Abstract
Cancer cells don’t exist as pure homogeneous populations in vivo. Instead they are embedded in “cancer cell nests” that are surrounded by stromal cells, especially cancer associated fibroblasts. Thus, it is not unreasonable to suspect that stromal fibroblasts could influence the metabolism of adjacent cancer cells, and visa versa. In accordance with this idea, we have recently proposed that the Warburg effect in cancer cells may be due to culturing cancer cells by themselves, out of their normal stromal context or tumor microenvironment. In fact, when cancer cells are co-cultured with fibroblasts, then cancer cells increase their mitochondrial mass, while fibroblasts lose their mitochondria. An in depth analysis of this phenomenon reveals that aggressive cancer cells are “parasites” that use oxidative stress as a “weapon” to extract nutrients from surrounding stromal cells. Oxidative stress in fibroblasts induces the autophagic destruction of mitochondria, by mitophagy. Then, stromal cells are forced to undergo aerobic glycolysis, and produce energy-rich nutrients (such as lactate and ketones) to “feed” cancer cells. This mechanism would allow cancer cells to seed anywhere, without blood vessels as a food source, as they could simply induce oxidative stress wherever they go, explaining how cancer cells survive during metastasis. We suggest that stromal catabolism, via autophagy and mitophagy, fuels the anabolic growth of tumor cells, promoting tumor progression and metastasis. We have previously termed this new paradigm “The Autophagic Tumor Stroma Model of Cancer Metabolism”, or the “Reverse Warburg Effect”. We also discuss how glutamine addiction (glutaminolysis) in cancer cells fits well with this new model, by promoting oxidative mitochondrial metabolism in aggressive cancer cells.
Keywords: caveolin-1, autophagy, cancer, tumor stroma, tumor microenvironment, recycled nutrients, metabolic coupling, glutamine addiction, ammonia, glutaminolysis
1. Caveolin-1 and Cancer Biomarker Studies
Caveolins are a family of scaffolding proteins that function in endocytosis, signal transduction, and cholesterol transport [1]. Caveolins 1 and 2 are ubiquitously expressed, while the expression of caveolin-3 is muscle-specific [1, 2]. In adipocytes, endothelial cells, and fibroblasts, caveolin-1 (Cav-1) plays a prominent role as an inhibitor of eNOS and iNOS, via interactions with the caveolin-scaffolding domain [2].
We have recently identified a loss of stromal Cav-1 as single independent predictor of clinical outcome in human breast cancer patients. More specifically, we see that a loss of stromal Cav-1 (in cancer associated fibroblasts) strictly correlates with early tumor recurrence, lymph node metastasis, increased tumor stage, tamoxifen-resistance, and overall poor clinical outcome [3, 4]. Importantly, the predictive value of a loss of stromal Cav-1 is independent of epithelial marker status, indicating that it is a useful biomarker in all the most common epithelial sub-types of human breast cancer, including ER+, PR+, HER2+, and triple-negative breast cancers. In fact, in triple negative breast cancers, patients with high stromal Cav-1 show an overall survival rate of >75.5 at 12 years post-diagnosis [5]. In contrast, triple negative patients with a loss of stromal Cav-1, have an overall survival rate of <10% at 5 years post-diagnosis. Similar results were obtained with DCIS patients, where a loss of stromal Cav-1 was predictive of disease recurrence and progression to invasive ductal carcinoma [6].
Furthermore, a loss of stromal Cav-1 in prostate cancer patients correlated with advanced prostate cancer and the presence of metastatic disease, as well as high Gleason score, another indicator of poor prognosis [7]. Thus, a loss of stromal Cav-1, in the cancer associated fibroblast compartment, may be a universal or widely-applicable biomarker for a host of different epithelial tumor types.
2. The Warburg Effect, PET Scanning, and the Tumor Stroma
Otto Warburg described mouse ascites cancer cells as having increased glycolysis and lactate production, compared to normal mouse liver and kidney cells in the presence of oxygen (for review see [8, 9]). This has been termed the Warburg effect or aerobic glycolysis. Studies have confirmed that aerobic glycolysis is a major contributor to total ATP production in certain types of cancer cells cultured under high oxygen conditions [10–13]. However, other cancer cell bioenergetic studies have shown that the tricarboxylic acid cycle (TCA) and oxidative phosphorylation are the most important pathways for ATP generation and aerobic glycolysis contributes much less to ATP generation [14, 15].
It is felt that the Warburg effect has important implications for cancer diagnosis and treatment. For example, PET scanning in cancer evaluation is thought to be useful because cancer cells have increased aerobic glycolysis and increased 2 deoxyglucose uptake. However, there is discordance between tumor PET avidity and cancer cell metabolism. Clear cell renal carcinoma is frequently associated with increased aerobic glycolysis, VHL gene mutations, HIF1a activation and increased glucose uptake. Despite increased aerobic glycolysis in clear renal carcinoma cells, PET scanning is not clinically useful in this disease with a substantial number of tumors being PET negative [16, 17]. In Hodgkin’s lymphoma, PET scanning is routinely used for disease evaluation and yet the neoplastic lymphoma cells are only a very small proportion of the tumor cells with the rest of the tumor being inflammatory and stromal cells [18]. It is also well known that inflammatory states can lead to false positive PET results when evaluating for cancer [19] [20]. These observations indicate that although PET scanning is effective in cancer staging, the basis for its diagnostic effectiveness may be related to the tumor stroma in some cancers.
In summary, although the traditional view of cancer metabolism is that cells are undergoing aerobic glycolysis, it has been shown that cancer cells have a broad spectrum of bioenergetic states ranging from predominance of aerobic glycolysis to predominance of oxidative phosphorylation. Since studies had not been carried out to our knowledge analyzing the bioenergetic state of the tumor microenvironment and differences between the microenvironment and the cancer epithelial cells we set out to further characterize this.
3. Mechanistic Studies on Cancer Metabolism and Autophagy in the Tumor Stroma
In order to begin to understand why a loss of stromal Cav-1 is a strong indicator of a lethal tumor microenvironment, we performed unbiased proteomic analysis on mesenchymal stem cells derived from WT and Cav-1 (−/−) deficient mice. As a result of this analysis, we showed that a loss of stromal Cav-1 expression upregulates the protein expression of i) 8 myofibroblast markers (such as vimentin, calponin, and collagen), ii) 8 glycolytic enzymes (LDHA and PKM2, as well as PGK1 and TPI), and iii) 2 anti-oxidants which are markers of oxidative stress (catalase and peroxiredoxin1) [21]. Thus, based on this and other unbiased transcriptional profiling studies, we proposed that a loss of stromal Cav-1 in cancer associated fibroblasts is associated with ROS (reactive oxygen species) production, and oxidative stress. This, in turn, is sufficient to activate certain key transcription factors, such as HIF1-alpha and NFkB, leading to the induction of aerobic glycolysis in cancer associated fibroblasts, under normoxic conditions (Figure 1). We then proceeded to validate this hypothesis, using a co-culture system employing MCF7 breast cancer cells and fibroblasts which harbor either NFkB-luc or HIF1a-luc transcriptional reporters [22].
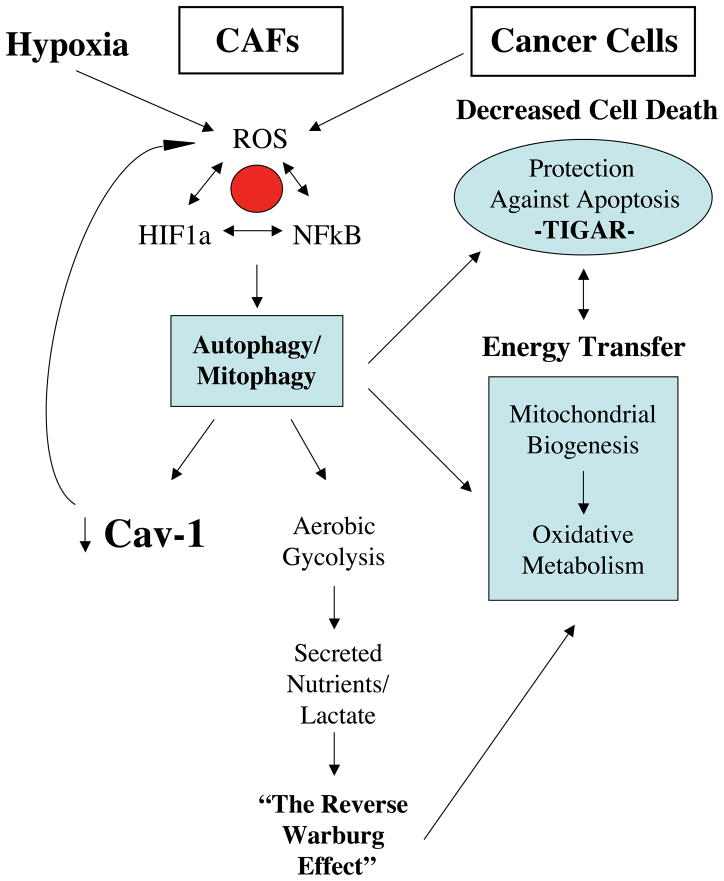
Figure 1. The Autophagic Tumor Stroma Model of Cancer.
In this model, cancer cells use oxidative stress as a “weapon” to extract recycled nutrients from cancer associated fibroblasts, via the induction of autophagy. Stromal autophagy, in turn, provides energy-rich recycled nutrients (such as lacatate, ketones, and glutamine) to fuel oxidative mitochondrial metabolism in cancer cells. Hypoxia, HIF1, and NFkB activation help drive autophagy in the tumor micronenvironment, while the upregulation of TIGAR (TP53-induced glycolysis and apoptosis regulator) in cancer cells protects them against apoptosis and confers autophagy resistance. TIGAR is a known inhibitor of both autophagy and apoptosis, and functionally shifts cells away from aerobic glycolysis, towards oxidative mitochondrial metabolism. [24–27]. This scenario allows for the vectorial and unilateral transfer of energy from the tumor stroma (catabolism) to cancer cells, thereby fueling anabolic tumor growth via oxidative mitochondrial metabolism in cancer cells. Modified with permission from [22, 23]. CAFs, cancer associated fibroblasts; ROS, reactive oxygen species.
Thus, based on the above studies, we proposed a new mechanism for understanding tumor metabolism with metabolic coupling between cancer cells and the cancer stroma. We proposed that in tumors with stromal Cav-1 downregulation, cancer cells induce oxidative stress in adjacent cancer associated fibroblasts leading to the onset of aerobic glycolysis in fibroblasts, which then drives the production of excess lactate and/or pyruvate. These high-energy metabolites could then be transferred to adjacent cancer cells where they enter the TCA cycle, resulting in increased oxidative phosphorylation and efficient ATP production. This effectively results in a new parasitic form of stromal-epithelial metabolic coupling, where fibroblasts directly feed cancer cells via the production of glycolytic intermediates. Secretion and re-uptake of lactate or pyruvate would be mediated by the monocarboxylate family of transporters, such as MCT1/4. We have previously termed this new paradigm “The Reverse Warburg Effect”, which is just the opposite of the conventional Warburg effect, in which cancer cells are thought to undergo aerobic glycolysis. Importantly, fibroblast oxidative stress leads to increased DNA damage and random mutagenesis in cancer cells [22, 23]. Interestingly, ROS makes cancer cells more aggressive with apoptosis resistance. We have shown that fibroblasts and high ROS lead to upregulation of the antiapoptotic protein TIGAR (TP53-induced glycolysis and apoptosis regulator) [22] (Figure 2). TIGAR is a known inhibitor of both autophagy and apoptosis, and functionally shifts cells away from aerobic glycolysis, towards oxidative mitochondrial metabolism. [24–27]. Other complementary studies have also shown that hypoxic and normoxic cancer cells develop a symbiotic relationship with transfer of lactate to normoxic cancer cells, thereby increasing oxidative phosphorylation in normoxic cancer cells [28].
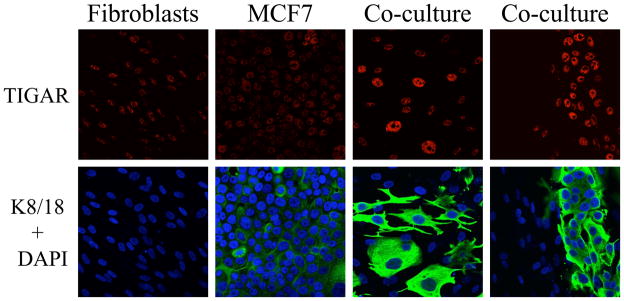
Figure 2. Fibroblasts Induce the Upregulation of TIGAR in Cancer Cells, Thereby Protecting Cancer Cells Against Apoptosis and Autophagy.
Fibroblasts or breast cancer cells (MCF7), were cultured separately or co-cultured. Note the fibroblast-induced upregulation of TIGAR selectively in the cancer cells, protects cancer cells against apoptosis and autophagy (shown in red). Cancer cells are labeled with anti-keratin antibodies (shown in green). All cell nuclei are also labeled (shown in blue), to allow visualization of the keratin-negative fibroblasts. Modified with permission from [22, 23]. TIGAR is a known inhibitor of both autophagy and apoptosis, and functionally shifts cells away from aerobic glycolysis, towards oxidative mitochondrial metabolism. [24–27].
In further support of this hypothesis, co-culture of cancer cells with fibroblasts induces dramatic changes in mitochondrial mass. Importantly, when cultured under homotypic conditions, cancer cells show very low mitochondrial mass (the conventional Warburg Effect) (Figure 3). However, co-culture with fibroblasts, which more closely mirrors the microenvironment of a naturally occurring tumor, promotes a very significant increase in mitochondrial mass in cancer cells, suggesting that the Warburg effect might be an in vitro artifact [23]. Importantly, lactate administration to homotypic cancer cell cultures similarly induces a significant increase in mitochondrial mass, suggesting that lactate administration (Figure 3) phenocopies the presence of reactive fibroblasts [23]. Our new data indicate that cancer cells and CAFs develop a parasitic relationship, with the unilateral energy transfer from glycolytic stromal cells to oxidative cancer cells
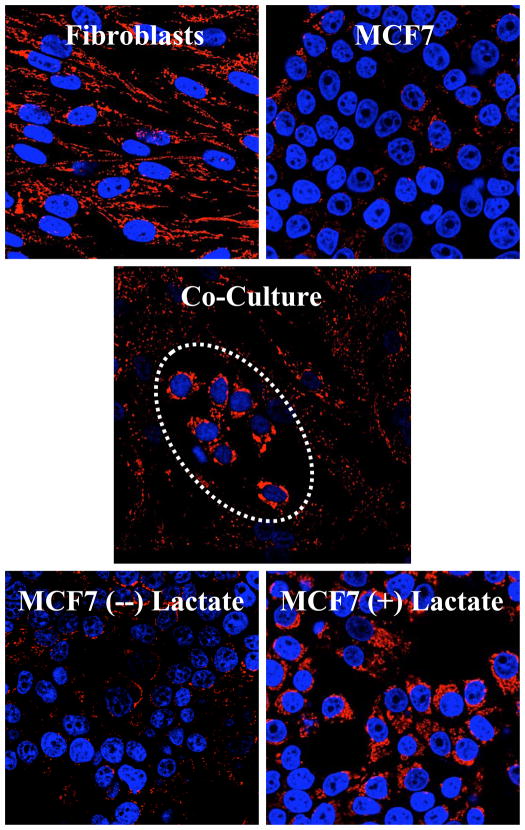
Figure 3. Visualizing the “Reverse Warburg Effect”.
Autophagy/mitophagy in fibroblasts promotes mitochondrial biogenesis in adjacent cancer cells.
(Upper) Homotypic cultures of MCF7 cells and hTERT-fibroblasts were immunostained with a mitochondrial membrane antibody (Red). Mitochondrial mass is lower in monocultures of MCF7 cells, as compared to fibroblasts.
(Middle) Co-culture with fibroblasts induces a significant increase in mitochondrial mass in the “central MCF7 cell colony”, encircled by a white oval. Conversely, mitochondrial mass decreases in co-cultured fibroblasts.
(Lower) Lactate treatment increases mitochondria mass in MCF7 cells, thus simulating the co-culture with fibroblasts. Reproduced with permission from Figures 9 and 12, in the following reference [23].
To better understand how a loss of Cav-1 occurs in cancer associated fibroblasts, we performed a series of inhibitor studies, which indicated that under conditions of oxidative stress, Cav-1 is targeted for autophagic/lysosomal degradation. More specifically, the degradation of Cav-1 was efficiently blocked using either, anti-oxidants (N-acetyl cysteine, metformin, or quercetin) or lysosomal inhibitors (chloroquine), directly implicating autophagy in this process. We also showed that Cav-1 downregulation in normal fibroblasts by RNA interference was sufficient to induce autophagy and mitophagy as seen by upregulation of the autophagy and mitophagy markers LC3A/B, ATG16L, BNIP3 and BNIP3L. Virtually identical results were also obtained simply by culturing fibroblasts under hypoxic conditions (see Figure 4). These results indicate that a loss of Cav-1 and/or hypoxia are indeed sufficient to confer the cancer-associated fibroblast phenotype.
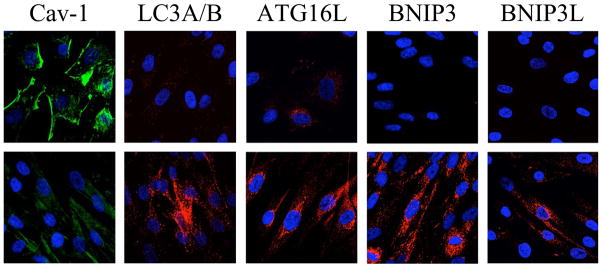
Figure 4. Hypoxia Induces a Loss of Stromal Caveolin-1 via Autophagy.
Fibroblasts were cultured under conditions of normoxia (upper panels) or hypoxia (lower panels). Note that hypoxia (lower panels) induces a loss of Cav-1 (shown in green), and the upregulation of autophagy markers (LC3, ATG16L, BNIP3, and BNIP3L; shown in red). Thus, hypoxia is sufficient to confer the cancer associated fibroblast phenotype. Modified with permission from [22, 23].
So, we believe that cancer cells induce oxidative stress in adjacent fibroblasts, which in turn leads to the induction of the autophagic program, via activation of HIF1a and NFkB. During autophagy, both caveolae (marked by Cav-1) and mitochodria are destroyed by lysosomal degradation, leading to the production of recycled nutrients to feed cancer cells. This also promotes the onset of aerobic glycolysis in cancer associated fibroblasts, via mitochondrial dysfunction. We have termed this new idea “The Autophagic Tumor Stroma Model of Cancer Metabolism”.
4. Autophagic Fibroblasts Promote Tumor Growth In Vivo, Independently of Angiogenesis
To further genetically validate this new hypothesis, we created constitutively autophagic fibroblasts, by recombinantly over-expressing a mutationally activated form of HIF1-alpha. As predicted, fibroblasts expressing activated HIF1a showed i) a loss of Cav-1, and ii) and a shift towards aerobic glycolysis, as evidenced by a loss of mitochondrial activity and increased lactate production. In this context, activated HIF1a also induced BNIP3 and BNIP3L, both well-known markers of mitophagy (the autophagic destruction of mitochondria). Interestingly, fibroblasts harboring activated HIF1a increased tumor growth by ~3-fold, without any increase in tumor vascularization. In this xenograft system, HIF1a-fibroblasts were co-injected with MDA-MB-231 cells, a human triple negative breast cancer cell line. Conversely, expression of activated HIF1a in MDA-MB-231 cells had just the opposite effect, resulting in a 3-fold reduction in tumor growth [29]. This may be due to the induction of apoptosis in epithelial cancer cells that harbor activated HIF1a.
In summary, we believe that autophagy in cancer associated fibroblasts fuels tumor growth, via the production of recycled nutrients, while autophagy directly within tumor cells retards tumor growth (likely via epithelial cell apoptosis).
Importantly, these studies are also directly supported by in vivo data obtained by the transcriptional profiling of human breast cancer tumor stroma. These results show that “The Reverse Warburg Effect” strictly correlates with tumor recurrence and metastasis, and is related to oxidative stress, DNA damage, and hypoxia in the tumor stroma of breast cancer patients [30–32].
Clinically, both autophagy inducers and autophagy inhibitors are effective anti-cancer therapies, and this has been referred to as the autophagy paradox. The inducers of autophagy, temsirolimus and everolimus are effective therapies for renal cell carcinoma [33, 34] and gliomas [35] used in clinical practice. Chloroquine which inhibits autophagy has shown prolongation in survival in patients with glioblastoma multiforme [36] and is being currently studied in clinical trials in many other malignancies. How can we explain this paradox. Using our new model, the systemic induction of autophagy will prevent epithelial cancer cells from using recycled nutrients, while the systemic inhibiton of autophagy will prevent stromal cells from producing recycled nutrients---both effectively “starving” cancer cells. Thus, energy transfer from the tumor stroma to cancer cells can be used to explain the “Autophagy Paradox”.
5. Glutamine Addiction, Ammonia Production, and Autophagy
In further support of our assertions that cancer cells use oxidative mitochondrial metabolism, many independent sources have reported that cancer cells are addicted to glutamine. In this regard, glutamine is then converted to glutamate, which then enters the TCA cycle as alpha-ketoglutarate, resulting in the high efficiency production of ATP via oxidative phosphorylation [9].
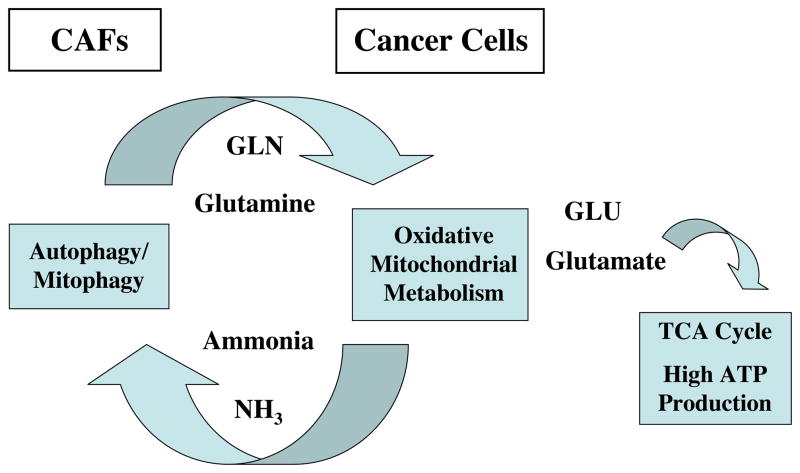
Interestingly, a by-product of glutaminolysis is ammonia. Recent studies have shown that this diffusible by-product can act as a potent inducer of autophagy [37–39]. Thus, the possible implications of these findings are that the glutamine addiction in cancer cells provides another related means for maintaining autophagy in the tumor microenvironment. Here, we speculate that glutamine is normally a product of autophagy occurring in cancer associated fibroblasts (Figure 5). In accordance with this hypothesis, we have previously shown that a loss of Cav-1 in the tumor microenvironment is indeed sufficient to induce autophagy, resulting in increased levels of glutamine in the stromal microenvironment [32].
Figure 5. Mitochondrial Glutamine Metabolism in Cancer Cells Provides Another Related Mechanism for Maintaining Autophagy in the Tumor Stroma.
Recent studies have shown that oxidative mitochondrial metabolism of glutamine in cancer cells produces ammonia. Ammonia is known to be sufficient to induce autophagy. Here, we propose that autophagy in cancer associated fibroblasts would provide cancer cells with an abundant source of glutamine. The ammonia produced would, in turn, help to maintain the autophagic phenotype of the cancer associated fibroblasts. CAFs, cancer associated fibroblasts.
This idea then provides the “kindling” for a new vicious cycle whereby autophagy in the tumor microenvironment provides glutamine to cancer cells, and the by-product of this de-amination reaction, ammonia, helps to maintain the autophagic production of glutamine. This model is consistent with our current findings and would also fit well with “The Autophagic Tumor Stroma Model of Cancer Metabolism”, that we have proposed [32] in which energy-rich recycled nutrients (lactate, ketones, and glutamine) fuel oxidative mitochondrial metabolism in cancer cells.
6. Cancer Cells are “Extracellular” Parasites: Parallels with Intracellular Parasites
Independent studies with infectious “intracellular” parasites (Plasmodium, Toxoplasma gondii, Trypanosoma cruzi) indicates that these parasites survive by inducing oxidative stress in infected host cells, resulting in the onset of an autophagic phenotype [40–47]. This then provides host-derived recycled nutrients to feed these intracellular parasites. Similarly, we have shown that cancer cells use essentially the same mechanism (oxidative stress and autophagy), to generate host-derived recycled nutrients, behaving as “extracellular” parasites.
Thus, it is perhaps not surprising that chloroquine, which is a well-known anti-malarial drug and an autophagy inhibitor, is also a very potent anti-tumor agent. As such, both anti-oxidants and autophagy inhibitors could be systematically developed as anti-cancer agents to directly uncouple the parasitic metabolic relationship between cancer cells and the tumor stromal microenvironment. This would provide promising new strategies for novel cancer chemotherapies.
Acknowledgments
M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-080250; R01-CA-098779; R01-CA-120876; R01-AR-055660), and the Susan G. Komen Breast Cancer Foundation. F.S. was supported by grants from the W.W. Smith Charitable Trust, the Breast Cancer Alliance (BCA), and a Research Scholar Grant from the American Cancer Society (ACS). R.G.P. was supported by grants from the NIH/NCI (R01-CA-70896, R01-CA-75503, R01-CA-86072, and R01-CA-107382) and the Dr. Ralph and Marian C. Falk Medical Research Trust. The Kimmel Cancer Center was supported by the NIH/NCI Cancer Center Core grant P30-CA-56036 (to R.G.P.). Funds were also contributed by the Margaret Q. Landenberger Research Foundation (to M.P.L.). This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This work was also supported, in part, by a Centre grant in Manchester from Breakthrough Breast Cancer in the U.K. (to A.H.) and an Advanced ERC Grant from the European Research Council.
Dr. M.P. Lisanti would especially like to thank Drs. James D. Watson, Craig B. Thompson, Lewis C. Cantley, Chi V. Dang, Mathew Vander Heiden, Joan Brugge, Tak W. Mak Eileen White, Karen Vousden, and Arnold Levine, for their feedback, thoughtful comments, and helpful suggestions.
Footnotes
Recently presented as a lecture at the Cold Spring Harbor Laboratory (CSHL; Banbury Center) Meeting on “Energy Metabolism, the Cell Cycle, and Cancer” on October 31 – November 3, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 2.Mercier I, Jasmin JF, Pavlides S, Minetti C, Flomenberg N, Pestell RG, Frank PG, Sotgia F, Lisanti MP. Clinical and translational implications of the caveolin gene family: lessons from mouse models and human genetic disorders. Lab Invest. 2009;89:614–623. doi: 10.1038/labinvest.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, Kleer CG, Brody JR, Lisanti MP. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, Henderson MA, Fanelli MA, Cuello-Carrion FD, Gago FE, Anderson RL. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, Sotgia F, Brody JR, Mitchell EP, Lisanti MP. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, Pestell RG, Sotgia F, Rui H, Lisanti MP. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1071–1079. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
- 7.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 9.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olavarria JS, Chico E, Gimenez-Gallego G, Nunez de Castro I. Effect of ammonium ions on the aerobic glycolysis in Ehrlich ascites tumor cells. Biochimie. 1981;63:469–475. doi: 10.1016/s0300-9084(81)80079-x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt H, Siems W, Muller M, Dumdey R, Rapoport SM. ATP-producing and consuming processes of Ehrlich mouse ascites tumor cells in proliferating and resting phases. Exp Cell Res. 1991;194:122–127. doi: 10.1016/0014-4827(91)90140-p. [DOI] [PubMed] [Google Scholar]
- 12.Balaban RS, Bader JP. Studies on the relationship between glycolysis and (Na+ + K+)-ATPase in cultured cells. Biochim Biophys Acta. 1984;804:419–426. doi: 10.1016/0167-4889(84)90069-7. [DOI] [PubMed] [Google Scholar]
- 13.Medina MA, Sanchez-Jimenez F, Marquez FJ, Perez-Rodriguez J, Quesada AR, Nunez de Castro I. Glutamine and glucose as energy substrates for Ehrlich ascites tumour cells. Biochem Int. 1988;16:339–347. [PubMed] [Google Scholar]
- 14.Kallinowski F, Vaupel P, Runkel S, Berg G, Fortmeyer HP, Baessler KH, Wagner K, Mueller-Klieser W, Walenta S. Glucose uptake, lactate release, ketone body turnover, metabolic micromilieu, and pH distributions in human breast cancer xenografts in nude rats. Cancer Res. 1988;48:7264–7272. [PubMed] [Google Scholar]
- 15.Guppy M, Leedman P, Zu X, Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J. 2002;364:309–315. doi: 10.1042/bj3640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadvar H, Kherbache HM, Pinski JK, Conti PS. Diagnostic role of [F-18]-FDG positron emission tomography in restaging renal cell carcinoma. Clin Nephrol. 2003;60:395–400. doi: 10.5414/cnp60395. [DOI] [PubMed] [Google Scholar]
- 17.Bouchelouche K, Oehr P. Positron emission tomography and positron emission tomography/computerized tomography of urological malignancies: an update review. J Urol. 2008;179:34–45. doi: 10.1016/j.juro.2007.08.176. [DOI] [PubMed] [Google Scholar]
- 18.Brepoels L, Stroobants S. PET scanning and prognosis in Hodgkin’s lymphoma. Curr Opin Oncol. 2008;20:509–516. doi: 10.1097/CCO.0b013e32830b88d3. [DOI] [PubMed] [Google Scholar]
- 19.Lim JW, Tang CL, Keng GH. False positive F-18 fluorodeoxyglucose combined PET/CT scans from suture granuloma and chronic inflammation: report of two cases and review of literature. Ann Acad Med Singapore. 2005;34:457–460. [PubMed] [Google Scholar]
- 20.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- 21.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Outschoorn TCUE, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Caro J, Lisanti MP, Sotgia F. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. Embo J. 2009;28:3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Jogl G. Structural and biochemical studies of TIGAR (TP53-induced glycolysis and apoptosis regulator) J Biol Chem. 2009;284:1748–1754. doi: 10.1074/jbc.M807821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 27.Green DR, Chipuk JE. p53 and metabolism: Inside the TIGAR. Cell. 2006;126:30–32. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.W-MD, Chiavarina B, Migneco G, Martinez Outschoorn UE, Pavlides S, Howell A, Tanowitz H, Casimiro MC, Wang C, Pestell RG, Grieshaber P, Caro J, Sotgia F, Lisanti MP. HIF 1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells. Cell Cycle. 2010;9:3534–3551. doi: 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling”. Aging (Albany NY) 2010;2:185–199. doi: 10.18632/aging.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: A transcriptional informatics analysis with validation. Cell Cycle. 2010;9 doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 32.Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP. The autophagic tumor stroma model of cancer: Role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9:3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 34.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 35.Krueger CMDA, Holland K. Everolimus for subependymal giant cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 36.Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 37.Eng CH, Abraham RT. Glutaminolysis yields a metabolic by-product that stimulates autophagy. Autophagy. 2010;6:968–970. doi: 10.4161/auto.6.7.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- 39.Marino G, Kroemer G. Ammonia: a diffusible factor released by proliferating cells that induces autophagy. Sci Signal. 2010;3:pe19. doi: 10.1126/scisignal.3124pe19. [DOI] [PubMed] [Google Scholar]
- 40.Andrade BB, Reis-Filho A, Souza-Neto SM, Raffaele-Netto I, Camargo LM, Barral A, Barral-Netto M. Plasma superoxide dismutase-1 as a surrogate marker of vivax malaria severity. PLoS Negl Trop Dis. 2010;4:e650. doi: 10.1371/journal.pntd.0000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dey S, Guha M, Alam A, Goyal M, Bindu S, Pal C, Maity P, Mitra K, Bandyopadhyay U. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic Biol Med. 2009;46:271–281. doi: 10.1016/j.freeradbiomed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 42.Elsheikha HM, El-Motayam MH, Abouel-Nour MF, Morsy AT. Oxidative stress and immune-suppression in Toxoplasma gondii positive blood donors: implications for safe blood transfusion. J Egypt Soc Parasitol. 2009;39:421–428. [PubMed] [Google Scholar]
- 43.Karaman U, Celik T, Kiran TR, Colak C, Daldal NU. Malondialdehyde, glutathione, and nitric oxide levels in Toxoplasma gondii seropositive patients. Korean J Parasitol. 2008;46:293–295. doi: 10.3347/kjp.2008.46.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen JJ, Gupta S, Guan Z, Dhiman M, Condon D, Lui C, Garg NJ. Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic Rats. J Am Coll Cardiol. 2010;55:2499–2508. doi: 10.1016/j.jacc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romano PS, Arboit MA, Vazquez CL, Colombo MI. The autophagic pathway is a key component in the lysosomal dependent entry of Trypanosoma cruzi into the host cell. Autophagy. 2009;5:6–18. doi: 10.4161/auto.5.1.7160. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Karnataki A, Parsons M, Weiss LM, Orlofsky A. 3-Methyladenine blocks Toxoplasma gondii division prior to centrosome replication. Mol Biochem Parasitol. 2010;173:142–153. doi: 10.1016/j.molbiopara.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Totino PR, Daniel-Ribeiro CT, Corte-Real S, de Fatima Ferreira-da-Cruz M. Plasmodium falciparum: erythrocytic stages die by autophagic-like cell death under drug pressure. Exp Parasitol. 2008;118:478–486. doi: 10.1016/j.exppara.2007.10.017. [DOI] [PubMed] [Google Scholar]