Abstract
BACKGROUND
The use of plasma-based resuscitation for trauma patients in hemorrhagic shock has been associated with a decrease in mortality. While some have proposed a beneficial effect through replacement of coagulation proteins, the putative mechanisms of protection afforded by plasma are unknown. We have previously shown in a cell culture model that plasma decreases endothelial cell permeability compared to crystalloid. The endothelial glycocalyx consists of proteoglycans and glycoproteins attached to a syndecan backbone, which together protect the underlying endothelium. We hypothesize that endothelial cell protection by plasma is due, in part, to its restoration of the endothelial glycocalyx and preservation of syndecan-1 after hemorrhagic shock.
METHODS
Rats were subjected to hemorrhagic shock to a mean arterial blood pressure of 30 mmHg for 90 minutes followed by resuscitation with either lactated Ringer’s solution (LR) or fresh plasma to a mean arterial blood pressure of 80 mm Hg and compared to shams or shock alone. After two hours, lungs were harvested for syndecan mRNA, immunostained with anti-syndecan-1, or stained with hematoxylin and eosin. To specifically examine the effect of plasma on the endothelium, small bowel mesentery was infused with a lanthanum-based solution, venules identified, and the glycocalyx visualized by electron microscopy. All data are presented as mean ±SEM. Results were analyzed by one-way ANOVA with Tukey post hoc tests.
RESULTS
Electron microscopy revealed degradation of the glycocalyx after hemorrhagic shock which was partially restored by plasma but not LR. Pulmonary syndecan-1 mRNA expression was higher in animals resuscitated with plasma (2.76 ± 0.03) compared to shock alone (1.39 ± 0.22) or LR (0.82 ± 0.03) and correlated with cell surface syndecan-1 immunostaining. Shock also resulted in significant lung injury by histopathology scoring (1.63 ± 0.26) which was mitigated by resuscitation with plasma (0.67 ± 0.17) but not LR (2.0 ± 0.25).
CONCLUSION
The protective effects of plasma may be due in part to its ability to restore the endothelial glycocalyx and preserve syndecan-1 after hemorrhagic shock.
INTRODUCTION
Data from both military and civilian studies have associated significant survival benefit after massive transfusion with resuscitation of high ratio plasma to red blood cells (≤ 1:2 plasma:RBCs).1–5 This change in resuscitation strategy centers on the early and increased use of plasma and has led to an increase in early survival, though the mechanism of protection is unknown. The purpose of the current study was to investigate the role of plasma on the endothelial glycocalyx after hemorrhagic shock.
The endothelial glycocalyx is a complex network of soluble components that projects from the cell surface of the endothelium into the vessel lumen.6 It consists of proteoglycans and glycoproteins attached to the cell membrane. The proteoglycans provide the structural support for the glycocalyx and consist of a core protein, either syndecans or glypicans, to which the glycosaminoglycans attach. Syndecans are the major source of heparan sulfate proteoglycans for all cell types. Endothelial cell adhesion molecules, primarily the selectins and immunoglobulin superfamily (ICAMs), are the major glycoproteins of the glycocalyx and play a key role in pathologic neutrophil-endothelial cell interactions that occur with injury to the glycocalyx.7 The glycocalyx lines the entire endothelium and its preservation has been implicated in multiple disease states. Other glycoproteins are important to coagulation, fibrinolysis, and hemostasis.
There is a dynamic equilibrium between the soluble components of the glycocalyx and the plasma component of blood. The area of the vessel lumen encompassed by the glycocalyx prohibits erythrocytes and leukocytes from interacting with the vessel wall and importantly reduces the flow of plasma, thus promoting plasma-endothelial cell interaction.8–10 We therefore hypothesized that the endothelial glycocalyx is injured after hemorrhagic shock and that resuscitation with plasma aids in restoring the glycocalyx. Injury to the endothelial glycocalyx has been demonstrated in laboratory models of ischemia/reperfusion but has not been investigated after hemorrhagic shock.11,12 This study now demonstrates for the first time that the endothelial glycocalyx is indeed injured after hemorrhagic shock and partially repaired by plasma compared to lactated Ringer’s solution (LR) resuscitation.
METHODS
Animal model of hemorrhagic shock
All procedures performed were protocols approved by the University of Texas Houston Medical School Animal Welfare Committee. The experiments were conducted in compliance with the National Institutes of Health (NIH) guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle with access to food and water ad libitum.
Male Sprague-Dawley rats weighing 200 to 300 g were fasted overnight with free access to water. Under isoflurane anesthesia, animals were placed on a heating blanket to maintain body temperature of 35°C to 37°C. Femoral arterial and venous catheters (Instech, Plymouth Meeting, PA) were placed then flushed with 0.3 mL of 1% heparin. No further heparin was used. The catheters were then connected to the corresponding fluid reservoir and blood pressure monitor (BPA-400; Micro-Med, Louisville, KY).
A pressure-controlled model of shock was used as previously described.13 Rats were bled to a mean arterial blood pressure (MAP) of 30 mmHg for 90 min then resuscitated with either LR or fresh plasma to a MAP of 80 mm Hg and compared to shams or shock alone (with no resuscitation) (n=5/group). Shams underwent anesthesia and placement of catheters but were not subjected to hemorrhagic shock. Five animals were used for each group. A computer-controlled, low-flow peristaltic pump (model P720; Instech) was connected to the venous cannula through which blood was withdrawn and resuscitation fluids were administered.14 Shed blood and resuscitation fluids were held in separate reservoirs placed on a balance with fluid weights recorded every 5 seconds using a LabVIEW program (National Instruments, Austin, TX). Fluid volume was calculated from its weight based on measured fluid density. After 2 hrs the animals were killed by exsanguination and lungs harvested. For animals resuscitated with plasma, a separate set (n=5) of Sprague-Dawley rats was used to obtain fresh plasma. Blood was obtained from an abdominal aortic puncture through a midline laparotomy. Blood was centrifuged at 500g for 10mins at 4°C to obtain plasma. Plasma was then warmed to 37°C then administered through the venous catheter.
Electron microscopy
Electron microscopy was performed on post capillary venules obtained from the small bowel mesentery in a separate set of animals using the same groups as described above (at least two/group). The small bowel mesentery was chosen as an easily accessible organ to perfuse and isolate vessels to study the endothelium. At the conclusion of resuscitation, the superior mesenteric artery was cannulated then infused with a fixative/staining solution of 2% lanthanum, 2% glutaraldehyde, 2% sucrose and 0.1 M sodium cacodylate buffer.15 Lanthanum is a trivalent cation that binds to negatively charged glycoprotein moieties of the glycocalyx.16 A section of mesentery ten cm from the ileocecal valve and adjacent to the bowel was excised and processed for electron microscopy following established protocols except for the addition of a 2% osmium tetroxide / 2% lanthanum staining before dehydration and embedding. Venules were identified using a JEOL 1200 II electron microscope in sections poststained with uranyl acetate and lead citrate for contrast. Electron microscope magnifications varied accordingly in order to frame differently sized venule images squarely onto either a Gatan 1k × 1k or TVIP F215 2k × 2k CCD. Images recorded at different magnifications were then normalized to the same magnification by scaling images to the same pixel size using the proc2d program within the EMAN image analysis software package.17
Quantitative real-time reverse-transcription polymerase chain reaction
Total RNA was extracted from lung tissue from each animal using TRI reagent (Sigma, St. Louis). Equal amount of RNA was taken from each sample then 1000-ng RNA from each group was reverse transcribed in a 20-µl reaction using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The real-time polymerase chain reaction (PCR) was performed in 20 µl of a reaction mixture containing 50ng cDNA (20ng/µl), 10 µl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems, Branchburg, New Jersey) 6 µL distilled water and 1µl of a primer and probe mixture which located at 4–5 exon boundary of syndecan-1 (Mm00448920_g1, Applied Biosystems). Actin expression (actin probe, Mm00607939_s1, Applied Biosystems) was used as the internal reference. Real-time PCR assays were performed in triplicate using Applied Biosystems Step-One-plus real-time PCR system (Applied Biosystems) with the following program: 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 seconds, and at 60°C for 1 min. A sequence detection program calculated the threshold cycle number (CT) at which the probe cleavage–generated fluorescence exceeded the background signal.18 Relative RNA expression levels were calculated using a comparative CT method. The set of gene primer and probe for syndecan-1was confirmed to have amplification efficiency equal to that of the internal reference gene. The relative expression level of syndecan-1 was normalized to that reference gene and to a calibrator sample that was run on the same plate.19 The normalized relative expression levels were automatically calculated using commercial software (StepOne Software V2.1, Applied Biosystems).
Cell surface syndecan-1 expression
Lungs were harvested and sections fixed with 4% formaldehyde then embedded in paraffin and sectioned. At least two sections from separate animals in each group were immunostained at the same setting with 1:200 rat antisyndecan-1 monoclonal antibody (BD, PharmingenTM) then incubated with 1:500 Alexa Fluor goat anti-rat IgG (H+L, Invitrogen). Images were obtained using an Olympus 1X71 microscope with SimplePCI6 software. The relative fluorescence intensity was quantified using Image J software (NIH) and reported as relative fluorescence units (RFU). A negative control was used as a technical control for staining without using primary antibody.
Histopathology
Lungs were harvested and sections fixed with 4% formaldehyde then embedded in paraffin and sectioned. At least two sections from separate animals in each group were stained with hematoxylin and eosin then scored using a three-point scale based on alveolar thickness, capillary congestion, and cellularity:20 alveolar wall’s thickness, 0 = normally thin alveolar walls; 1 = mild thickening; 2 = clearly thickened walls and; 3 = thickening of the wall, with 50–100% extremely thick. For capillary congestion of the alveolar wall: 0 = exhibiting a normal red cell density; 1 = mildly congested; 2 = moderately congested; 3 = severely congested. For cellularity of the alveolar wall: 0 = normal variable but small number of cells; 1 = mild increase in cellularity; 2 = mild to moderately increased cell number in 50–75% the section; and 3 = an increase in cellularity of all alveolar walls. The overall lung injury score was calculated by averaging the three indices of injury.
Statistical analysis
All data are presented as mean ±SEM, with significance at p<0.05. Results were analyzed by one-way ANOVA with Tukey post hoc tests, n= 5/group. Means notated with letters indicate statistical difference between groups.
RESULTS
Hemorrhagic Shock Model
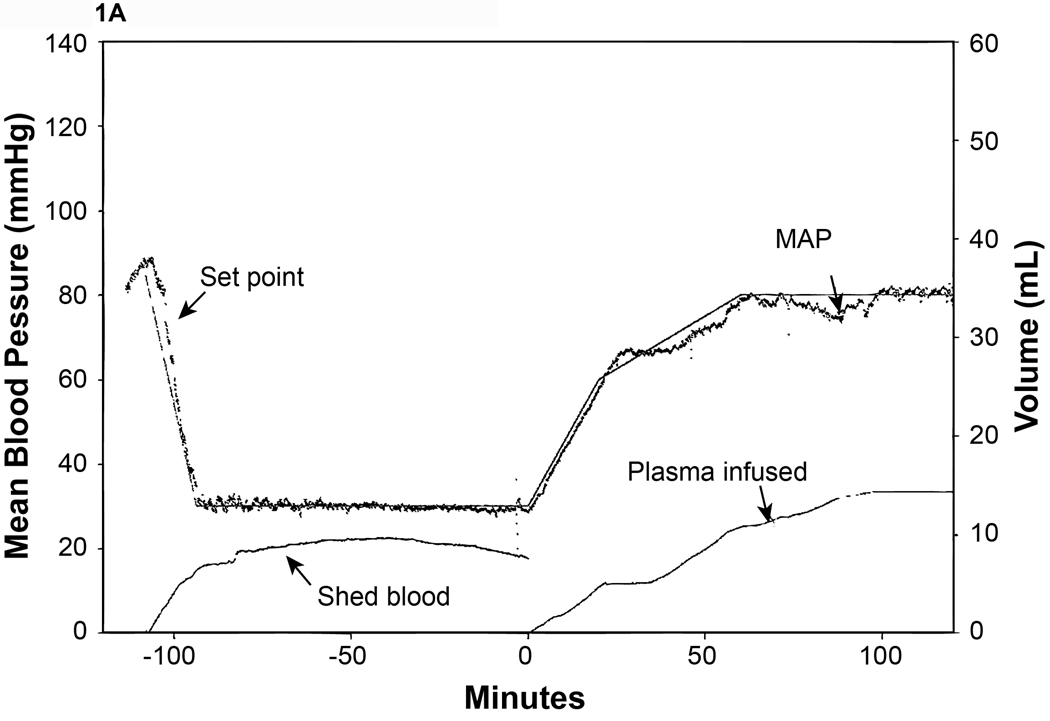
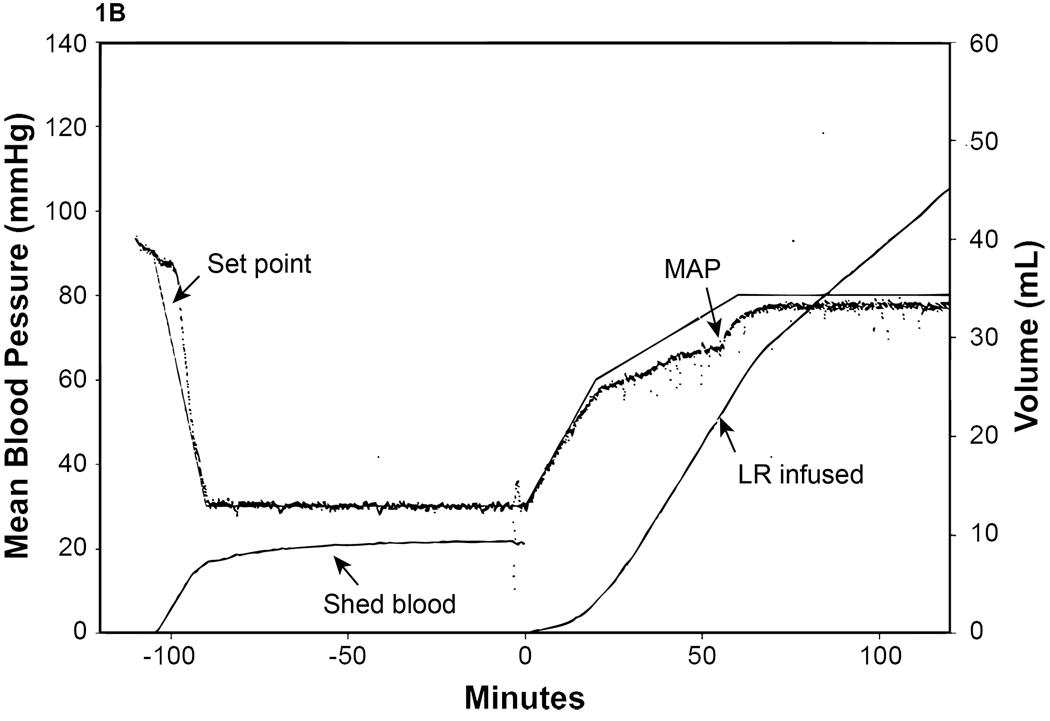
All experimental groups had approximately 50% of total blood volume withdrawn during the shock period (50.2%–53.8%) to achieve a MAP= 30 mm Hg. Shams had no blood withdrawn. The plasma group required significantly less volume (29.5 ±2.8 mls/kg/hr) to maintain the MAP during resuscitation compared to the LR group (73.9 ±10.0 mls/kg/hr) (Fig. 1A and 1B).
Figure 1. Mean arterial blood pressure (MAP) response to hemorrhagic shock.
MAP and fluid infusion from representative animals are shown. Animals were attached to the computerized pressure-controlled system then bled to a MAP of 30 mmHg for 90 minutes and resuscitated with either A.) plasma or B.) lactated Ringer’s solution (LR) to a MAP of 80 mm Hg. The set point (the target MAP) was calculated by using a LabVIEW program based on our target MAP of 30 mm Hg. The recorded MAP was compared with the set point and the computer converted the difference as a electric signal to drive a low-flow peristaltic pump through which blood was withdraw and/or fluid was administered.
Glycocalyx is partially restored by plasma resuscitation
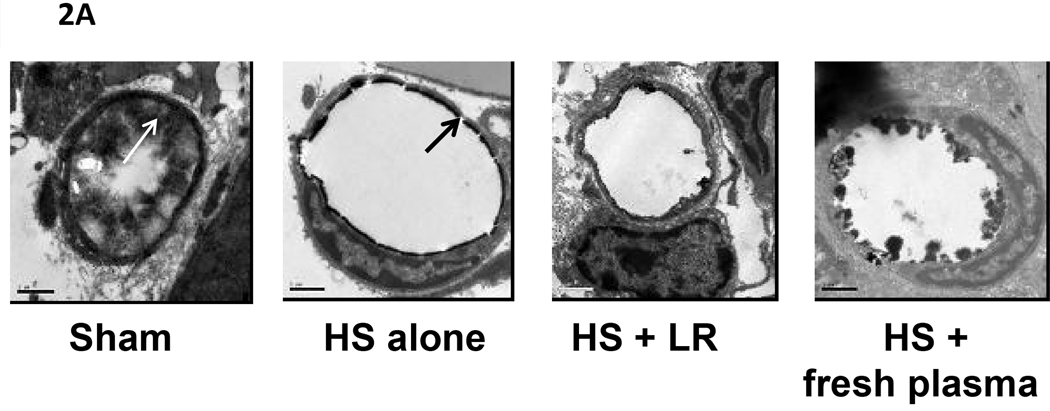
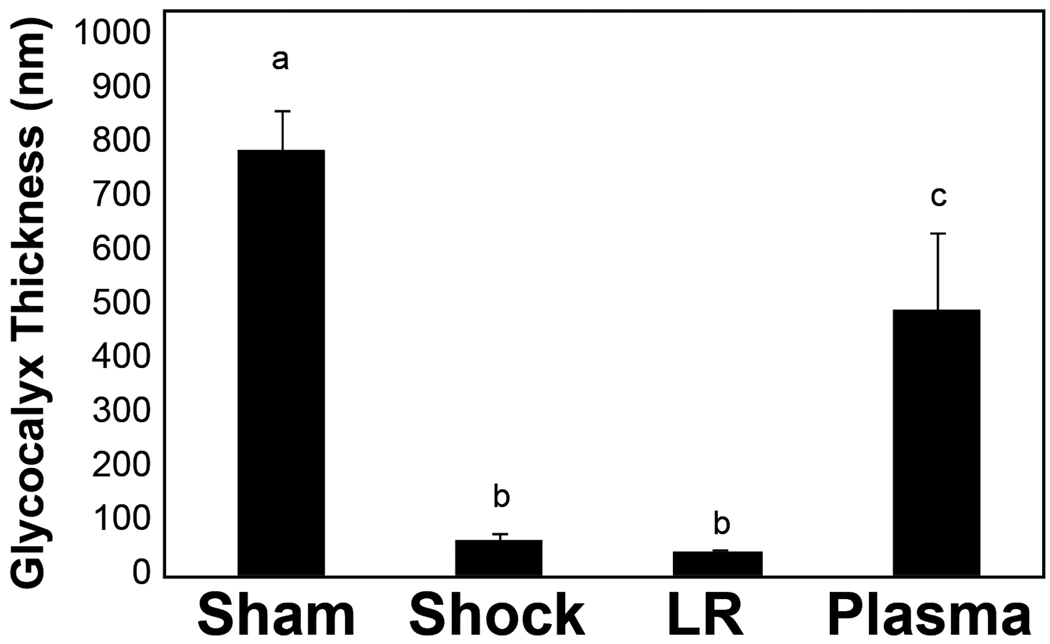
Electron microscopic images (Figure 2) of the glycocalyx are shown in Figure 2A and the quantitative data in Figure 2B. Images revealed that shams had an intact glycocalyx which was virtually ablated by hemorrhagic shock. LR resuscitation did not result in repair while plasma resuscitation demonstrated early signs of restoration of the glycocalyx by two hours of resuscitation. Sham versus shock, p<0.001; sham versus LR p=<0.001; sham versus plasma p= 0.01; shock versus plasma p<0.001; LR versus plasma p<0.001.
Figure 2. Electron microscopic (EM) images of rat mesenteric venules stained to reveal the endothelial glycocalyx.
Rats were subjected to hemorrhagic shock (HS) alone or resuscitation with either lactated Ringer’s solution (LR) or plasma and compared to shams. After 2 hours of resuscitation, small bowel mesentery was isolated, the superior mesenteric artery cannulated and infused with a fixative/staining solution to visualize the glycocalyx. Venules were identified and sections poststained with uranyl acetate and lead citrate for contrast. A. Magnification is 20,000× at 0.6 CCD demagnification. Representative images are shown from sham (A), shock (B), LR (C), and plasma (D) resuscitated groups. The bar represents 1 micron. The black arrows indicate the cell membrane and the white arrows the glycocalyx. B. Using the correct pixel size, the boxer program in the EMAN image processing suite was used to measure the thickness of the glycocalyx layer around the circumference of vessel. Means notated with letters indicate statistical difference between groups. Sham versus shock, p<0.001; sham versus LR p=<0.001; sham versus plasma p= 0.01; shock versus plasma p=0.001; LR versus plasma p<0.001.
Syndecan-1 expression in lung is enhanced by plasma
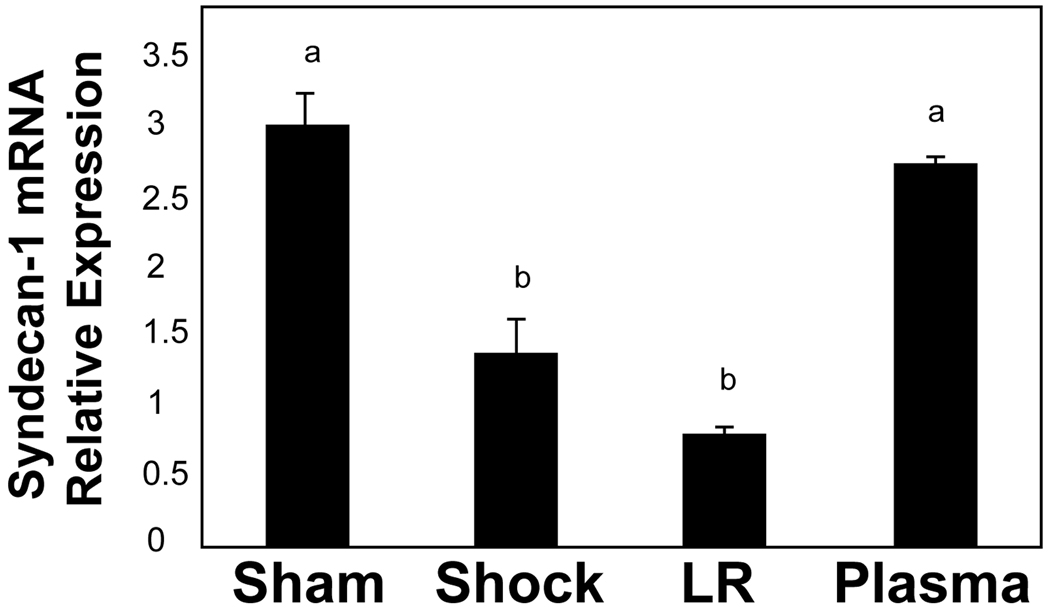
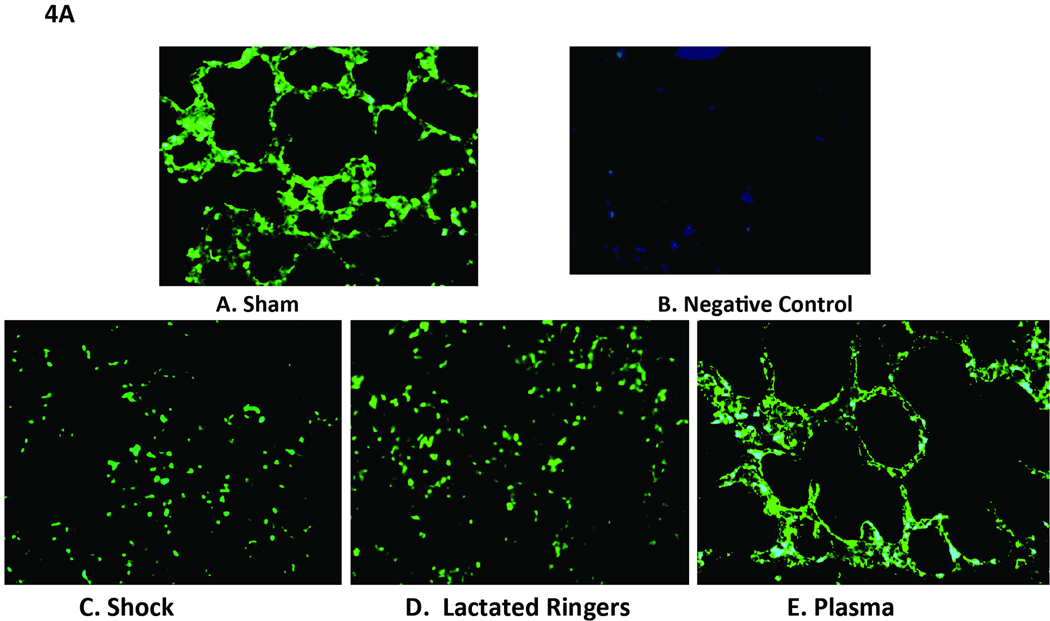
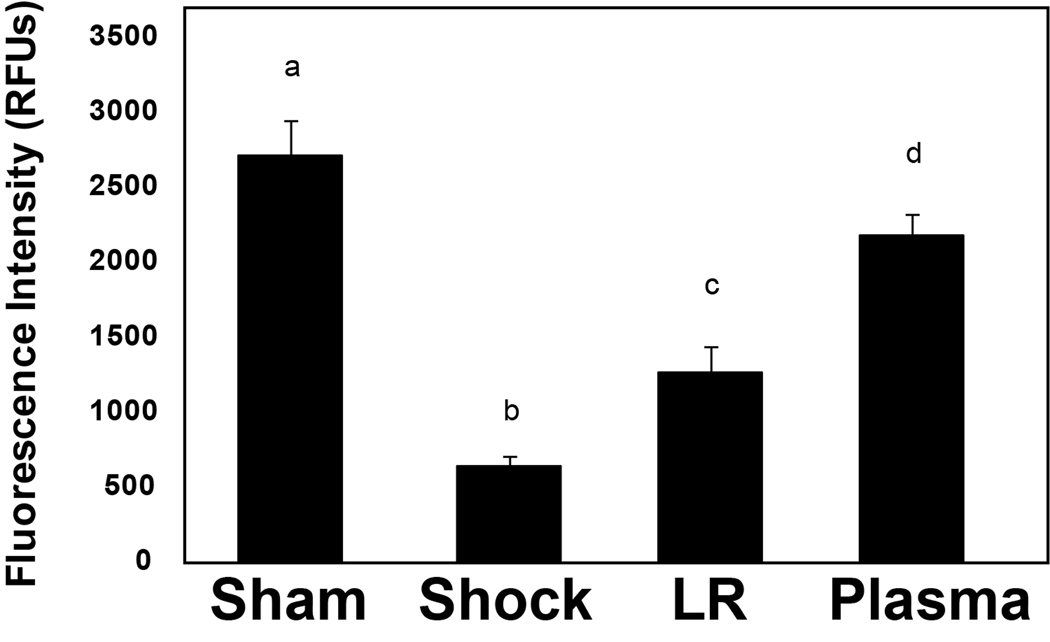
Compared to shams (3.03 ± 0.22), the relative expression level of lung syndecan-1 mRNA was significantly reduced by shock alone (1.39 ± 0.22, p=0.02) and even more so by LR (0.82 ± 0.03, p<0.001) but enhanced by plasma resuscitation (2.76 ± 0.03), back to sham levels. Both the shock (p=0.03) and LR (p<0.001) groups had significantly less mRNA than the plasma group, suggesting that plasma restores the backbone of the endothelial glycocalyx (Figure 3). Similar findings were demonstrated after tissue immunostaining and were consistent with plasma restoration of syndecan-1 (Figure 4A and B). Both sham (2707 ± 226 RFU) and plasma (2172 ± 146 RFU) had significantly more syndecan-1 staining than either shock (624 ± 72 RFU, p<0.001 sham versus shock and p<0.001 plasma versus shock) or LR (1262 ± 173 RFU, p=0.002 sham versus LR and p=0.014 plasma versus LR).
Figure 3. Syndecan-1 mRNA in lung.
Rats were subjected to hemorrhagic shock (HS) alone or two hours of resuscitation with either lactated Ringer’s solution (LR) or plasma and compared to shams. mRNA was extracted from lung tissue and analyzed by quantitative real-time polymerase chain reaction (PCR). The relative expression level of syndecan-1 was normalized to a reference gene and to a calibrator sample that was run on the same plate.18 Compared to shams, lung syndecan mRNA was significantly reduced by shock alone, further decreased by LR, but enhanced by plasma resuscitation , suggesting that plasma restores the backbone of the endothelial glycocalyx. Means notated with letters indicate statistical difference between groups. Sham versus shock, p=0.02; sham versus LR p=<0.001; shock versus plasma p=0.03; LR versus plasma p<0.001.
Figure 4. Cell surface syndecan-1 expression in pulmonary alveolar cells.
After hemorrhagic shock and resuscitation, lungs were immunostained with antisyndecan-1 monoclonal antibodies (magnification = original × 200). The negative control is a technical control for staining without using primary antibody. Immunostaining revealed that cell surface syndecan-1 is expressed abundantly in sham animals (A) compared to negative controls (B) but expression is markedly decreased after hemorrhagic shock (C), and by lactated Ringer’s solution (D), but further enhanced by plasma (E) resuscitation. Sham versus shock, p<0.001; sham versus LR, p =0.002; shock versus plasma, p<0.001; LR versus plasma, p=0.014.
Lung injury is lessened by plasma resuscitation
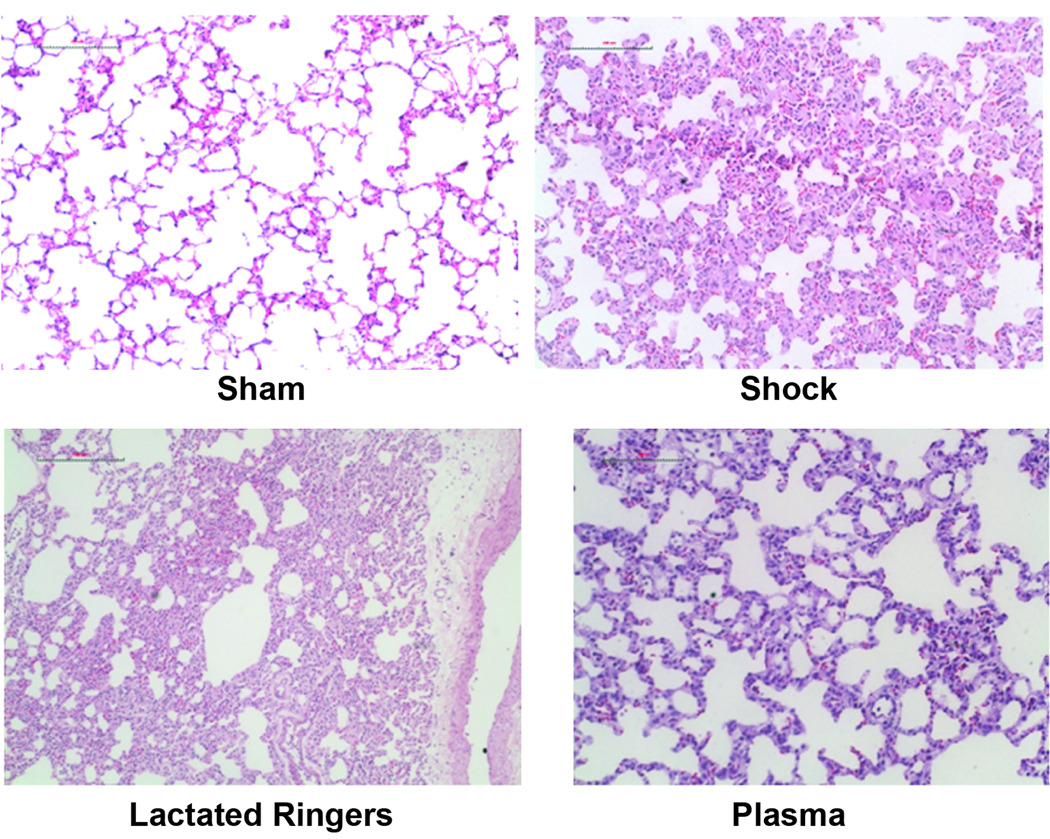
Shock animals had significantly increased lung injury, as assessed by alveolar wall thickness, capillary congestion, and cellularity. Compared to shams, shock and LR resuscitation worsened lung injury while injury was attenuated by plasma (Table 1 and Figure 5).
Table 1.
Lung histopathologic injury score.
| Group | Alveolar Thickness | Capillary Congestion | Cellularity | Overall Score |
|---|---|---|---|---|
| Sham | 0.3±0.19 | 0.2±0.19 | 0.1±0.09 | 0.20 ± 0.01 a |
| Shock | 1.5±0.45 | 1.7±0.52 | 1.4±0.48 | 1.63 ± 0.26 b |
| LR | 2.3±0.64 | 1.9±0.33 | 1.8±0.34 | 2.00 ± 0.25 b |
| Plasma | 0.4±0.18 | 0.9±0.33 | 0.7±0.38 | 0.67 ± 0.17 c |
LR= lactated Ringer’s solution
Histopathologic injury is based on alveolar wall thickness, capillary congestion, and cellularity using a score from 0–3, with 0 being the lowest and 3 the highest injury score. The overall lung injury score was calculated by averaging the three indices of injury.
sham versus shock, p<0.001; sham versus LR, p<0.001; sham versus plasma, p=0.02; shock versus plasma, p=0.004; LR versus plasma, p<0.001.
Figure 5. Lung histopathology.
After hemorrhagic shock and resuscitation for two hours, lungs were stained with hematoxylin and eosin and sections scored based on alveolar thickness, capillary congestion, and cellularity. Injury scores are shown in Table 1. Representative sections are shown for sham (A), shock (B), lactated Ringer’s solution (C), and plasma (D) resuscitated animals. The bar represents 100µm.
DISCUSSION
In this study we demonstrated for the first time that hemorrhagic shock injures the endothelial glycocalyx which is partially restored by plasma but not LR resuscitation. Similarly, pulmonar y syndecan-1 mRNA and alveolar cell surface syndecan-1 expression were higher in animals resuscitated with plasma compared to LR and correlated with reduced lung injury.
Syndecan is the major cell membrane protein of the glycocalyx. Its extracellular domain is substituted with heparan sulfate chains and promotes interaction with plasma proteins.21 Syndecans are expressed on virtually every cell in the body and there are four members (syndecan 1–4) that comprise the syndecan family.22 Syndecan-1 has been detected in patients immediately after aortic surgery and we have published preliminary data demonstrating that it is shed in patients after hemorrhagic shock.15,23
Injury to syndecan-1 and the glycocalyx
Injury to syndecan-1 and the glycocalyx has important implications. The glycocalyx serves as an effective barrier to leukocyte-endothelial cell and platelet adhesion by providing steric hindrance between receptor and ligand, thereby blocking adhesion to the underlying endothelium.24 Processes which degrade the glycocalyx, such as cytokines, tumor necrosis factor-α, ischemia/reperfusion, and now hemorrhagic shock, uncover adhesion molecules which allow direct interaction between the endothelial cell surface and circulating neutrophils and plasma proteins.25,26 Importantly, Chappell et al recently demonstrated protection of the glycocalyx by hydrocortisone and antithrombin which reduced postischemic leukocyte adhesion 27, suggesting that interventions to preserve or repair the glycocalyx may have important therapeutic implications.
The syndecan -1/ glycocalyx endothelial layer also plays a key role in maintaining vascular stability. Injury after shock can lead to enhanced vascular permeability and tissue edema which may predispose to organ failure and death.28 Hayasida et al reported increased lung edema after lipopolysacharide administration in syndecan-1 knockout mice compared to wild type mice 29 while van den Berg et al showed that enzymatic degradation of the glycocalyx in myocardial capillaries led to myocardial edema.30 Preserving the microvascular barrier as a therapeutic target for sepsis is being explored.31 A recent in vitro study by our group demonstrated that plasma preserved pulmonary endothelial cell permeability compared to LR in a cell culture model of hypoxia/reoxygenation.32 Whether plasma’s potential vascular stabilizing properties translate into reduced morbidity or mortality is unclear but will be the subject of future investigation. Using the lung as an end organ to study does have an important limitation; the lung is comprised of both endothelial and epithelial cells and therefore we cannot determine the contribution of endothelial syndecan-1 in pulmonary protection by plasma. Data from mesenteric venules, however, suggest that endothelial cells play an important role.
Plasma partially restores syndecan-1 and glycocalyx: potential mechansims
Lastly, we have demonstrated that plasma resuscitation is effective in partially restoring syndecan-1 and the glycocalyx, though the precise mechanism by which this occurs is unknown. It is feasible that systemic syndecan-1 or other soluble plasma components physically interact with the endothelial membrane to repair components of the syndecan backbone. There are soluble components of the glycocalyx composed of proteins and proteoglycans which may be derived from either the endothelium or from blood constituents directly.6 It is also conceivable that plasma is not directly contributing to the structural integrity of the glycocalyx but rather is restoring cell surface syndecan-1 by mobilizing an intracellular pool of preformed syndecan-1.
Finally, plasma may also stimulate endothelial cell syndecan-1 transcription but with signs of restitution demonstrated by two hours of resuscitation, which is a less plausible explanation. Additional investigation is clearly needed. However, one limitation of the current study is that only MAP was used as an end point of resuscitation in this pilot study. Future studies should include more traditional end points such as hemoglobin and base deficit.
In conclusion, we have demonstrated in a rodent model that hemorrhagic shock degrades the endothelial glycocalyx. LR resuscitation resulted in few signs of repair while plasma resuscitation demonstrated early signs of restoration. In support of these findings, pulmonary syndecan-1 mRNA and alveolar cell surface syndecan-1 expression were higher in animals resuscitated with plasma compared to LR and correlated with reduced lung injury. Taken together, our findings support the protective effects of plasma seen clinically and may be due in part to its ability to restore the endothelial glycocalyx and preserve syndecan-1 after hemorrhagic shock.
ACKNOWLEDGMENTS
The authors thank Bernard Becker from Ludwig-Maximilians-University Munich, Munich, Germany, and Patricia Navarro and Glenn Decker from the University of Texas, Houston, Texas, for their assistance with glycocalyx staining and Min Ye for her technical assistance with scoring lung injury.
Funding: This study was supported by the National Institutes of Health P50 GM038529.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
DISCLOSURES
Name: Rosemary A. Kozar, MD, PhD
Contribution: Study design, data analysis, manuscript preparation
Name: Zhanglong Peng, MD, PhD
Contribution: Study conduct, data analysis
Name: Rongzhen Zhang, MD, PhD
Contribution: Study conduct
Name: John B. Holcomb, MD
Contribution: Study design, manuscript preparation
Name: Shibani Pati, MD, PhD
Contribution: Study design, manuscript preparation
Name: Pyong Park, PhD
Contribution: Study design and manuscript preparation
Name: Tien C. Ko, MD
Contribution: Study design and manuscript preparation
Name: Angel Paredes, PhD
Contribution: Conduct of the study and manuscript preparation
Contributor Information
Rosemary A Kozar, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
Zhanglong Peng, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
Rongzhen Zhang, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
John B Holcomb, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
Shibani Pati, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
Pyong Park, Division of Respiratory Diseases, Children’s Hospital, Harvard Medical School, Boston, MA.
Tien C Ko, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
Angel Paredes, Department of Surgery and Pathology, University of Texas Health Science Center at Houston.
REFERENCES
- 1.Borgman M, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. Blood product replacement affects survival in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 2.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, Wade CE, Holcomb JB. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64(2):S69–S78. doi: 10.1097/TA.0b013e318160ba2f. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal L, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 4.Gunter OL, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez EA, Moore FA, Holcomb JB, Miller C, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients who require massive transfusion. J Trauma. 2007;62(1):112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 6.Reitsma S, Slaaf DW, Vink H, van Zandvoort M, Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulivor AW, Lipowsky HH. Inflammation and ischemia-induced shedding of venular glycocalyx. Am J Physiol. 2004;286:1672–1808. doi: 10.1152/ajpheart.00832.2003. [DOI] [PubMed] [Google Scholar]
- 8.Pries AR, Secomb TW, Sperandio M, Gaehtgens P. Blood flow resistance during hemodilution: effect of plasma composition. Cardiovasc Res. 1998;37:225–235. doi: 10.1016/s0008-6363(97)00226-5. [DOI] [PubMed] [Google Scholar]
- 9.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch Eur J Physiol. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 10.Rehm M, Zahler S, Lotsch M, Welsch U, Conzen P, Jacob M, Friedrich B. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100:1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Gayosso IR, Platts SH, Duling BR. Reactive oxygen species mediate modifications of glycocalyx during ischemia-reperfusion. Am J Physiol. 2006;290:2247–2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 12.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 13.Yang R, Han X, Uchiyoma T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol. 2003;285:621–629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Hunter RL, Gonzalez EA, Moore FA. Poloxamer 188 prolongs survival of hypotensive resuscitation and decreases vital tissue injury after full resuscitation. Shock. 2009;32(4):442–450. doi: 10.1097/SHK.0b013e31819e13b1. [DOI] [PubMed] [Google Scholar]
- 15.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Bruno R, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 16.Vogel J, Sperandio M, Pries AR, Linderkamp O, Gaethgens P, Kuschinsky W. Influence of the endothelial glycocalyx on cerebral blood flow in mice. J Cerebral Blood Flow Metab. 2000;20:1571–1578. doi: 10.1097/00004647-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128(1):82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shureiqi I, Wu Y, Chen D, Yang XL, Guan B, Morris JS, Yang P, Newman RA, Broaddus R, Hamilton SR, Lynch P, Levin B, Fischer SM, Lippman SM. The critical role of 15-lipoxygenase-1 in colorectal epithelial cell terminal differentiation and tumorigenesis. Cancer Res. 2005;65(24):11486–11492. doi: 10.1158/0008-5472.CAN-05-2180. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q1. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 21.Fux L, Ilan N, Sanderson RD, Vlodavsky I. Heparanase: busy at the cell surface. Trends in Biochemical Sciences. 2009;34:511–519. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambaerts K, Wilcox-Adelman SA, Zimmermann P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opin Cell Biol. 2009;21:662–669. doi: 10.1016/j.ceb.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haywood-Watson RJL, II, Wang W, Pati S, Kozar RA, Faz G, Holcomb JB, Gonzalez EA. Human micro-vascular barrier disruption after hemorrhagic shock. J Surg Res. 2010;158:313. [Google Scholar]
- 24.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol. 2002;283:1282–1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 26.Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion. Am J Physiol. 2006;290:2247–2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 27.Chappell D, Dorfler N, Jacob M, Rehm M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 28.Lee WL, Slutsky AS. Sepsis and endothelial permeability. NEJM. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 29.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 31.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo-4 dependent slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2(23) doi: 10.1126/scitranslmed.3000678. 23ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pati S, Matijevic N, Doursout MF, Ko T, Cao Y, Deng X, Kozar RA, Hartwell E, Conyers J, Holcomb JB. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. Shock. 2010;29:S55–S63. doi: 10.1097/TA.0b013e3181e453d4. [DOI] [PMC free article] [PubMed] [Google Scholar]