Abstract
Pheromones regulate male social behaviors in Drosophila, but the identities and behavioral role(s) of these chemosensory signals, and how they interact, are incompletely understood. Here we show that (Z)-7-tricosene (7-T), a male-enriched cuticular hydrocarbon (CH) previously shown to inhibit male-male courtship, is also essential for normal levels of aggression. The opposite influences of 7-T on aggression and courtship are independent, but both require the gustatory receptor Gr32a. Surprisingly, sensitivity to 7-T is required for the aggression-promoting effect of 11-cis-vaccenyl acetate (cVA), an olfactory pheromone, but 7-T sensitivity is independent of cVA. 7-T and cVA therefore regulate aggression in a hierarchical manner. Furthermore, the increased courtship caused by depletion of male CHs is suppressed by a mutation in the olfactory receptor Or47b. Thus, male social behaviors are controlled by gustatory pheromones that promote and suppress aggression and courtship, respectively, and whose influences are dominant to olfactory pheromones that enhance these behaviors.
INTRODUCTION
Social interactions between male Drosophila involve relatively high levels of aggression, and low levels of courtship1, 2. How the normal balance between these two behaviors is achieved is poorly understood. Previous studies have implicated pheromones detected by both the gustatory and olfactory systems in regulating these social interactions. Males lacking all gustatory sensilla3, specific gustatory receptors4, 5, or their normal complement of CHs6, 7, non-volatile pheromones that include ligands of gustatory receptors8, exhibit elevated levels of male-male courtship. These data suggest that gustatory pheromones play a suppressive role in male-male courtship. Conversely, a genetic manipulation that eliminates CHs6 has recently been suggested to reduce male-male aggression9. Whether the same or different CH molecules inversely regulate these two male social behaviors is not clear.
A single olfactory pheromone, cVA, has been implicated in both suppressing male-male courtship and enhancing male-male aggression. Mutants lacking Or67d10, a receptor for cVA10–12, have been reported to exhibit elevated male-male courtship, implying that cVA may ordinarily suppress this behavior. However, a subsequent study is unable to replicate this observation3, and exogenously applied cVA does not reduce male-male courtship13. In contrast, cVA strongly promotes male-male aggression, in an Or67d-dependent manner13. Although detection of cVA by Or67d receptor is not essential for aggression between isolated pairs of male flies, it may play a role under conditions of high male population density13.
Despite this recent progress, the nature of the chemosensory signals that control the normal balance between male-male aggression and courtship is incompletely understood. Furthermore, the extent to which gustatory and olfactory systems function independently vs. interdependently or hierarchically to regulate these behaviors has not been investigated. In the present study, we have investigated the interplay between the gustatory and olfactory pheromones in the regulation of male-male social behaviors (Supplementary Fig. 1).
RESULTS
Male CHs control the balance between male social behaviors
We first asked whether male CHs were required for male-male aggression. Previous studies show that the production of these non-volatile pheromones requires oenocytes, a group of cells located beneath the abdominal cuticle of male flies6, 14. Male oenocytes can be selectively ablated in adults using genetic tools6 (see Methods). This manipulation eliminated most if not all male CHs6, including the two most abundant male-enriched CH molecules8, 15, 16 (7-T and (Z)-7-pentacosene, 7-P), without affecting the levels of cVA, an olfactory pheromone synthesized in the male ejaculatory bulb17 (Supplementary Fig. 2a, b and Fig. 1a, orange vs. blue bars). Strikingly, pairs of oenocyte-eliminated (oe−) males exhibited significantly reduced levels of male-male aggression, compared to pairs of control (oe+) males (Supplementary Fig. 3a), in addition to higher levels of male-male courtship6 (Supplementary Fig. 3b). However, aggression between such oe− males was not completely eliminated, consistent with a recent report9.
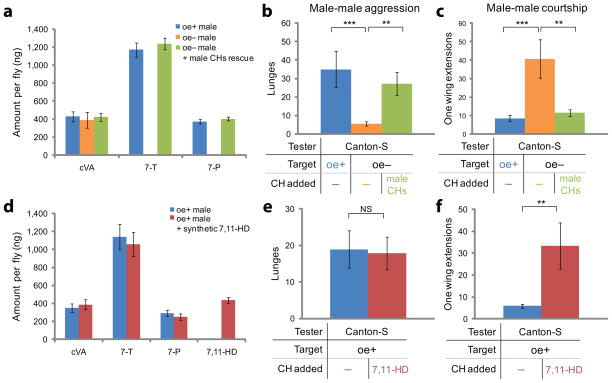
Figure 1. Male CHs are important for the normal balance of male-male social behaviors.
(a) Quantification of cVA and major CH molecules from oe+ (blue), oe− (orange) or oe− males perfumed with male CHs (green) (n=10). (b, c) Quantification of aggression (b) and courtship (c) performed by Canton-S tester males towards target males of the indicated genotypes and CH perfuming treatments (n=20). (d) Quantification of cVA and major CH molecules from oe+ (blue) or oe+ males perfumed with synthetic 7,11-HD (red) (n=9). (e, f) Quantification of aggression (e) and courtship (f) performed by Canton-S tester males towards target males of the indicated genotypes and CH perfuming treatments (n=16). Error bars are s.e.m. in this and all subsequent figures. NS: p>0.05, *p<0.05, **p<0.01 and ***p<0.001.
To distinguish whether eliminating male CHs influenced the social behaviors of oe− males in a fly-autonomous or non-autonomous manner, we paired a wild-type Canton-S “tester” male with either an oe+ or an oe− “target” male. Canton-S testers performed significantly lower levels of male-male aggression towards oe− than towards oe+ targets, as measured by the occurrence of lunges (Fig. 1b, orange vs. blue bars; also see Supplementary Fig. 4). Wing threat, a behavioral display exhibited during male-male aggressive encounters1, 18, 19, was not affected by the elimination of male CHs (data not shown). Canton-S tester males also performed significantly higher levels of courtship towards oe− than towards oe+ target males, as measured by the occurrence of unilateral wing extensions (Fig. 1c, orange vs. blue bars) and circling episodes2 (data not shown). To prove that the alteration of tester male behaviors was due to the lack of CHs on oe− target males, and not to some other, unknown effect of this genetic manipulation, we restored normal levels of male CHs to oe− males by passive transfer from control males7 (Supplementary Fig. 2c and Fig. 1a, green bars; see Methods). This manipulation rescued both the decreased male-male aggression and increased male-male courtship (Fig. 1b, c, green bars; also see Supplementary Fig. 4) exhibited by the testers. These data indicate that male CHs not only inhibit male-male courtship, but positively regulate aggression as well.
The reciprocal influences of male CHs could reflect independent and opposite effects on male-male aggression vs. male-male courtship, or a primary effect exclusively on one behavior, which then indirectly inhibits the performance of the other. cVA can strongly enhance aggression without causing a concomitant decrease in male-male courtship13, implying that aggression does not behaviorally inhibit male-male courtship. To ask whether, conversely, enhancing male-male courtship indirectly decreased aggression, oe+ target males were perfumed with synthetic (Z,Z)-7,11-heptacosadiene (7,11-HD), a typical female-specific CH molecule6, 8 (Supplementary Fig. 5 and Fig. 1d). This treatment elevated courtship by wild-type tester males towards the targets, without reducing their level of aggression (Fig. 1e, f). Thus elevated male-male courtship does not behaviorally inhibit aggression. The reciprocal effects of CHs on male-male aggression vs. male-male courtship therefore reflect parallel, direct influences of such pheromones on these two social behaviors.
7-T reciprocally regulates male aggression and courtship
Male CHs are comprised of multiple classes of compounds8, 16; among these, 7-T has been shown to suppress courtship6, 20. The requirement of CHs for normal levels of male-male aggression may therefore reflect the influence of a different male-enriched CH(s). Alternatively, 7-T might both promote aggression and suppress male-male courtship. To distinguish between these alternatives, we chemically synthesized 7-T and 7-P, the two most abundant male-enriched CHs8, and asked whether perfuming oe− targets with either of them was sufficient to restore the normal balance of social behaviors by male testers (Supplementary Fig. 6 and Fig. 2a; see Methods). Remarkably, synthetic 7-T was sufficient both to restore normal levels of aggression, as well as to suppress courtship (Fig. 2b, c, green bars), by wild-type tester males. In contrast, synthetic 7-P exhibited no behavioral effect in this assay (Fig. 2b, c, purple bars). These data indicate that a single CH species can exert opposite-direction influences on aggression and male-male courtship, in the absence of other CH molecules synthesized by oenocytes.
Figure 2. 7-T reciprocally regulates both male-male aggression and courtship.
(a) Quantification of cVA and major CH molecules from oe+ (blue), oe− (orange) or oe− males perfumed with synthetic 7-T (green) or 7-P (purple) (n=11–12). (b, c) Quantification of aggression (b) and courtship (c) performed by Canton-S tester males towards target males of the indicated genotypes and CH perfuming treatments (n=20). (d) Relative levels of 7-T carried by oe− males incubated after 7-T transfer for various periods of time (green), shown as a percentage of wild-type levels of 7-T (blue) (n=9–11). Absolute quantification is shown in Supplementary Fig. 6. Green shading represents relative amount of synthetic 7-T carried by oe− males (darker = higher). (e, f) Quantification of aggression (e) and courtship (f) performed by Canton-S tester males towards oe− targets (orange), oe+ targets (blue), or oe− target males carrying different amounts of synthetic 7-T (green) (n=18). NS: p>0.05, *p<0.05, **p<0.01 and ***p<0.001.
We next investigated whether these distinct influences of 7-T might be exerted at different concentrations of the pheromone. We generated oe− target males carrying different amounts of 7-T, ranging from ~20% to ~2 fold of the amount of 7-T present on oe+ males (Supplementary Fig. 7 and Fig. 2d; see Methods). These flies were then paired with wild-type tester males. Surprisingly, as little as ~30% of the wild type amount of 7-T applied to oe− males could produce a significant increase in aggression and exhibited a trend to inhibit courtship (Fig. 2e, f). Further increasing the level of 7-T, to ~2-fold of the normal level, did not cause additional changes in either social behavior. These data indicate that 7-T oppositely influences aggression and inter-male courtship over a similar concentration range.
Gr32a mediates the behavioral effects of 7-T
Next, we investigated the chemosensory receptor(s) that mediates the behavioral effects of 7-T. Previous work has shown that 7-T can activate bitter-sensing GRNs20, which express multiple gustatory receptors21–23, but no specific receptor for 7-T has yet been identified. The fact that Gr32a is expressed in bitter-sensing GRNs21–25, and that Gr32a−/− mutant males exhibit increased courtship towards decapitated males4, suggests Gr32a as a candidate receptor mediating the behavioral effects of 7-T.
We first asked whether Gr32a was required for normal levels of aggression. When paired with oe+ target males, Gr32a−/− mutant tester males4 showed a diminished aggression level compared to Gr32a+/− heterozygous control males (Fig. 3a, Gr32a+/− vs. Gr32a−/−; also see Supplementary Fig. 8). This Gr32a mutant phenotype was reverted by expression of a Gr32a genomic rescue construct4 (Fig. 3a, Gr32a−/− Rescue; also see Supplementary Fig. 8). In contrast, the Gr32a−/− mutation did not affect the level of courtship towards oe+ target males (Fig. 3b), although it did impair courtship towards oe− target males perfumed with 7-T (see below). Although a previous study reports that Gr32a−/− mutant males show increased male-male courtship towards decapitated target males4, a result that we independently replicated (Supplementary Fig. 9), such decapitated male targets may fail to provide additional signals, such as behavioral feedback3, that intact males normally provide to suppress male-male courtship. It has also been reported that Gr32a−/− mutant males exhibit increased bilateral wing extension behaviors towards females26, but we did not observe such behavior towards oe+ male targets.
Figure 3. Gr32a mediates the behavioral effects of 7-T and permits the aggression-promoting effect of cVA.
(a, b) Quantification of aggression (a) and courtship (b) performed by tester males of the indicated genotypes towards oe+ target males (n=26–28). (c, d) Quantification of aggression (c) and courtship (d) performed by tester males of the indicated genotypes towards oe− target males (orange) or oe− males perfumed with synthetic 7-T (green) (n=26–30). Dashed lines represent control levels of social behaviors (performed by Gr32a+/− testers towards oe+ targets (from Fig. 3a, b)). (e) Quantification of aggression performed by pairs of males of the indicated genotypes, in the presence of acetone alone (blue), or in the presence of 500 μg synthetic cVA (green) (n=18–20). Note the flies were group-housed prior to the behavioral assays to better reveal the effect of cVA (see Methods). (f) Quantification of aggression performed by tester males of the indicated genotypes towards oe− target males (orange) or oe− males perfumed with 7-T (green) (n=20). NS: p>0.05, *p<0.05, **p<0.01 and ***p<0.001.
We then asked whether Gr32a is required for the effects of 7-T to promote aggression and inhibit male-male courtship. Indeed, Gr32a−/− mutant tester males failed to show increased aggression and decreased courtship towards oe− targets perfumed with 7-T (Fig. 3c, d, Gr32a−/−, orange vs. green bars). In contrast, control Gr32a+/− tester males, like wild-type males, exhibited increased aggression and decreased courtship towards such target males (Fig. 3c, d, Gr32a+/− , green bars), at levels comparable to those displayed towards oe+ targets (Fig. 3c, d, Gr32a+/−, orange bars vs. dashed lines). The phenotype of Gr32a−/− mutants could be reverted by Gr32a genomic rescue (Fig. 3c, d, Gr32a−/− Rescue, orange vs. green bars). These data indicate that Gr32a is required for the inverse effects of 7-T on aggression and male-male courtship (Supplementary Fig. 1, green), suggesting that Gr32a may encode a 7-T receptor. Whether Gr32a is required for the electrophysiological response to 7-T by GRNs20 is an interesting question that remains to be investigated.
Notably, as fly GRNs often co-express >1 gustatory receptor21–23, and as detection of bitter compounds in flies may require multiple receptors25, 27, our data do not exclude the possibility that other gustatory receptors besides Gr32a are involved in the response to 7-T. Moreover, the fact that Gr32a is required for the suppression of male-male courtship by 7-T, but not by the full complement of male CHs present on oe+ target males (Fig. 3b), likely indicates the existence of additional, functionally redundant CH species (e.g., CH50328), and receptor(s) other than Gr32a5, that are involved in the suppression of male-male courtship (Supplementary Fig. 1, grey).
To test whether Gr32a+ GRNs directly mediate both of the behavioral effects of 7-T, or simply play an indirect, permissive role, we asked whether artificial activation of these GRNs would be sufficient to restore aggression and suppress courtship towards oe− target males. To do this, we ectopically expressed TRPV1, a mammalian capsaicin receptor29 previously used to activate Drosophila GRNs24, in Gr32a+ GRNs. We paired Gr32a-GAL4/UAS-TRPV1 tester males with oe− target males, and asked whether applying capsaicin to these target males restored the normal balance of social behaviors exhibited by the TRPV1-expressing testers. Indeed, the presence of capsaicin on oe− targets elicited aggression and suppressed courtship towards these targets by the Gr32a-GAL4/UAS-TRPV1 testers (Supplementary Fig. 10), although the magnitude of this behavioral rescue was much lower than that obtained using 7-T. Control tester lines carrying either Gr32a-GAL4 or UAS-TRPV1 did not respond to capsaicin (Supplementary Fig. 10). Thus, artificial activation of Gr32a+ GRNs in tester males partially mimicked the behavioral effects of 7-T. The incomplete penetrance of the capsaicin/TRPV1 manipulation likely reflects the expression of the Gr32a-GAL4 driver21 in only a subset of all Gr32a+ GRNs23 (M. H. and Kristin Scott, personal communications). However a requirement for activation of additional, Gr32a− GRNs to mimic a full behavioral response to 7-T, could also explain the difference. Whatever the case, the data suggest that 7-T is likely to act directly on Gr32a+ GRNs to exert its opposing effects on aggression and male-male courtship (Supplementary Fig. 1, green).
7-T and cVA hierarchically regulate male aggression
The observation that 7-T plays a key role in aggression raised the question of how it interacted with cVA, an olfactory pheromone that regulates the intensity of male-male aggression13. Exogenous, synthetic cVA did not promote aggression in Gr32a−/− males, although it did so in Gr32a+/− control flies and in Gr32a−/− genomic rescue flies (Fig. 3e). The inability of synthetic cVA to promote aggression in Gr32a−/− mutant flies was particularly striking in light of the fact that these mutants still showed residual (yet greatly reduced) levels of aggression (Fig. 3a). Therefore, the failure of synthetic cVA to promote aggression of Gr32a−/− mutant flies cannot be simply ascribed to the absence of this behavior per se. Rather, the results imply that Gr32a-mediated signaling is required for (i.e., gates) the aggression-promoting effect of cVA. In contrast, the male-female courtship-suppressing effect of cVA10, 30 was undiminished in Gr32a−/− mutant males (data not shown). Thus the inability of cVA to promote aggression in Gr32a−/− mutants does not simply reflect a general insensitivity to cVA caused by Gr32a mutation.
We then asked whether, conversely, cVA sensitivity was required for the ability of 7-T to restore aggression towards oe− targets. Or67dGAL4/GAL4 mutant males are anosmic to cVA10 and are insensitive to its aggression-promoting effect13. We paired them with oe− targets or oe− males perfumed with synthetic 7-T. Or67dGAL4/GAL4 mutant males and the control Or67d+/+ males showed comparable aggressive responses to 7-T (Fig. 3f). Similarly, ectopic expression of Kir2.1 in Or67d+ ORNs, which has been shown to eliminate the aggression-promoting effect of cVA13, did not interfere with the aggressive responses to 7-T (Supplementary Fig. 11). Thus, sensitivity to cVA is not required for the ability of 7-T to restore normal levels of aggression towards oe− males. This finding confirms and extends our previous observation that sensitivity to cVA is not required for normal levels of aggression between pairs of wild-type male flies13. Taken together, these data indicate that the aggression-promoting effect of cVA is dependent upon signaling through 7-T/Gr32a, but not vice versa, suggesting a hierarchical interaction between the gustatory and olfactory systems in regulating aggression (Supplementary Fig. 1, green and blue).
Or47b mutations suppress courtship toward oe− males
The foregoing observations raised the question of whether hierarchical interactions between the gustatory and olfactory systems might also regulate male-male courtship. Specifically, we investigated whether the elevated levels of male-male courtship caused by genetic depletion of male CHs might require detection of olfactory pheromones. Previous studies have identified two olfactory receptors, Or47b and Or88a, that respond to odors present on both males and females12. Or47b+ ORNs express FruM and project to a sexually dimorphic glomerulus, VA1lm (also known as VA1v)31, 32, suggesting a potential role in regulating social behaviors. Consistent with this idea, disruption of GABAergic signaling in Or47b+ ORNs has suggested a possible role for Or47b+ ORNs in the location of females by males33. We therefore investigated a possible role for Or47b in the elevated courtship exhibited towards oe− target males.
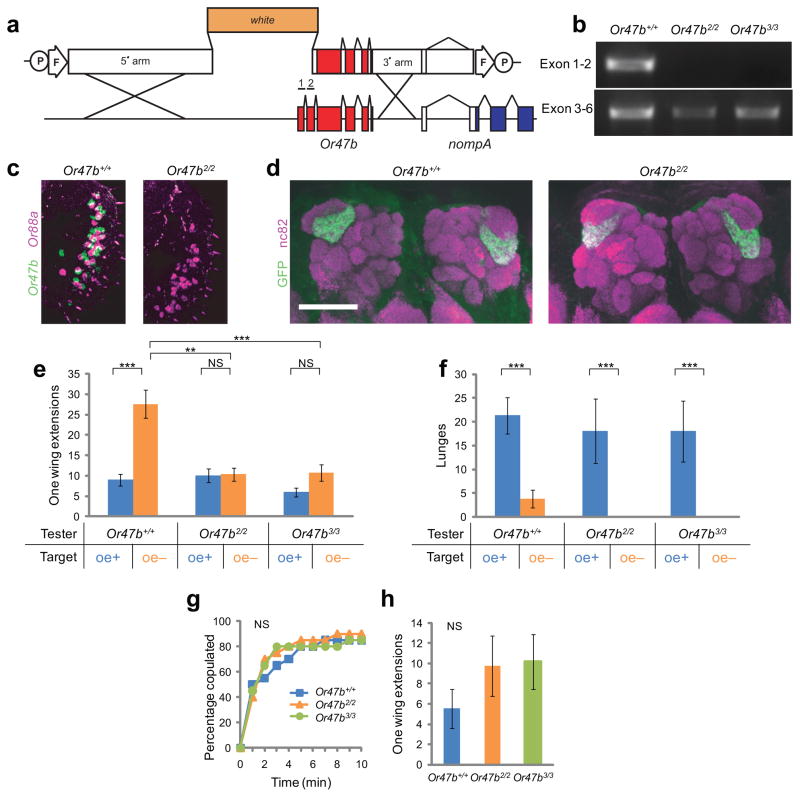
We examined two independent Or47b null alleles, Or47b2/2 and Or47b3/3, generated by homologous recombination (see Methods) (Fig. 4a, b). In situ hybridization confirmed the elimination of Or47b mRNA in the third antennal segment, where it is normally expressed (Fig. 4c). The projections of Or47b+ ORNs to the VA1lm/VA1v glomerulus31, 32 were unaffected by the mutation (Fig. 4d), indicating that lack of Or47b does not perturb proper targeting of these ORNs. Elimination of Or47b in tester males suppressed the elevated courtship exhibited towards oe− target males (Fig. 4e), but did not affect the level of aggression (Fig. 4f). To determine whether this phenotype reflected a general deficit in courtship behavior, we also tested these mutants in male-female interactions. Both Or47b2/2 and Or47b3/3 mutant males exhibited normal latencies to copulate with virgin females, compared to Or47b+/+ controls (Fig 4g). The frequency of unilateral wing extensions towards females also did not show a statistically significant difference among genotypes, although there was a trend, if anything, to a slightly higher level in the mutants (Fig. 4h).
Figure 4. Or47b is required for elevated male-male courtship caused by depletion of male CHs.
(a) Schematic illustration of the targeting construct (top) and site of homologous recombination (bottom) in Or47b locus. The flanking gene (nompA) included in the targeting construct is not disrupted. (b) PCR validation of two independently recovered mutant alleles lacking the first two exons of Or47b. (c) RNA in situ hybridization for Or47b (green) and Or88a (magenta). (d) Projections of Or47b+ ORNs to the VA1lm glomeruli visualized using Or47b-GAL4; UAS-mCD8GFP in flies of the indicated genotypes; nc82, neuropil counter-stain. Scale bar = 50 mm in (c, d). (e, f) Quantification of aggression (e) and courtship (f) performed by tester males of the indicated genotypes towards oe+ (blue) or oe− (orange) target males (n=20). (g, h) Cumulative latency to copulation (g; n=20) and unilateral wing extension frequency (h; n=17–18) by males of the indicated genotypes towards virgin females. NS: p>0.05, *p<0.05, **p<0.01 and ***p<0.001.
These data reveal that the increased levels of male-male courtship caused by genetic depletion of male CHs can be suppressed by a mutation in Or47b. This implies the existence of one or more male courtship-promoting cues detected by this receptor, whose influence is normally subordinate to the courtship-suppressing effects of male CHs6, 7 (Supplementary Fig. 1, green and red). It is also possible that the presence of male CHs suppresses the synthesis/release of pheromone(s) detected by Or47b. However this is an unlikely explanation given that odors from wild type males can activate Or47b12. The normal behavioral role of Or47b is not clear. Or47b and its unknown ligand may function in male-male behavior, e.g. by promoting social interactions that facilitate the detection of short-range chemosensory cues, such as 7-T. Effects of the Or47b mutation on male-female courtship have not yet been detected (J. D. L. and Leslie Vosshall, personal communications), but this receptor could play a role that is redundant with that of other olfactory (or non-chemosensory) cues (Fig. 4g, h).
DISCUSSION
The interplay between different chemosensory systems in the regulation of specific social behaviors is poorly understood. Here we provide evidence that non-volatile pheromones detected by the gustatory system dominantly control behavioral responses to olfactory cues that promote male-male social interactions. On the one hand, a male CH (specifically 7-T) is essential for the aggression-promoting influence of cVA; on the other hand, 7-T and other gustatory pheromones inhibit a courtship-promoting signaling pathway dependent upon Or47b.
The epistatic influences of male CHs on the behavioral effects of olfactory pheromones may be an indirect consequence of behavioral state changes, or may involve more direct sensory gating mechanisms (Supplementary Fig. 1, solid vs. dashed arrows). One way that male CHs could indirectly regulate the influence of olfactory pheromones is through a principal role in sex discrimination6 (Supplementary Fig. 1, dashed arrows). According to this view, courtship and aggression reflect behavioral states that are automatically engaged as a consequence of recognizing the opponent fly as female vs. male, respectively. Consistent with this idea, masculinization of CH profiles can alter sex-specific patterns of male-female social interactions9. However, in the case of male-male social interactions, while oenocyte ablation increases male-male courtship6 it does not fully eliminate male-male aggression (Fig. 1b, c). Since male aggression towards normal females almost never occurs9, this residual aggression implies that male testers still recognize oenocyte-ablated targets as male. Therefore, instances of male-male courtship exhibited towards CH-depleted targets do not necessarily imply sex mis-identification (unless such identification must be made repeatedly upon each social encounter). The functional significance of courtship displays in male-male social encounters in Drosophila remains to be understood. In some arthropod species, male-male mating plays a role in establishing dominance34, as it does in some human populations35.
The foregoing considerations suggest that the effects of CH-depletion on male-male social interactions, and sensitivity to olfactory pheromones, may not be an indirect consequence of behavioral state changes caused by impaired sex discrimination. In that case, these gustatory pheromones may regulate olfactory influences on these social behaviors via a more direct gating mechanism (Supplementary Fig. 1, solid green and black arrows). The circuit-level mechanisms by which such gating occurs will be an interesting topic for future investigation.
The chemosensory “logic” of male social interactions revealed here has some intriguing parallels in mice36. A mutation in TrpC2, which impairs the detection of pheromones by the vomeronasal organ (VNO37), results in both decreased male-male aggression and increased male-male courtship38, 39. By contrast, mutations affecting the main olfactory epithelium (MOE) reduce male-male aggression without increasing inter-male courtship40, 41. These phenotypes are similar to those caused by manipulating CH and cVA signaling, respectively, in Drosophila. This similarity suggests a division-of-labor between these two insect pheromonal systems that may be analogous to that between the accessory and main olfactory systems in vertebrates42. Major urinary proteins (MUPs) have recently been shown to function as murine aggression pheromones, and are detected by a subset of VNO neurons43. However their molecular receptor(s) remain to be identified. The identification and genetic manipulation of these and other pheromone-receptor pairs regulating aggression and courtship in mice should further clarify whether the hierarchical interactions revealed here represent a conserved “logic” for the chemosensory regulation of social behaviors.
METHODS
Fly stocks
Fly stocks were raised in vials containing standard fly medium made of yeast, corn and agar. The stocks were maintained in fly incubators at 25 °C and 60% humidity on a 12h:12h light-dark circle. In most cases, flies for behavioral assays were collected within 8 h after eclosion and were raised individually (“tester” males), or at 30 males/vial (“target” males) for 5–7 d before behavioral assays.
Canton-S flies were obtained from M. Heisenberg. Gr32a−/−, the genomic rescue strains, and the Gr32a-GAL4 strains4 were from T. M. and H. A.. The fly strains for the ablation of oenocytes were from J-C. B. and J. D. L.. The Or47b mutant alleles were generated and characterized by J. M. at L. Vosshall’s laboratory, and were backcrossed into the Canton-S background by J-C. B. and J. D. L..
Genetic elimination of male CHs
Ablation of male oenocytes6 was achieved by crossing male “+; PromE(800)-GAL4, tub-GAL80TS; +” flies to female “+; UAS-StingerII, UAS-hid/CyO; +” or female “+; UAS-StingerII; +” at 18 °C to generate oe− or oe+ male progeny, respectively. Adult male progeny were collected within 8 h after eclosion and kept at 25 °C for 1 d. Subsequently, both oe+ and oe− males were maintained at 30 °C during the daytime and at 25 °C during the nighttime for 3 more d. The flies were then maintained at 25 °C for 1–2 d before use. The expression of UAS-hid44 in oenocytes specifically in the adult stage (under the control of tub-GAL80TS)45 eliminates most if not all male CHs.
Behavioral assays
Unless otherwise indicated, the behavioral assays were performed using mixed fly pairs consisting of one single-housed “tester” male and one group-housed “target” male. As previously shown, group housing suppresses both aggression and courtship by male flies2, 46. Using a single-housed tester and a group-housed target male forces aggression and courtship to be conducted predominantly by the tester males. This uni-directional bias in the initiation of aggressive or courtship behaviors by the tester towards the target male facilitated analysis of the effects of experimental manipulations on the tester vs. the target.
To measure social interactions, one tester male and one target male were introduced into the behavior chamber (see below) by gentle aspiration. Their behavioral interactions were videotaped for 20 min and analyzed manually or by using custom CADABRA software2. To distinguish testers and targets, a blue dot was painted on the thorax of target males under CO2 anesthesia, 1–2 d before behavioral assays were performed.
The design of the behavior arena was adapted from previous reports2, 47. Briefly, a rectangular chamber (4 cm × 5 cm × 12 cm) was placed on top of an acrylic base. In the center of the base, a 1 cm × 1 cm × 0.5 cm hole was filled with apple juice-agar-sucrose medium, surrounded by a 0.5 cm wide border containing 1% agar medium. The behavior arena was illuminated by a ring-shaped fluorescent lamp. Videotaping was performed using a commercial camcorder (Sony DCR-HC38) placed on top of the arena.
For experiments involving synthetic cVA, the experimental design was as previously reported13. Briefly, before and during behavioral assays, a small piece of filter paper containing 5 μl solvent (acetone) or 5 μl solvent containing 500 μg cVA was placed at one corner of the behavior arena. Male flies of the indicated genotypes were housed at 10 flies/vial for 5–7 d before behavioral assays. A pair of male flies of identical genotype and age was introduced into the behavior arena and their behavioral interactions were recorded for 20 min and analyzed by using custom CADABRA software.
For courtship assays between males and females, virgin Canton-S females were group-housed (10 flies/vial) for 5–7 d before behavioral assays. One tester male fly and one virgin Canton-S female were introduced into the behavior arena and their behavioral interactions were recorded for 10 min. The latency to copulate and the occurrence of one wing extensions were scored if applicable.
Chemical synthesis of CH molecules
(Z)-7-pentacosene (7-P) was synthesized as follows. n-BuLi (2.5 M in hexanes, 8.86 ml, 22.2 mmol) was added drop-wise to a solution of 1-octyne (2.22 g, 20.2 mmol) in THF (100 ml) at 78 °C and the mixture was stirred at 78 °C for 30 min and at 0 °C for 30 min. The resulting solution was treated with a solution of 1-bromoheptadecane (5.10 g, 16.0 mmol) in THF (10 ml), tetrabutylammonium iodide (0.74 g, 2.02 mmol), and refluxed for 15 h. The reaction mixture was quenched with saturated aqueous NH4Cl and extracted with ether. The combined ether extracts were washed with brine, dried (MgSO4), and concentrated under vacuum. The residue was chromoatographed (hexanes) to give 7-pentacosyne as a colorless viscous oil (2.93 g, 42 %).
To a solution of 7-pentacosyne (0.920 g, 2.64 mmol) in hexanes (35 ml) was added quinoline (0.853 g, 6.60 mmol) and Lindlar catalyst (0.562 g). The resulting suspension was vigorously stirred under a hydrogen balloon for 2 h. The catalyst was filtered off through a pad of Celite. The solvent was evaporated and the residue was purified by flash chromatography (hexanes) to afford (Z)-7-pentacosene as a colorless oil (0.823 g, 89%) with greater than 98% purity by gas chromatography.
(Z)-7-tricosene (7-T, greater than 98% purity by gas chromatography) was synthesized from 1-octyne and 1-bromopentadecane employing a procedure analogous to that used to synthesize (Z)-7-pentacosene.
(Z,Z)-7,11-heptacosadiene (7,11-HD) was prepared employing a modification of published procedures48, 49. A solution of methylmagnesium bromide (3.0 M in ether, 2.11 ml, 6.34 mmol) was added drop-wise to a stirring suspension of (dppp)NiCl2 (1.56 g, 2.88 mmol) in benzene (40 ml) and the mixture was refluxed for 15 min. A solution of hexylmagnesium bromide (2.0 M in ether, 36.0 ml, 72.0 mmol) was added and most of the ether was removed by distillation under N2. Benzene (100 mL) and dihydropyran (9.09 g, 108 mmoL) were added and mixture was refluxed for 16 h. The reaction mixture was quenched with saturated aqueous NH4Cl and extracted with ether. The combined ether extracts were washed with brine, dried (MgSO4), and concentrated under vacuum. The residue was twice chromatographed (hexanes/EtOAc = 20/1 to 10/1), the second time on silica gel embedded with AgNO3 (10% w/w) to give (Z)-4-undecan-1-ol as a colorless viscous oil (0.675 g, 6 %).
A mixture of (Z)-4-undecan-1-ol (0.635 g, 3.73 mmol) and PCC (1.21 g, 5.60 mmol) was stirred in CH2Cl2 (10 ml) for 1 h. The suspension was diluted with ether, filtered through a pad of Celite, and concentrated under vacuum. The residue was chromoatographed (hexanes/EtOAc=10/1) to give (Z)-4-undecanal as a colorless oil (0.338 g, 54 %).
n-BuLi (2.5 M in hexanes, 0.63 ml, 1.57 mmol) was added drop-wise to a solution of hexadecyltriphenylphosphonium bromide (0.975 g, 1.72 mmol) in THF (30 ml) at −30 °C. The reaction mixture was allowed to warm to room temperature for 20 min, then cooled to −30 °C, HMPA (5 ml) was added, and cooled to −60 °C. A solution of (Z)-4-undecanal (0.240 g, 1.43 mmol) in THF (10 ml) was added drop-wise and the mixture was allowed to warm to room temperature. The reaction mixture was quenched with water and extracted with ether. The combined ether extracts were washed with brine, dried (MgSO4), and concentrated under vacuum. The residue was chromoatographed (hexanes) to give (Z, Z)-7,11-heptacosadiene as a colorless oil (0.361 g, 67%) with greater than 98% purity by gas chromatography.
Quantification of male CHs
The quantification method was adapted from earlier reports6, 14. Briefly, individual male flies were CO2 anesthetized and washed for 5 min in 25 μl of iso-octane containing 20 ng/μl of octadecane as an internal standard. The iso-octane extracts were analyzed by gas chromatography. 1 μl of each male CH extract was injected into a Hewlett-Packard 5890 II gas chromatograph coupled with a HP 5972 mass selective detector system. The injector was held at 300 °C and operated in splitless mode for 0.75 min after injection. A 30 m × 0.25 mm ID × 0.25 μm film thickness RTX-5MS column from Restek Corporation (Bellafonte, Pennsylvania, US) was operated with a flow of 0.9 ml/min helium corresponding to a linear velocity of 34.4 cm/sec. The oven temperature began at 55 °C for 1.5 min and was ramped at 40 °C/min to 135 °C and then at 25 °C/min to 235 °C and then at 3 °C/min to 275 °C where it was held for 1 min. Electron impact spectra (70 eV electron energy) were recorded from 50 to 550 m/z at a rate of 1.5 scans per sec. HP Chemstation G1701 BA version B.01.00 software was used to calculate the retention time, the total peak area, and the identity of each compound.
Perfuming of male flies with CH molecules
The procedure for perfuming live oe− males with male CHs was based on a passive transfer protocol adapted from a previous report7. Briefly, ten 5–6 d old oe− male flies were mixed with 100 oe+ males in small vials (~10 cm3). The vials were placed upside-down in a 25 °C incubator for 1 d before behavioral assays or gas chromatography. Such a protocol ensured that a wild type-equivalent amount of male CH molecules was transferred to individual oe− males. For behavioral assays, these oe− males were marked by a blue dot on the thorax, which could be used to sort them out from oe+ males without anesthesia. For gas chromatography, these males were instead marked by cutting off one wing before mixing.
The procedure for perfuming male flies with synthetic CH molecules was also adapted from a previous report6. Briefly, the compound of interest (2.5 μl for 7-T, 1 μl for 7-P and 1.5 μl for 7,11-HD, or no compound for control) was applied directly onto a small piece of filter paper in a 5 ml glass vial. Groups of 5–8 males were introduced into the vial by gentle aspiration, and vortexed twice at medium speed, each for 20 sec. The male flies were then transferred to fresh vials containing fly food. The vials were placed upside-down in the 25 °C incubator for 24 h before behavioral assays or gas chromatography. Such a protocol ensured that a wild type-equivalent amount of 7-T, 7-P or 7,11-HD was transferred to individual males.
To perfume different amounts of synthetic 7-T, an identical procedure was followed, except that the oe− males carrying synthetic 7-T were allowed to recover in vials for 6 h, 24 h, 72 h and 96 h before behavioral assays or gas chromatography, resulting in the oe− males carrying progressively smaller amounts of 7-T as a function of recovery time. Target males flies were used at a comparable age for behavioral assays or gas chromatography, independent of their post-transfer recovery times.
For experiments involving TRPV1, capsaicin (Sigma M2028) was dissolved in ethanol at 400mM, and subsequently diluted at 1:25 in acetone (adapted from ref. 24). 0.5 μl of this solution was carefully pipetted onto the abdomen of individual male flies under CO2 anesthesia. For control males, the same procedure was applied, except that no capsaicin was added in the ethanol:acetone solution. Flies were transferred back to vials and were allowed to recover for ~12 h before behavioral experiments.
Generation of Or47b mutant alleles
Mutants for Or47b were generated by homologous recombination, using the “ends-out” technique50, which replaces the exons of interest with a selectable marker, in this case the eye color pigmentation gene, white. Regions 5′ and 3′ of the gene were amplified by PCR from genomic DNA as follows:
5′ ARM 5.218 kb: Or47b.up-for and Or47b.up-rev
-
3′ ARM 3.369 kb: Or47b.dn-for and Or47b.dn-rev
Or47b.up-for TCGCTTTTCGGCTTGTCT Or47b.up-rev TTGCGATGGATGGATAGG Or47b.dn-for CACCCACTCGCAAATGAA Or47b.dn-rev CATTTTCACCGCAACCTG
Fragments were subcloned into the CM105 (S. Chen and G. Struhl) vector which contains two polylinkers flanking the mini-white gene with a unique I-SceI site 5′ of the white gene, flanked by FRT sites and containing conventional P element repeats. The 5′ arm was cloned into the AvrII site and the 3′ arm was cloned into the NotI site. The construct was designed to delete sequences containing the first two exons and 1kb of DNA upstream of the translation start site. Virgin female flies carrying one targeting construct were crossed to w118, 70FLP, 70I-SceI, Sco/CyO and 3-day-old progeny were heat shocked at 38oC for 60 min. Homozygous transgenic lines were created by standard techniques. To check that the targeted homologous recombination took place, PCR primers Or47b.2-for and Or47b.2-rev were used to amplify sequence containing the first 2 exons of the Or47b gene, which were deleted in the null mutant. Primers Or47b.3-for and Or47b.3-rev were used to amplify sequence containing the last 4 exons of the Or47b gene, which is intact in the Or47b null mutants.
| Or47b.2-for | CATGTGCAATGTGATGACCA |
| Or47b.2-rev | CGATGCAAAGCAACTTGAGA |
| Or47b.3-for | TCAAGTTGCTTTGCATCGAG |
| Or47b.3-rev | ATGCAAATGGCCAGAAAAAG |
Statistical analysis
Most of the behavioral data were non-parametrically distributed. Mann–Whitney U tests (for pair-wise comparisons) and Kruskal-Wallis analysis of variance (ANOVA) (for comparisons among >2 groups) were applied. Significant difference among groups detected by Kruskal-Wallis ANOVA was analyzed using Dunn’s post hoc test (with corrections for multiple comparisons) to identify groups with statistically significant differences. Two-way ANOVA was applied for comparisons among cumulative copulation latency curves.
Supplementary Material
Acknowledgments
We thank Anderson lab members for helpful discussions, G. Mancuso for administrative assistance, G. Mosconi for lab management, and K. Scott and L. Vosshall for critical comments on the manuscript. Assistance from Dr. Nathan F. Dalleska and use of GC-MS instrumentation in the Environmental Analysis Center at the California Institute of Technology is gratefully acknowledged. We thank L. Vosshall for generously providing the Or47b mutant alleles generated by J.M. in her laboratory. D.J.A. is an investigator of the Howard Hughes Medical Institute.
Footnotes
AUTHOR CONTRIBUTIONS
L.W. carried out the experiments and performed the data analysis. L.W. and D.J.A. together conceived the research and wrote the manuscript. X.H. synthesized CH molecules. J. M. generated and characterized Or47b mutant alleles. M. H. characterized the expression of Gr32a-GAL4. J-C.B., T.M., H.A. and J.D.L. contributed fly strains.
References
- 1.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 2.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krstic D, Boll W, Noll M. Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE. 2009;4:e4457. doi: 10.1371/journal.pone.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 7.Savarit F, Sureau G, Cobb M, Ferveur JF. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc Natl Acad Sci USA. 1999;96:9015–9020. doi: 10.1073/pnas.96.16.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 9.Fernández MP, et al. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 11.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupp JJ, et al. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 15.Everaerts C, Farine JP, Cobb M, Ferveur J-Fo. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE. 2010;5:e9607. doi: 10.1371/journal.pone.0009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jallon JM. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet. 1984;14:441–478. doi: 10.1007/BF01065444. [DOI] [PubMed] [Google Scholar]
- 17.Butterworth FM. Lipids of Drosophila: a newly detected lipid in the male. Science. 1969;21:1356–1357. doi: 10.1126/science.163.3873.1356. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dow MA, von Schilcher F. Aggression and mating success in Drosophila melanogaster. Nature. 1975;254:511–512. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- 20.Lacaille F, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marella S, et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koganezawa M, Haba D, Matsuo T, Yamamoto D. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr Biol. 2010;20:1–8. doi: 10.1016/j.cub.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yew JY, et al. A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol. 2009;19:1245–1254. doi: 10.1016/j.cub.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 30.Ejima A, et al. Generalization of Courtship Learning in Drosophila Is Mediated by cis-Vaccenyl Acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 32.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Root CM, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Issa FA, Edwards DH. Ritualized submission and the reduction of aggression in an invertebrate. Curr Biol. 2006;16:2217–2221. doi: 10.1016/j.cub.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 35.Wolff N, Jing S. Contextualization of physical and sexual assault in male prisons: incidents and their aftermath. J Correct Health Care. 2009;15:58–77. doi: 10.1177/1078345808326622. quiz 80–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 37.Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 38.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 39.Leypold BG, et al. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 41.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 42.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 43.Chamero P, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, et al. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad USA. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci USA. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 48.Wenkert E, Ferreira VF, Michelotti EL, Tingoli M. Synthesis of acyclic, cis olefinic pheromones by way of nickel-catalyzed Grignard reactions. J Org Chem. 1985;50:719–921. [Google Scholar]
- 49.Davis TL, Carlson DA. Synthesis of 7,11-dienes from enol ether and grignard reagents under nickel catalysis: sex pheromones of Drosophila melanogaster. Synthesis. 1989;12:936–938. [Google Scholar]
- 50.Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad USA. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.