS and 5 μg/ml Hoechst nuclear stain. After a 45-min incubation, the fixative and nuclear stain were removed and replaced with 200 μl PBS. The INCell Analyzer 3000 (GE Healthcare Biosciences, Little Chalfont, UK), a laser-based, confocal imaging system, was employed to quantitate the arrestin-GFP translocation response, as described previously (42, 43). In brief, images were quantitated using the granularity analysis GRN1 algorithm that identifies fluorescent spots or grains of arrestin-GFP localization based on size and fluorescence intensity. A grain size setting of 5 and an intensity gradient setting of 1.25 were employed for this analysis. Only GFP-positive cells, selected using a cell-intensity signal threshold of 100, were quantitated. The reported parameter “Fgrains” represents the average fluorescence intensity of the spots or grains of arrestin-GFP localization.
Receptor internalization assay
HEK293 or COS-7 cells transiently expressing the HA-tagged CRF1 receptor alone or together with K44A, β-arrestin-1, or β-arrestin-2 were plated in six-well plates and incubated overnight. Afterward, the cells were incubated with CRF (100 nM-1 μM) or media (control) for 30–90 min, and receptor sequestration was assessed by flow cytometry as described previously (1). Specificity of the receptor internalization signal detected by the FITC-labeled HA.11 monoclonal antibody (no. FITC-101L; Covance Research Products, Denver, PA) was verified by demonstrating that cells transfected with empty vector exhibited very low immunostaining. In addition, no appreciable immunostaining of HA-tagged CRF1 receptor-expressing cells was measured using a FITC-labeled IgG to detect nonspecific binding.
CRF1 receptor phosphorylation assay
Phosphorylation of the CRF1 receptor was determined, as previously described (17, 19). Briefly, transfected HEK293 cells in 10-cm dishes were metabolically labeled for 4 h at 37°C in 5 ml of Pi-free DMEM containing 0.1% (wt/vol) BSA and 100 μCi/ml 32Pi. Cells were then treated with vehicle or CRF for 5 min, which is the time point for maximum agonist-induced phosphorylation of the CRF1 receptor (19). After cells were ruptured in lysis buffer (LB: 50 mM Tris, pH 8.0, 100 mM NaCl, 20 mM NaF, 10 mM sodium pyrophosphate, 5 mM EDTA, 10 mg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor, 10 μg/ml pepstatin, 10 μg/ml benzamidine, 1 mM phenylmethylsulfonyl fluoride, and 1 μM okadaic acid), they were preextracted by the addition of LB containing 2 M NaCl and 8 M urea followed by overnight tumbling at 4°C. Membranes were then solubilized in LB supplemented with 1% (vol/vol) Nonidet P-40, 1% (wt/vol) sodium deoxycholate, and 0.1% (wt/vol) SDS. After clarification at 14,000 g, solubilized membranes were precleared by incubating them with 2% (vol/vol) protein A-Sepharose for 1 h at 4°C. Immunoprecipitation of CRF1 receptors was performed by adding 1 μl of HA.11 monoclonal anti-HA antibody and 2% (vol/vol) protein A-Sepharose and incubating overnight at 4°C. After the Sepharose-bound immune complexes were washed in LB lacking protease inhibitors, 32P-labeled phospho-HA-CRF1 receptors were eluted in Laemmli sample buffer for 1 h at 48°C and resolved by SDS-PAGE (8–16% gradient resolving gel) with equal protein loading of each lane. Phospho-HA-CRF1 receptor bands were visualized and quantified as previously described (17, 19).
Immunoblotting of HA-tagged CRF1 receptors
Membranes prepared from HEK293 cell lysates were solubilized in LB buffer (see above). After equal amounts of membrane protein were added to each lane, proteins were resolved using SDS-PAGE with a 8–16% Trisglycine gradient resolving gel as previously described (19). After Western transfer of resolved proteins on to polyvinylidene difluoride membranes was completed, the Western blots were immunoprobed with the HA.11 monoclonal anti-HA antibody (19). Chemiluminscent detection of immune complexes was performed using ECL+ Plus (Amersham Pharmacia Biotech, Piscataway, NJ).
CRF1 receptor binding assay
HEK293 cells were resuspended in 50 mM Trizma base with 5 mM MgCl2, 2 mM EGTA, 1 mM DTT, and 5 μg/ml aprotinin (pH 7.4) and disrupted by brief polytroning. Cell nuclei and debris were pelleted by centrifugation at 600 g for 5 min at 0°C. The resulting supernatant was then centrifuged at 20,000 g for 30 min at 0°C as previously described (8). Afterward, HEK293 membranes (5 μg protein/tube) were combined with wheat germ agglutinin scintillation proximity beads (0.1–1 mg; Amersham Pharmacia Biotech) and 125I-labeled SVG (no. NEX306, 100 pM; Perkin-Elmer, Boston, MA). The binding reaction was incubated for 120 min at 22°C with shaking. Afterward, 125I radioactivity was measured in a TopCount scintillation counter (Packard Instruments). Nonspecific binding was determined as residual radioactivity in the presence of 10 μM oCRF. Under these conditions, <10% of the total radioactivity was specifically bound by wild-type and mutant CRF1 receptors.
Data reduction and statistical analyses
Data reduction for the cAMP RIA was performed using a log-logit program. Sequestration was defined as the fraction of total cell surface receptors that were removed from the plasma membrane of cells following CRF agonist exposure compared with that of vehicle-treated cells. FlowJo software, version 7.1.3 (Tree Star, Ashland, OR) was used to analyze internalization experiments. Scatchard analysis of the equilibrium displacement binding data was performed using the XLfit curve-fitting software (ID Business Solutions; Guilford, Surrey, UK; see Ref. 8). ANOVAs across experimental groups were performed using PRISM, version 4.0 (GraphPad Software, San Diego, CA). If the one-way ANOVA were statistically significant, planned post hoc analyses were performed using Bonferroni's multiple-comparison tests to determine individual group differences. CRF1 receptor phosphorylation bands were quantitated and analyzed as previously described (17).
RESULTS
Characteristics of β-arrestin interactions with wild-type CRF1 receptors
GFP-labeled β-arrestin-1 (β-arrestin-1-GFP) and β-arrestin-2 (β-arrestin-2-GFP) were employed to investigate the interaction of the two nonvisual arrestin isoforms with CRF1 receptors in real time and in living cells. HEK293 cells were transiently transfected with CRF1 receptors and either β-arrestin-1-GFP or β-arrestin-2-GFP. The distribution of the GFP-labeled β-arrestins was then monitored in the same cells before and after treatment with oCRF or h/rCRF. In the absence of agonist, β-arrestin-1-GFP was diffusely distributed in the cytoplasm of transfected cells, with a small amount present in the nucleus (Fig. 1). Upon addition of oCRF, β-arrestin-1-GFP translocated to the CRF1 receptor at the plasma membrane. The redistribution of β-arrestin-1-GFP was first detected 1–2 min after agonist addition and steadily increased in magnitude, reaching a maximum level by ~5 min (Fig. 1). As the cytoplasmic pool of β-arrestin-1-GFP redistributed to the plasma membrane, the pool of β-arrestin-1-GFP residing in the nucleus was revealed (Fig. 1).
In the absence of agonist, β-arrestin-2-GFP was diffusely distributed throughout the cytoplasm of the cells. Unlike β-arrestin-1, however, β-arrestin-2-GFP was excluded from the nucleus (Fig. 2A). We and others have previously observed this difference in subcellular distribution of the two β-arrestin isoforms (39, 52). Treatment with oCRF resulted in a considerably more rapid translocation of β-arrestin-2-GFP to CRF1 receptors at the plasma membrane (Fig. 2A) compared with β-arrestin-1-GFP (Fig. 1). Translocation was first observed within 30 s and was complete by 2 min, as indicated by the depletion of the cytoplasmic pool of β-arrestin-2-GFP. For both β-arrestin–GFP isoforms, the punctate pattern of fluorescence at the plasma membrane reflected their localization with the receptor in clathrin-coated pits (28, 29). Similar results were also found in CHO-K1 cells. As shown in Fig. 3, β-arrestin-2-GFP was again recruited more rapidly than β-arrestin-1-GFP to oCRF-activated CRF1 receptors at the plasma membrane. These data indicate that the CRF1 receptor can bind both nonvisual arrestins, but it has a marked preference for the β-arrestin-2 isoform.
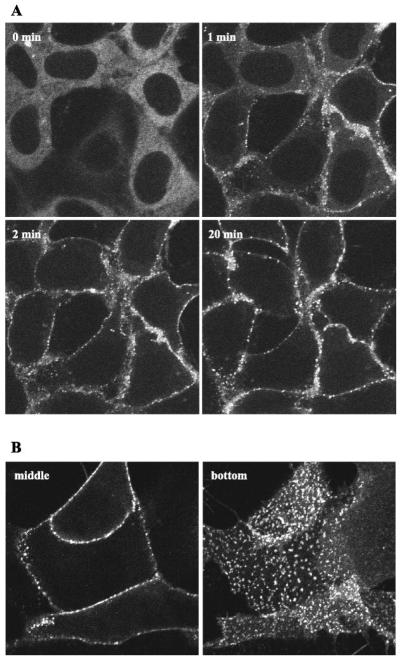
Fig. 2.
Recruitment of β-arrestin-2 by the agonist-activated full-length wild-type CRF1 receptor in transfected HEK293 cells. Confocal microscopy was used to evaluate the interaction of β-arrestin-2-GFP with the full-length CRF1 receptors transiently expressed in HEK293 cells. A: distribution of β-arrestin-2-GFP is shown in cells before (0 min) and after (1, 2, and 20 min) treatment with 200 nM CRF in a representative experiment. B: imaging of the β-arrestin-2-GFP distribution in the cells following a 30-min treatment with CRF. Confocal images were taken from both the middle (image on left) and bottom (image on right) of HEK293 cells in a representative experiment. Note the localization of β-arrestin-2-GFP at the plasma membrane in clathrin-coated pits and the absence of β-arrestin-2-GFP inside the cell.
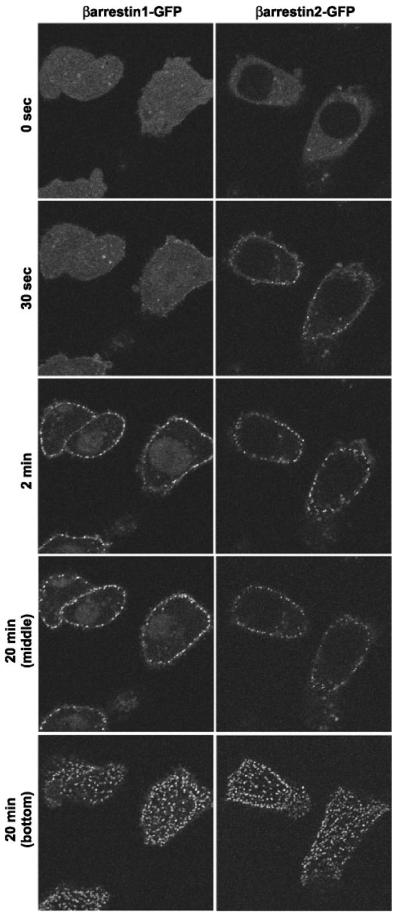
Fig. 3.
Recruitment of β-arrestin-1 and β-arrestin-2 by the agonist-activated full-length wild-type CRF1 receptor in transfected CHO-K1 cells. Confocal microscopy was used to evaluate the interaction of β-arrestin-1-GFP and β-arrestin-2-GFP with full-length CRF1 receptors transiently expressed in CHO-K1 cells. The distribution of each β-arrestin isoform is shown in CHO-K1 cells before (0 s) and after (30 s, 2 min, and 20 min) treatment with 200 nM CRF in a representative experiment. For the 20-min time point, confocal images were taken from both the middle and bottom of the same cells. Note the localization of each β-arrestin isoform at the plasma membrane in clathrin-coated pits and the absence of each isoform inside the cell.
Although CRF1 receptors internalized in response to agonist (see below), neither β-arrestin isoform trafficked with the receptor inside the cell. Instead, both β-arrestin-1-GFP and β-arrestin-2-GFP remained at the plasma membrane even after 1 h of oCRF treatment (Figs. 1-3 and data not shown). Confocal images taken from the middle and bottom of the same cells reveal the exclusive plasma membrane distribution of each β-arrestin isoform (Figs. 2B and 3). An identical time course and pattern of β-arrestin recruitment was also observed for cells stimulated with h/rCRF (data not shown). This finding agrees with data showing h/rCRF and oCRF bind to and activate the CRF1 receptor with equal affinities and potencies (5, 8, 15, 46). Our data and studies by Dautzenberg et al. (6, 7) and Teli et al. (53) have shown that GRK3 is important for homologous desensitization of CRF1 receptors. Overexpressing GRK3 did not alter, however, the pattern of β-arrestin-2-GFP recruitment or prevent dissociation of the CRF1 receptor-β-arrestin-2 complex (data not shown).
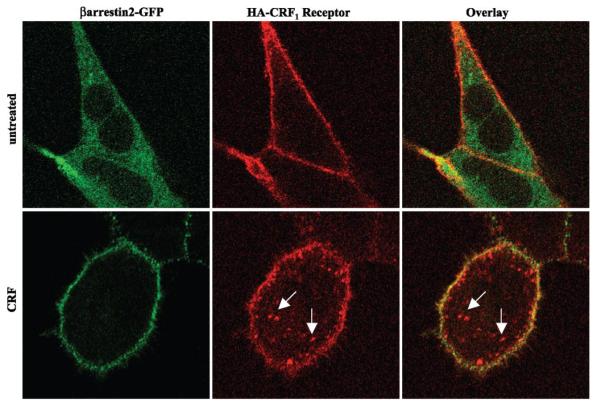
To investigate further CRF1 receptor-arrestin interactions, we performed colocalization experiments in the same cells using GFP-labeled β-arrestin-2 and Texas Red-labeled CRF1 receptors. In the absence of agonist, CRF1 receptor immunofluorescence was localized predominantly to the plasma membrane, whereas β-arrestin-2-GFP was distributed in the cytoplasm (Fig. 4, top). Following a 30-min treatment with CRF, CRF1 receptor immunofluorescence was localized both at the plasma membrane and inside the cell in vesicles, indicative of receptor internalization (Fig. 4, bottom). The GFP-labeled β-arrestin-2 colocalized with CRF1 receptors at the plasma membrane but did not colocalize with the pool of internalized CRF1 receptors (Fig. 4, bottom). Taken together, these data indicate that the CRF1 receptor is a class A GPCR in terms of its interaction with β-arrestin-1 and β-arrestin-2. The CRF1 receptor recruits β-arrestins to the membrane, but the complex dissociates and β-arrestin is excluded from receptor-containing intracellular vesicles.
Fig. 4.
Colocalization of β-arrestin-2-GFP with cell surface but not internalized full-length wild-type CRF1 receptors. The distribution of β-arrestin-2-GFP and Texas Red-labeled full-length hemagglutinin (HA)-tagged CRF1 receptors was evaluated by confocal microscopy in transiently transfected HEK293 cells. Top: distribution of β-arrestin-2-GFP fluorescence (green) and CRF1 receptor immunofluorescence (red) before CRF treatment. Bottom: distribution of β-arrestin-2-GFP fluorescence (green) and CRF1 receptor immunofluorescence (red) in HEK293 cells stimulated with 200 nM CRF for 30 min. The overlay image indicates colocalization (yellow) of β-arrestin-2 with CRF1 receptors at the plasma membrane but not inside the cell in a representative experiment. Arrows point to vesicles containing internalized CRF1 receptors.
Agonist-induced CRF1 receptor internalization
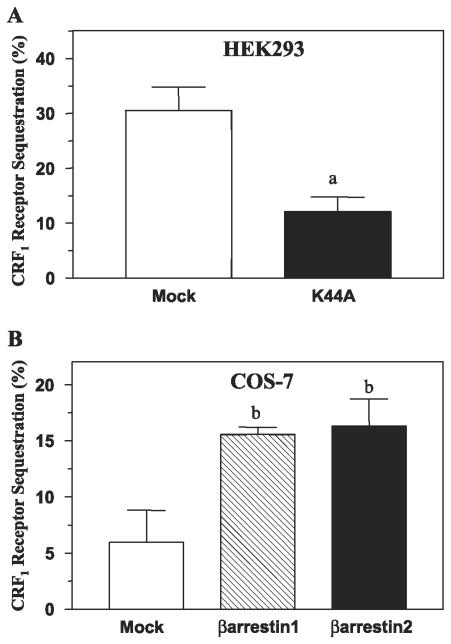
Next, we examined internalization of wild-type CRF1 receptors in transfected HEK293 cells using flow cytometry. Exposing cells to oCRF for 30 min decreased cell surface CRF1 receptors ~30% because of sequestration of the internalized receptor into early endosomes (Fig. 5A). CRF1 receptor internalization was inhibited ~60% (P < 0.005) by cotransfection of the dynamin dominant-negative mutant K44A (Fig. 5A). The dynamin dependence of CRF1 receptor internalization indicates that receptors were internalized via clathrin-coated pits. To investigate whether CRF1 receptor sequestration is arrestin-dependent, we used COS-7 cells, which express very low levels of endogenous β-arrestins (35). Agonist-induced internalization of CRF1 receptors was approximately threefold greater (P < 0.05) in COS-7 cells overexpressing β-arrestin-1 (15.55 ± 0.65%) or β-arrestin-2 (16.30 ± 2.40%) compared with the low levels of internalization observed in control cells (5.95 ± 2.85%; Fig. 5B). The results of these internalization experiments are consistent with the confocal microscopy observations and suggest that both clathrin- and arrestin-dependent endocytosis mechanisms are involved in internalization of CRF1 receptors.
Fig. 5.
Dynamin and arrestin dependence of agonist-induced full-length wild-type CRF1 receptor internalization in transfected HEK293 or COS-7 cells. Flow cytometry was used to detect internalization of full-length CRF1 receptors. A: CRF1 receptor sequestration measured in HEK293 cells with and without coexpression of the dynamin dominant-negative mutant K44A following a 30-min treatment with CRF (400 nM). aP < 0.005 vs. mock (empty vector). B: CRF1 receptor internalization measured in COS-7 cells in the presence and absence of overexpressed full-length β-arrestin-1 or β-arrestin-2 following a 30-min treatment with CRF (200 nM). By one-way ANOVA, the following post hoc differences were found to be statistically significant: bP < 0.05 vs. mock.
Membrane expression and cellular signaling of HA-tagged wild-type and mutant CRF1 receptors
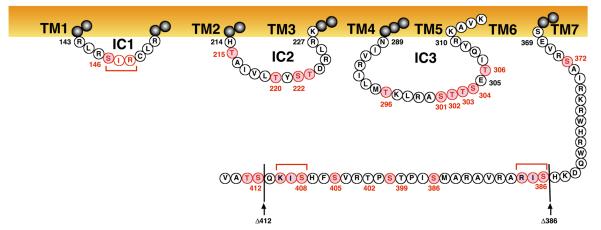
The human CRF1 receptor carboxyl terminus has seven serines and three threonines that are putative sites for GRK-mediated phosphorylation (Fig. 6) and a TSPT motif (residue nos. 399–402) that may function as an arrestin binding site (44). We truncated the receptor carboxyl terminus at Ser412 to create a mutant receptor (Δ412). We also truncated the carboxyl terminus at Ser386 (Δ386) to eliminate putative GRK phosphorylation sites and the potential arrestin-binding motif. First, we characterized ligand-binding and cAMP-signaling properties of the two mutant receptors by completing membrane saturation binding and intracellular cAMP stimulation experiments. Scatchard analyses of the saturation binding data demonstrated that agonist affinities (i.e., Kd) for the Δ412 and Δ386 mutants were equivalent to the Kd value for the full-length wild-type CRF1 receptor (Table 1). Importantly, the Bmax values for the full-length wild-type, Δ412, and Δ386 CRF1 receptors were similar (Table 1). Furthermore, membrane protein levels for wild-type, Δ412, and Δ386 CRF1 receptors measured by Western blot were also similar in HEK293 cell membranes (data not shown). Thus the Δ412 and Δ386 truncations did not reduce the expression of these two receptor mutants on the plasma membrane. Concentration-response curves for agonist-stimulated cAMP accumulation were also measured in HEK293 cells transiently expressing Δ412, Δ386, or the full-length wild-type CRF1 receptors. The sensitivity (EC50) of oCRF-, UCN1-, and SVG-stimulated cAMP accumulation generated by Δ412, Δ386, and full-length wild-type CRF1 receptors did not differ significantly (Fig. 7 and Table 1). Furthermore, the maximum levels of cAMP accumulation generated by Δ412, Δ386, and full-length wild-type receptors following activation by oCRF (Fig. 7), UCN1 (data not shown), or SVG (data not shown) were relatively similar (Fig. 7). Hence the membrane expression, agonist binding, and cAMP signaling function of the CRF1 receptor were not altered by truncating its carboxy terminus at Ser412 or Ser386.
Fig. 6.
Diagram of the intracellular sequences of the human CRF1 receptor. The full-length wild-type CRF1 receptor protein is 415 amino acids in length. The transmembrane-spanning domains are highlighted in orange while intracellular serines and threonines are highlighted in red. Locations of the Δ412 and Δ386 truncations of the carboxy terminus are indicated by black arrows. In the third intracellular loop, the red-highlighted serine/threonine cluster located at residue nos. 301–396 may conform to a G protein-coupled receptor kinase (GRK) phosphorylation site because of the presence of an acidic residue (Glu305; see Refs. 13 and 23). GRK2 and GRK3 have been shown to be acidotropic kinases (13, 23). Within this third intracellular loop sequence, a potential protein kinase A (PKA) phosphorylation site (R299A300S301) may also be present. In the carboxy terminus, the T399S400P401T402 motif may represent an arrestin binding site after agonist-induced phosphorylation has occurred (44). In the second intracellular loop, the basic amino acid His214 and the adjacent hydrophobic sequence (A216I217V218L219) may also be involved in arrestin recruitment and binding (33). The red brackets indicate two potential protein kinase C (PKC) phosphorylation sites in the carboxy terminus.
The IC3 of the CRF1 receptor also contains a putative site for GRK phosphorylation, namely a cluster of serine and threonine residues in close proximity to an acidic amino acid (STTSET motif; Fig. 6). Therefore, we substituted alanines for the serine/threonine cluster to produce the IC3-5ST/A mutant receptor. We also deleted the same IC3 motif from the Δ386 mutant to create the Δ386-IC3-5ST/A mutant. The maximum and EC50 values for CRF-stimulated cAMP accumulation did not significantly differ for these two mutants (data not shown) and were similar to CRF-stimulated cAMP signaling by wild-type CRF1 receptors.
Agonist-induced phosphorylation of wild-type and mutant CRF1 receptors
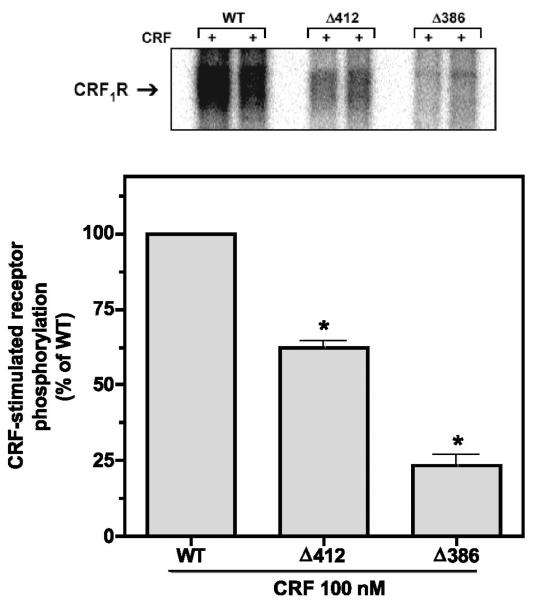
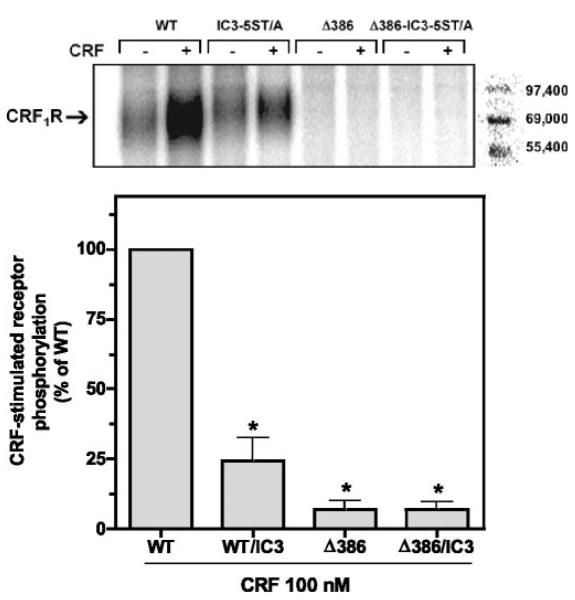
Previously, we reported that CRF-induced phosphorylation of HA epitope-tagged full-length wild-type CRF1 receptors transiently expressed in COS-7 cells increased dramatically in a time- and concentration-dependent manner (17, 19). In the present study, we also observed a rapid, large increase in wild-type CRF1 receptor phosphorylation detected as a dense phosphoprotein band with a relative molecular mass of 60–70 kDa in membranes prepared from HEK293 cells treated with a saturating concentration of CRF (100 nM) for 5 min (Fig. 8). Phosphorylation of the Δ412 mutant was ~35% (P < 0.001) less than that of the wild-type CRF1 receptor (Fig. 8). A much greater reduction in phosphorylation was observed, however, for the Δ386 receptor mutant (Fig. 8). Computerized analysis of phosphoprotein bands confirmed that Δ386 phosphorylation was 80–90% less (P < 0.001) than that of wild-type CRF1 receptors. CRF-stimulated phosphorylation of the Δ386 mutant was also significantly (P < 0.001) lower than the magnitude of Δ412 phosphorylation (Fig. 8).
Fig. 8.
Effect on CRF-stimulated phosphorylation of truncating the carboxyl terminus of the CRF1 receptor. HEK293 cells were transiently transfected with the following HA-tagged receptor cDNAs: 1) full-length wild-type CRF1 receptor (2 lanes on left); 2) Δ412 mutant (2 lanes in center); or 3) Δ386 mutant (2 lanes on right). After cells were metabolically labeled with 32Pi, they were exposed to 100 nM CRF for 5 min (+). Phosphoreceptors were immunoprecipitated and resolved by SDS-PAGE after loading equal amounts of protein in each lane. In four separate experiments, the 60,000–70,000 relative molecular mass (Mr) band representing the phosphorylated CRF1 receptor was ~35% less (P < 0.001) in cells expressing the Δ412 mutant and ~90% less (P < 0.001) in cells expressing the Δ386 mutant (lane on right) compared with cells expressing the full-length wild-type receptor.
We also tested whether the IC3 serine/threonine cluster of the CRF1 receptor contributes to the level of agonist-induced phosphorylation. When transfected HEK293 cells were exposed to CRF (100 nM) for 5 min, a large reduction in phosphorylation of the IC3-5ST/A mutant was measured compared with that of the wild-type CRF1 receptor (Fig. 9). In the same experiment, phosphorylation of the carboxyl-terminal truncation mutant Δ386 was again markedly deficient, and no further reduction was found when both mutations were incorporated into the same receptor (Δ386-IC3-5ST/A; Fig. 9). These data indicate that serines and threonines in both the carboxyl terminus and IC3 are phosphorylated upon agonist activation of the CRF1 receptor.
Fig. 9.
Effect on CRF-stimulated phosphorylation of mutagenizing the serine/threonine cluster in the third intracellular loop (IC3) on the CRF1 receptor. HEK293 cells were transiently transfected with the following HA-tagged receptor cDNAs: 1) full-length, wild-type CRF1 receptor (2 lanes on far left); 2) mutant in which alanines were substituted for a serine/threonine cluster (Ser301 to Thr306) in the IC3 loop (IC3-5ST/A; 2 lanes in left center); 3) Δ386 truncation mutant (2 lanes in right center); or 4) Δ386 mutant with the mutagenized IC3 serine/threonine cluster (Δ386-IC3-5ST/A; 2 lanes on far right). After cells were metabolically labeled with P32 and exposed to media (−) or 100 nM CRF (+) for 5 min, phosphoreceptors were immunoprecipitated and resolved by SDS-PAGE. In three separate experiments, the 60,000–70,000 Mr band representing the phosphorylated CRF1 receptor was decreased in the IC3-5ST/A mutant by ~75% (P < 0.01), and minimally detectable in cells expressing the Δ386 or Δ386-IC3-5ST/A mutants (P < 0.001), compared with phosphorylation of the full-length wild-type CRF1 receptor.
Recruitment of β-arrestin-2 by mutant CRF1 receptors
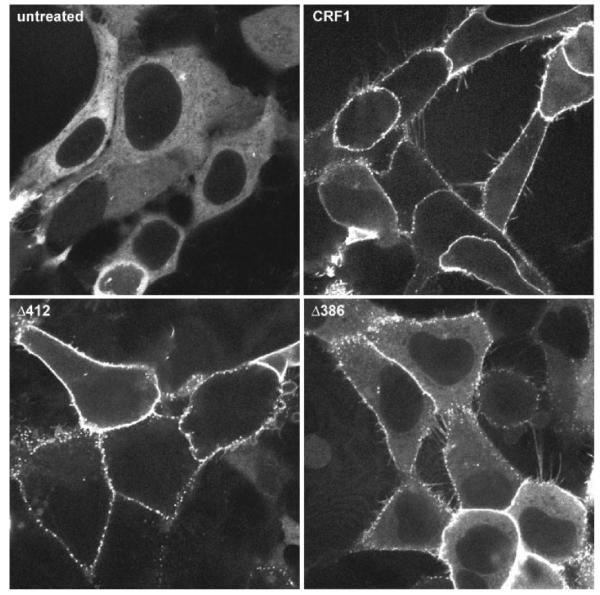
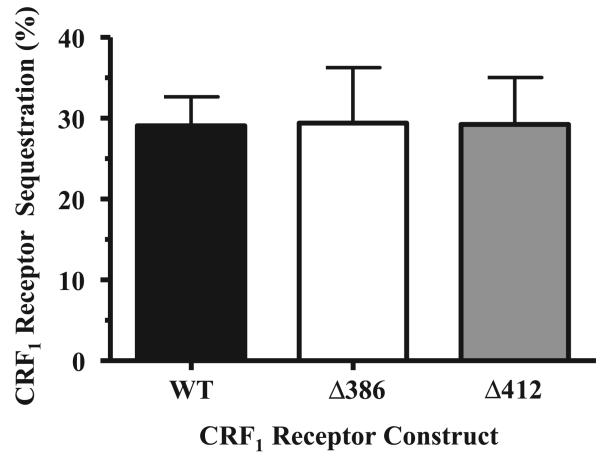
We next investigated whether the CRF1 receptor carboxyl terminus plays a role in the recruitment of arrestin. For these experiments, we employed β-arrestin-2-GFP because the CRF1 receptor preferentially interacts with this arrestin isoform (see Figs. 1-4). HEK293 cells were transiently transfected with β-arrestin-2-GFP and either the full-length wild-type CRF1 receptor, the Δ412 truncation mutant, or the Δ386 truncation mutant. In the absence of CRF, β-arrestin-2-GFP was evenly distributed in the cytoplasm of cells (Fig. 10, top left). Upon addition of CRF, pronounced translocation of cytoplasmic β-arrestin-2 to full-length wild-type CRF1 receptors on the membrane and localization of β-arrestin-2 in clathrin-coated pits were again observed (Fig. 10, top right). Although CRF-induced phosphorylation of Δ412 mutants was 35% less than that of wild-type CRF1 receptors (Fig. 8), Δ412 receptors induced the same strong recruitment of β-arrestin-2-GFP to the cell surface as did its full-length wild-type counterpart (Fig. 10, cf. top right and bottom left). Exposing the Δ386 mutant to oCRF (Fig. 10) or h/rCRF (data not shown), however, resulted in appreciably weaker recruitment of β-arrestin-2-GFP to the cell membrane than did equivalent CRF activation of full-length wild-type CRF1 receptors, as indicated by the significant amount of β-arrestin-2-GFP remaining in the cytoplasm (Fig. 10, cf. top right and bottom right). When we assessed the ability of wild-type and mutant CRF1 receptors to undergo agonist-induced internalization, our experiments revealed that wild-type, Δ412, and Δ386 CRF1 receptors internalized to a similar extent in transfected HEK293 cells during CRF exposure (Fig. 11).
Fig. 10.
Effect on β-arrestin-2 recruitment of truncating the carboxyl terminus of the CRF1 receptor. Confocal microscopy was used to evaluate the interaction of β-arrestin-2-GFP with the full-length wild-type CRF1 receptor (top right), Δ412 (bottom left), and Δ386 (bottom right) transiently expressed in HEK293 cells. Shown are representative confocal images of the distribution of β-arrestin-2-GFP following a 20-min treatment with 400 nM CRF. Top left: distribution of β-arrestin-2-GFP in CRF1 receptor-expressing cells not treated with CRF.
Fig. 11.
Agonist-induced internalization of wild-type and mutant CRF1 receptors in transfected HEK293 cells. HEK293 cells were transiently transfected with cDNA for HA-tagged full-length wild-type, Δ412, or Δ386 mutant CRF1 receptors in four initial experiments. Flow cytometry was then used to detect CRF-stimulated internalization of full-length and carboxy terminally truncated CRF1 receptors during a 90-min treatment period (vehicle vs. 1 μM CRF). By one-way ANOVA, no significant group differences were detected (F = 0.057; P > 0.05). In preliminary experiments, the time course and magnitudes of agonist-induced internalization of WT, Δ412, or Δ386 mutant CRF1 receptors were similar following 30, 45, 60, and 90 min of CRF exposure.
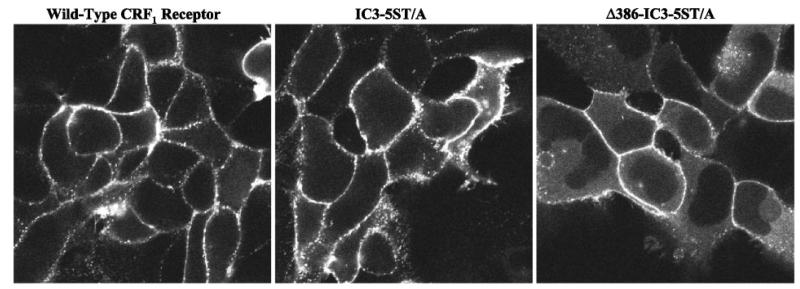
Having observed that deletion of putative GRK phosphorylation sites in the carboxyl terminus of the CRF1 receptor decreased translocation of cytosolic β-arrestin-2 to membrane-bound receptors, we next evaluated whether mutation of the STTSET motif in the IC3 of the CRF1 receptor would alter β-arrestin-2 recruitment. Activation of IC3-5ST/A mutant receptors produced a redistribution of β-arrestin-2-GFP to the cell membrane that was as robust as that induced by activation of full-length wild-type receptors (Fig. 12, cf. left and middle), despite the reduced CRF-induced phosphorylation of the IC3-5ST/A mutant (Fig. 9). In contrast, β-arrestin-2-GFP recruitment was noticeably weaker in the Δ386-IC3-5ST/A mutant receptor lacking both the carboxyl-terminal tail and the IC3 serine/threonine cluster as evidenced by the pool of β-arrestin-2-GFP remaining in the cytoplasm (Fig. 12, cf. left and right).
Fig. 12.
Effect on β-arrestin-2 recruitment of mutagenizing the serine/threonine cluster in the third intracellular loop on the CRF1 receptor. Confocal microscopy was used to evaluate the interaction of β-arrestin-2-GFP in HEK293 cells transiently expressing the full-length wild-type CRF1 receptor (left), the IC3-5ST/A mutant (middle), or Δ386-IC3-5ST/A mutant (right). Shown are representative confocal images of the distribution of β-arrestin-2-GFP following a 20-min treatment with 100 nM CRF.
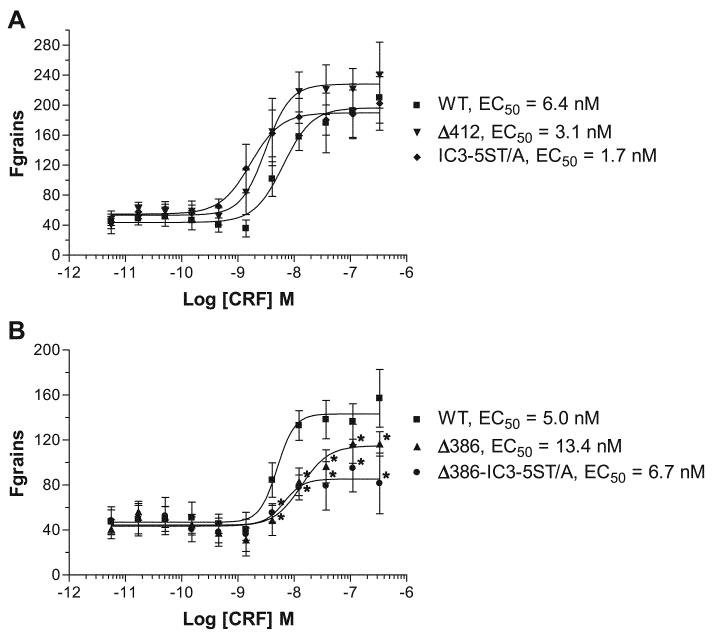
To quantify the observed differences in β-arrestin-2-GFP recruitment, we transiently expressed the full-length wild-type and mutant CRF1 receptors in U-2 OS cells and then analyzed the arrestin translocation response using the INCell Analyzer 3000 (42). U-2 OS cells were necessary for these quantitation experiments because of their superior adherent and morphological properties that allow for more sensitive measurements (43). CRF concentration-response curves for β-arrestin-2-GFP translocation to the wild-type and mutant CRF1 receptors are depicted in Fig. 13. The calculated EC50 values (see Fig. 13) for β-arrestin-2 recruitment by full-length wild-type and mutant CRF1 receptors are similar to the Kd values reported in the literature for CRF1 receptors transiently expressed in HEK293 cells (8). Compared with the full-length wild-type CRF1 receptor, no significant reduction was observed in the maximum agonist-induced recruitment of β-arrestin-2-GFP to the Δ412 truncation mutant and the IC3-5ST/A intracellular loop mutant (Fig. 13A). In contrast, significantly less β-arrestin-2-GFP was recruited to the Δ386 and Δ386-IC3-5ST/A mutants (P < 0.001; Fig. 13B). No significant differences were observed between recruitment of β-arrestin-2-GFP to the Δ386 and Δ386-IC3-5ST/A mutants. These results are consistent with the qualitative assessment of β-arrestin-2 translocation made in HEK293 cells (Figs. 10 and 12). These data suggest that phosphorylation of serines and/or threonines in the CRF1 receptor carboxyl terminus, but not IC3, is important for β-arrestin-2 recruitment. Moreover, they demonstrate the involvement of additional phosphorylation-independent receptor motifs that are exposed upon agonist binding and mediate the strong association of arrestin with CRF1 receptors.
Fig. 13.
Quantitation of β-arrestin-2 translocation to the agonist-activated full-length wild-type and mutant CRF1 receptors in transfected U-2 OS cells. U-2 OS cells transiently coexpressing β-arrestin-2-GFP and either full-length wild-type or mutant CRF1 receptors were plated in 96-well plates and cultured overnight. Cells were then stimulated with CRF (0–1,000 nM) for 30 min and analyzed on the INCell Analyzer 3000. The reported parameter “Fgrains” represents the fluorescence intensity of β-arrestin-2-GFP localized with the receptor in spots or grains. Data represent means ± SE for 3 independent experiments performed in triplicate. A: CRF concentration-response curves for full-length wild-type CRF1 receptor, Δ412 mutant, and IC3-5ST/A mutant. B: CRF concentration-response curves for full-length wild-type CRF1 receptor, Δ386 mutant, and Δ386-IC3-5ST/A mutant. By one-way ANOVA, there were statistically significant differences (P < 0.001) across the groups for changes in CRF-stimulated recruitment of β-arrestin-2 by the activated receptor. The following post hoc differences were found to be statistically significant between groups: *P < 0.001 vs. the wild type.
DISCUSSION
In the present study, we investigated the phosphorylation and arrestin-binding properties of agonist-activated CRF1 receptors. We found that CRF1 receptors activated by either oCRF or h/rCRF preferentially recruit β-arrestin-2 over β-arrestin-1 in multiple cell lines, in agreement with a recent study (22). In addition, our arrestin overexpression experiments show that increasing the cellular level of β-arrestin can upregulate CRF1 receptor internalization. However, because neither β-arrestin isoform traffics with the receptor inside the cell, the CRF1 receptor is a “class A” GPCR in terms of its interaction with arrestin proteins. Employing receptor mutagenesis, we found that agonist-induced phosphorylation of the CRF1 receptor occurs in two distinct receptor domains, one located in the IC3 and one in the carboxyl-terminal tail. Importantly, we discovered that recruitment and binding of β-arrestin-2 by the CRF1 receptor is mediated by both phosphorylation-dependent and phosphorylation-independent intracellular motifs.
A common feature of GPCRs is rapid recruitment of β-arrestin to agonist-activated receptors at the plasma membrane, although the fate of the receptor-β-arrestin complex differs (31, 39, 40). Class A receptors form transient complexes with β-arrestin that dissociate at or near the plasma membrane. Consequently, β-arrestin does not internalize with these receptors inside the cell. Class B receptors form stable complexes with β-arrestin that internalize as a unit into intracellular vesicles and persist inside the cell. Conflicting data exist over the classification of the CRF1 receptor, since it has been reported to be class A in HEK293 and mouse fetal cortical cells (22, 48) but class B in CHO-K1 cells (47). Our results with β-arrestin-1-GFP and β-arrestin-2-GFP in HEK293, CHO-K1, and U-2 OS cells indicate that CRF1 receptors are class A. We have also observed that the CRF1 receptor has a marked preference for β-arrestin-2 based on the kinetics of the trans-location event. Neither β-arrestin isoform internalized with the CRF1 receptor into endocytic vesicles, even after 1 h of CRF treatment in our study. Perry et al. (47) and Markovic et al. (34) have reported that the CRF1 receptor-β-arrestin complex internalizes together into cytosolic vesicles. The use of fixed rather than live cells may account for the discrepancy between their findings and data from our experiments (Figs. 1-4) and the study by Holmes et al. (22), indicating that the CRF1 receptor exhibits a class A interaction with β-arrestins.
The transient interaction of β-arrestin with class A receptors appears to allow their more rapid recycling and resensitization by facilitating receptor dephosphorylation (41). Consistent with a class A grouping are reports showing that CRF1 receptors endogenously expressed in rat anterior pituitary cells or recombinantly overexpressed in HEK293 cells resensitize within 1–2 h after being desensitized by CRF treatment (22, 49, 53). However, studies in human retinoblastoma Y79 and neuroblastoma IMR-32 cells have reported a much slower time course of 24 h for complete restoration of CRF1 receptor cAMP signaling (18, 51). These divergent results may be explained by Y79 cells expressing β-arrestin-1 but not β-arrestin-2 (Dautzenberg and Hauger, unpublished observations), whereas HEK cells express both β-arrestins (35). Recently, GRK3 overexpression was reported to increase the interaction of β-arrestin-1 (but not β-arrestin-2) with the CRF1 receptor measured by bioluminescence resonance energy transfer (22). In our study, we found that overexpressing GRK3 did not alter β-arrestin-2 recruitment or prevent dissociation of the CRF1 receptor-arrestin complex. Thus future research should elucidate the regulatory effects of selective GRK and arrestin mechanisms operating on CRF1 receptor function in different cell types.
Defining intracellular binding sites for β-arrestins has become an important focus of research because of their multi-faceted roles in regulating GPCR signaling. The carboxyl terminus of many GPCRs contains critical domains for GRK-mediated phosphorylation that regulate β-arrestin translocation and binding (3, 4, 12, 21, 26, 27, 31, 32, 38, 39, 41, 44, 45, 50, 55). Importantly, several reports have shown that serine/threonine clusters in the carboxyl-terminal tail of certain GPCRs function as β-arrestin binding sites (41, 44). GRK-induced phosphorylation of these serine/threonine clusters facilitates the tight (class B) binding of β-arrestin to the activated receptor that leads to β-arrestin internalizing with the receptor inside the cell. Deletion of carboxyl-terminal serine/threonine clusters markedly diminishes agonist-induced phosphorylation of a class B receptor and the stability of the GPCR-arrestin complex, effectively changing a class B into a class A phenotype (41, 44). Furthermore, support for the idea that GRK-mediated phosphorylation plays a critical role in the strength of arrestin binding comes from kinetic studies showing that GRK recruitment to the activated neurokinin-1 receptor precedes translocation of β-arrestin-GFP (2).
We have localized the sites of agonist-induced CRF1 receptor phosphorylation to serine/threonine residues in both the IC3 and the carboxyl terminus. Receptor phosphorylation is reduced ~75% upon mutation of the putative GRK phosphorylation site in the IC3 and completely abolished upon truncation of the carboxyl-terminal tail at position 386. The nearly complete absence of CRF1 receptor phosphorylation observed for the Δ386 truncation mutant was surprising given that this receptor construct still contains the native IC3 domain. This finding suggests that an intact carboxyl terminus is required for phosphorylation of the CRF1 receptor IC3 site, similar to recent observations by Kim et al. (25) and Oakley et al. (39) for agonist-induced phosphorylation of the dopamine D1 receptor, another class A receptor with preference for β-arrestin-2. They propose the intriguing hypothesis that the dopamine D1 receptor is regulated by hierarchical phosphorylation that first occurs in the receptor carboxyl terminus and then, in turn, occurs in the IC3 (25). In a similar manner, phosphorylation of specific serine and/or threonine residues in the CRF1 receptor carboxyl terminus may be necessary for subsequent phosphorylation of the serine/threonine cluster in the IC3 loop.
The phosphorylation-defective Δ386 mutant was also impaired in its ability to recruit β-arrestin-2 to the plasma membrane. In contrast, truncation of the CRF1 receptor at serine-412 produced no defect in CRF-induced β-arrestin-2 translocation. Recently, Thr399, which resides in a possible arrestin-binding motif (TSPT; see Refs. 40 and 44), was reported to be a critical site of GRK-mediated phosphorylation and desensitization of CRF1 receptors (53). Because reducing cellular expression of GRK3 or blocking the action of GRK3 inhibits homologous desensitization of CRF1 receptors (6, 53), Thr399 may be phosphorylated preferentially by GRK3, thereby transforming the TPST motif into an active site for β-arrestin binding. In addition, we observed that CRF-induced internalization of wild-type, Δ412, and Δ386 CRF1 receptors did not differ significantly. This finding is in agreement with a recent study in which a CRF1 receptor mutant truncated at Lys384 internalized to the same extent as the wild-type CRF1 receptor (48). The lesser amount of β-arrestin-2 translocating to the agonist-activated Δ386 mutant we detected (see Fig. 10) may have been sufficient to promote internalization of Δ386 receptors. Alternatively, other cellular mechanisms may internalize CRF1 receptors (11). For example, the Ras-like small GTP-binding proteins Rab4 and Rab5 were recently found to be involved in CRF-induced internalization and resensitization of CRF1 receptors (22).
Additional receptor domains, however, must also participate in the CRF1 receptor-β-arrestin-2 interaction based on our finding that the Δ386 truncation mutant can still recruit β-arrestin-2 to the plasma membrane to some extent. Phosphorylation of the IC3 has been shown to be important for β-arrestin recruitment and binding to some GPCRs, including the dopa-mine D1 (25), muscarinic M2 (30), and α2b-adrenergic (9) receptors, but this does not appear to be the case for the CRF1 receptor. Mutation of the serine/threonine cluster in the CRF1 receptor IC3 (IC3-5ST/A) markedly reduced receptor phosphorylation but had no effect on β-arrestin-2 recruitment compared with the full-length wild-type CRF1 receptor. In addition, combining the IC3 and Δ386 deletions (Δ386-IC3-5ST/A) had no significant impact on β-arrestin-2 recruitment compared with the Δ386 deletion alone. These data suggest that the CRF1 receptor contains an arrestin-binding domain in one or more of the intracellular loops that is dependent on receptor activation but independent of GRK phosphorylation. The presence of phosphorylation-independent arrestin-binding motifs has been suggested for several GPCRs, including the vasopressin V2 (41), luteinizing hormone (36), parathyroid hormone (54), neurotensin (44), and substance P (44) receptors. For these GPCRs, elimination of the carboxyl-terminal phosphorylation sites inhibited receptor phosphorylation by 90% or more, but only partially impaired the recruitment of arrestin. Moreover, Marion et al. (33) recently identified a 10-amino-acid motif in the proximal portion of the second intracellular loop of rhodopsin family GPCRs that functions as a phosphorylation-independent structural determinant for β-arrestin binding. The analogous region of the CRF1 receptor, a secretin family GPCR, retains several of the key features of this motif, namely a basic residue at position 1 (His214) and hydrophobic residues at positions 4–7 (A216I217V218L219) of the second intracellular loop (see Fig. 6).
In summary, we conclude that β-arrestin-2 recruitment by agonist-activated membrane CRF1 receptors is mediated by at least two distinct domains as follows: 1) a phosphorylation-dependent motif in the carboxyl terminus and 2) a phosphorylation-independent motif in one or more of the intracellular loops. Furthermore, we posit that CRF1 receptor signaling will be sensitive to changes in the relative expression of β-arrestin isoforms. Under conditions in which expression of β-arrestin-2 predominates over β-arrestin-1, we predict that desensitization of CRF1 receptors will occur more rapidly to limit the extent and duration of Gs-coupled signaling. Furthermore, CRF1 receptors may strongly signal via the ERK-MAP kinase cascade in cells or neurons with a predominance of β-arrestin-2 during prolonged agonist exposure. Thus understanding arrestin-mediated processes regulating the kinetics and transduction pathway selectivity of CRF1 receptor signaling will most likely lead to more effective pharmacotherapies for stress-induced anxiety and depressive disorders in which central CRF neuro-transmission is excessively active (15, 16, 24, 37).
ACKNOWLEDGMENTS
We thank Dr. Marc Caron (Department of Cell Biology, Duke University) for invaluable scientific input and financial support. We likewise thank Dr. Kevin Catt (Branch Chief, Endocrinology and Reproductive Research, National Institutes of Child Health and Human Development, National Institutes of Health) for generous and helpful advice during this study. We gratefully acknowledge Judith Hernandez-Aranda (Departamento de Bioquimica, CINVESTAV) and Sandra Braun [University of California San Diego (UCSD)] for performing cell biology experiments, Alan Turken (UCSD) for completing cAMP assays, and Dr. Ana M. Barral (Research Scientist, Nereus Pharmaceuticals, San Diego, CA) for providing helpful advice about flow cytometry methods. We gratefully acknowledge Susan Shew for editorial assistance.
GRANTS
R. L. Hauger received support from Department of Veterans Affairs Merit Review Grant no. 0015; the Veterans Affairs (VA) Mental Illness Research, Education, and Clinical Center of the 22nd Veterans Integrated Service Networks; the VA Center of Excellence for Stress and Mental Health (CESAMH); National Institutes of Health RO1 Grants AG-022982 and MH-074697 RO1; and the University of California Institute for Mexico and the United States-Consejo Nacional de Ciencia y Tecnologia grant for collaborative projects. A. Olivares-Reyes was supported by the Centro de Investigacion y de Estudios Avanzados del Instituto Politecnico Nacional, a UC MEXUSCONACYT grant for collaborative projects, and CONACYT Grant 39485-Q.
REFERENCES
- 1.Barak LS, Tiberi M, Freedman NJ, Kwatra MM, Lefkowitz RJ, Caron MG. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist mediated beta2-adrenergic receptor sequestration. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 2.Barak LS, Warabi K, Feng X, Caron MG, Kwatra MM. Real-time visualization of the cellular redistribution of G protein-coupled receptor kinase 2 and beta-arrestin 2 during homologous desensitization of the substance P receptor. J Biol Chem. 1999;274:7565–7569. doi: 10.1074/jbc.274.11.7565. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier M, Hausdorff WP, De Blasi A, O'Dowd BF, Kobilka BK, Caron MG, Lefkowitz RJ. Removal of phosphorylation sites from the 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature (Lond) 1988;333:370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- 4.Braun L, Christophe T, Boulay F. Phosphorylation of key serine residues is required for internalization of the complement 5a (C5a) anaphylatoxin receptor via a β-arrestin, dynamin, and clathrin-dependent pathway. J Biol Chem. 2003;278:4277–4285. doi: 10.1074/jbc.M210120200. [DOI] [PubMed] [Google Scholar]
- 5.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 6.Dautzenberg FM, Braun S, Hauger RL. GRK3 mediates desensitization of CRF1 receptors: a potential mechanism regulating stress adaptation. Am J Physiol Regul Integr Comp Physiol. 2001;280:R935–R946. doi: 10.1152/ajpregu.2001.280.4.R935. [DOI] [PubMed] [Google Scholar]
- 7.Dautzenberg FM, Wille S, Braun S, Hauger RL. GRK3 regulation during CRF- and urocortin-induced CRF1 receptor desensitization. Biochem Biophys Res Commun. 2002;298:303–308. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- 8.Dautzenberg FM, Kilpatrick GJ, Wille S, Hauger RL. The ligand-selective domains of corticotropin-releasing factor type 1 and type 2 receptors reside in different extracellular domains: generation of chimeric receptors with a novel ligand-selective profile. J Neurochem. 1999;73:821–829. doi: 10.1046/j.1471-4159.1999.0730821.x. [DOI] [PubMed] [Google Scholar]
- 9.DeGraff JL, Gurevich VV, Benovic JL. The third intracellular loop of 2-adrenergic receptors determines subtype specificity of arrestin interaction. J Biol Chem. 2002;277:43247–42352. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich KD, Grigoriadis DE, DeSouza EB. Homologous desensitization of human corticotropin-releasing factor1 receptor in stable transfected mouse fibroblast cells. Brain Res. 1996;710:287–292. doi: 10.1016/0006-8993(95)01480-2. [DOI] [PubMed] [Google Scholar]
- 11.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 13.Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human β2-adrenergic receptor. J Biol Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- 14.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 15.Grigoriadis DE. The corticotropin-releasing factor receptor: a novel target for the treatment of depression and anxiety-related disorders. Expert Opin Ther Targets. 2005;9:651–684. doi: 10.1517/14728222.9.4.651. [DOI] [PubMed] [Google Scholar]
- 16.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. CRF receptor signaling in the central nervous system: new molecular targets for affective disorders. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauger RL, Olivares-Reyes JA, Braun S, Catt KJ, Dautzenberg FM. Regulation of corticotropin releasing factor type 1 receptor phosphorylation and desensitization by protein kinase C: a possible role in stress adaptation. J Pharmacol Exp Therap. 2003;306:794–803. doi: 10.1124/jpet.103.050088. [DOI] [PubMed] [Google Scholar]
- 18.Hauger RL, Dautzenberg FM, Flaccus A, Liepold T, Spiess J. Regulation of corticotropin-releasing factor receptor function in human Y-79 retinoblastoma cells: rapid and reversible homologous desensitization but prolonged recovery. J Neurochem. 1997;68:2308–2316. doi: 10.1046/j.1471-4159.1997.68062308.x. [DOI] [PubMed] [Google Scholar]
- 19.Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Commun. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman AR, Ceda G, Reisine TD. Corticotropin-releasing factor desensitization of adrenocorticotropic hormone release is augmented by arginine vasopressin. J Neurosci. 1985;5:234–242. doi: 10.1523/JNEUROSCI.05-01-00234.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holliday ND, Lam CW, Tough IR, Cox HM. Role of the C terminus in neuropeptide Y Y1 receptor desensitization and internalization. Mol Pharm. 2005;67:655–664. doi: 10.1124/mol.104.006114. [DOI] [PubMed] [Google Scholar]
- 22.Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- 23.Jewell-Motz E, Liggett SB. An acidic motif within the third intracellular loop of the α2C2 adrenergic receptor is required for agonist-promoted phosphor-ylation and desensitization. Biochemistry. 1995;34:11946–11953. doi: 10.1021/bi00037a036. [DOI] [PubMed] [Google Scholar]
- 24.Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–889. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Kim OJ, Gardner BR, Williams DB, Marinec PS, Cabrera DM, Peters JD, Mak CC, Kim KM, Sibley DR. The role of phosphorylation in D1 dopamine receptor desensitization. J Biol Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 27.Krasel C, Vilargdaga JP, Bunemann M, Lohse MJ. Kinetics of G-protein-coupled receptor signaling and desensitization. Biochem Soc Trans. 2004;32:1029–1031. doi: 10.1042/BST0321029. [DOI] [PubMed] [Google Scholar]
- 28.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/beta-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- 30.Lee KB, Ptasienski JA, Pals-Rylaarsdam R, Gurevich VV, Hosey MM. Arrestin binding to the M2 muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop. J Biol Chem. 2000;275:9284–9289. doi: 10.1074/jbc.275.13.9284. [DOI] [PubMed] [Google Scholar]
- 31.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 32.Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins: kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta2-adrenergic receptor and rhodopsin. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 33.Marion S, Oakley RH, Kim KM, Caron MG, Barak LS. A beta-arrestin binding determinant common to the second-intracellular loops of Rhodopsin-family G protein-coupled receptors. J Biol Chem. 2006;281:2932–2938. doi: 10.1074/jbc.M508074200. [DOI] [PubMed] [Google Scholar]
- 34.Markovic D, Papadopoulou N, Teli T, Randeva H, Levine MA, Hillhouse EW, Grammatopoulos D. Differential responses of CRH receptor type 1 variants to PKC phosphorylation. J Pharmacol Exp Ther. 2006;319:1032–1042. doi: 10.1124/jpet.106.107441. [DOI] [PubMed] [Google Scholar]
- 35.Menard L, Ferguson SSG, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of β2-adrenergic receptor kinase and β-arrestin determine kinetics of internalization. Mol Pharm. 1997;51:800–808. [PubMed] [Google Scholar]
- 36.Min L, Ascoli M. The association of arrestin-3 with the human lutropin/ choriogonadotropin receptors depends mostly on receptor activation rather than on receptor phosphorylation. J Biol Chem. 2002;277:702–710. doi: 10.1074/jbc.M106082200. [DOI] [PubMed] [Google Scholar]
- 37.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 38.Neuschafer-Rube F, Hermosilla R, Rehwald M, Ronnstrand L, Schulein R, Wernstedt C, Puschel GP. Identification of a Ser/Thr cluster in the C-terminal domain of the human prostaglandin receptor EP4 that is essential for agonist-induced beta-arrestin1 recruitment but differs from apparent principal phosphorylation site. Biochem J. 2004;379:573–585. doi: 10.1042/BJ20031820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, β-arrestin1, and β-arrestin2 with G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 40.Oakley RH, Barak LS, Caron MG. Real time imaging of GPCR-mediated arrestin translocation as a strategy to evaluate receptor-protein interactions. In: George SR, O'Dowd BF, editors. G Protein Coupled Receptor-Protein Interactions. Wiley; Hoboken, NJ: 2005. pp. 53–80. [Google Scholar]
- 41.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 42.Oakley RH, Hudson CC, Cruickshank RD, Meyers DM, Payne RE, Rehm SM, Loomis CR. The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay Drug Develop Tech. 2002;1:21–30. doi: 10.1089/154065802761001275. [DOI] [PubMed] [Google Scholar]
- 43.Oakley RH, Cowan CL, Hudson CC, Loomis CR. Transfluor provides a universal cell-based assay for screening G protein coupled receptors. In: Minor L, editor. Handbook of Assay Development in Drug Discovery. CRC; Boca Raton, FL: 2006. pp. 431–453. [Google Scholar]
- 44.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled-β-arrestin complexes after receptor endocytosis. J Biol Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 45.Penn RB, Benovic JL. Mechanisms of receptor regulation. In: Conn PM, editor. Handbook of Physiology. Endocrinology. Cellular Endocrinology. sect. 7, chapt. 7. I. Am Physiol Sci; Bethesda, MD: 1998. p. 145. [Google Scholar]
- 46.Perrin MH, Vale W. Corticotropin-releasing factor receptors. In: Pangalos MN, Davies CH, editors. Understanding G Protein-coupled Receptors and Their Role in the CNS. Oxford Univ Press; New York, NY: 2002. [Google Scholar]
- 47.Perry SJ, Junger S, Kohout TA, Hoare SRJ, Struthers RS, Grigoriadis DE, Maki RA. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2005;280:11560–11568. doi: 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- 48.Rasmusson TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of β-arrestin 1 recruitment. Eur J Biochem. 2004;271:4366–4374. doi: 10.1111/j.1432-1033.2004.04371.x. [DOI] [PubMed] [Google Scholar]
- 49.Reisine T, Hoffman A. Desensitization of corticotropin releasing factor receptors. Biochem Biophys Res Commun. 1983;111:919–925. doi: 10.1016/0006-291x(83)91387-6. [DOI] [PubMed] [Google Scholar]
- 50.Reiter E, Lefkowitz RJ. GRKs and β-arrestins: roles in receptor silencing, trafficking, and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Roseboom PH, Urben CM, Kalin N. Persistent corticotropin-releasing factor1 receptor desensitization and downregulation in the human neuroblastoma cell line IMR-32. Brain Res Mol Brain Res. 2001;92:115–127. doi: 10.1016/s0169-328x(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 52.Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A. Differential nucleocytoplasmic shuttling of beta-arrestins: characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem. 2002;277:37693–37701. doi: 10.1074/jbc.M207552200. [DOI] [PubMed] [Google Scholar]
- 53.Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone (CRH) receptor type 1α signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- 54.Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate β-arrestin/receptor association. J Biol Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- 55.von Zastrow M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]