The endoplasmic reticulum (ER) is the main site of protein and lipid synthesis, membrane biogenesis, xenobiotic detoxification and cellular calcium storage, and perturbation of ER homeostasis leads to stress and the activation of unfolded protein response (UPR)1. Chronic activation of ER stress has been shown to play an important role in the development of insulin resistance and diabetes in obesity2. However, mechanisms that lead to chronic ER stress in a metabolic context in general, and obesity in particular, are not understood. Here, we comparatively examined the proteomic and lipidomic landscape of hepatic ER purified from lean and obese mice to explore the mechanisms of chronic ER stress in obesity. We found suppression of protein but stimulation of lipid synthesis in the obese ER without significant alterations in chaperone content. Alterations in the ER fatty acid and lipid composition results in the inhibition of sarco/endoplasmic reticulum calcium ATPase (SERCA) activity and ER stress. Correcting the obesity-induced alteration of ER phospholipid composition or hepatic Serca over-expression in vivo both reduced chronic ER stress and improved glucose homeostasis. Hence, we established that abnormal lipid and calcium metabolism are important contributors to hepatic ER stress in obesity.
It has been generally accepted that a surplus of nutrients and energy stimulates synthetic pathways and may lead to client overloading in the ER. However, it has not been demonstrated whether increased de novo protein synthesis and client loading into the ER and/or a diminished productivity of ER in protein degradation or folding leads to ER stress in obesity. Intriguingly, dephosphorylation of eukaryotic translation initiation factor 2α (eIF2α) in the liver of high-fat-diet fed mice reduced ER stress response3, suggesting that additional mechanisms other than translational up-regulation may also contribute to ER dysfunction in obesity. To address these mechanistic questions, we first fractionated ER from lean and obese liver tissues (Supplementary Fig.1a-b) and then extracted ER proteins for comparative proteomic analysis to examine the status of this organelle in obesity. We identified a total of 2,021 unique proteins (Supplementary Table 1). Among them, 120 proteins were differentially regulated in obese hepatic ER samples (Supplementary Fig.1c, Supplementary Table 2a-b). We independently validated the differential regulation when possible by immunoblot analyses and verified the fidelity of the system (Supplementary Fig.1d). Gene Ontology analysis identified the enrichment of metabolic enzymes, especially ones involved in lipid metabolism, in the obese ER proteome, while protein synthesis and transport functions were over-represented among down-regulated ER proteins (Fig.1a). Consistently, we found that ER associated protein synthesis was down-regulated in the obese liver as demonstrated by polysome profiling (data not shown), whereas the expression of genes involved in de novo lipogenesis (Fas, Scd1, Ces3, Dgat2 and Dak2) and phospholipid synthesis (Pcyt1a and Pemt) were broadly up-regulated (Fig.1b, c). We also observed upregulation of protein degradation pathway but did not find a broad change in the quantity of ER chaperones (Supplementary Fig.2, Supplementary Table 2a). Taken together, these data revealed a fundamental shift in hepatic ER function in obesity from protein to lipid synthesis and metabolism.
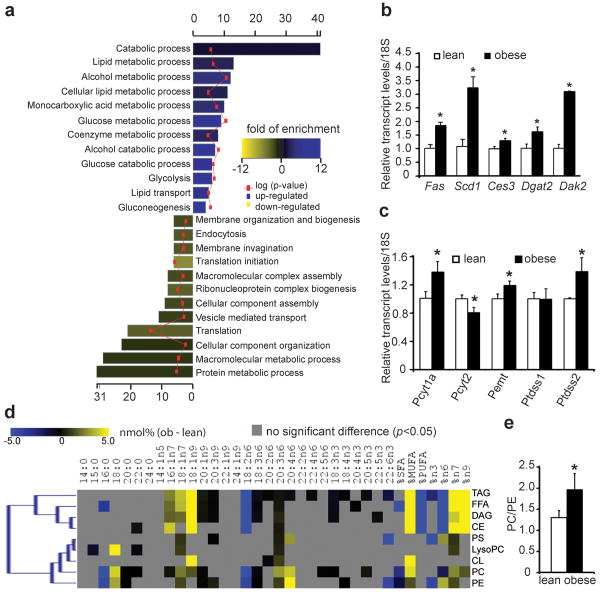
Figure 1. Proteomic and lipidomic landscape of the lean and obese ER.
a, Biological pathways associated with significantly regulated proteins in the obese ER proteome. Bar colors indicate the fold enrichment with significance values (negative log of p-values) superimposed. b,c, Transcript levels of genes involved in lipid metabolism in the lean and obese mouse liver. d, Alterations of liver ER lipidome. Heatmap display of all significant (p<0.05) alterations present between lean and obese ER lipidomes. The color corresponds to differences in the relative abundance (nmol%) of each fatty acid among individual lipid groups detected in the lean and obese liver ER. e, The relative abundance of PC and PE in lean and obese liver ER samples. Values are mean±SEM (n=6 for each group). “*” denotes p<0.05, Student's t-test.
The presence of chronic ER stress in obese liver (Supplementary Fig.2) despite reduction in ER-associated protein synthesis led us to postulate that the ER stress in obesity may not simply be invoked by protein overloading but also driven by compromised folding capacity, in which lipid metabolism may have a function4. For example, the ability of palmitate and cholesterol to induce ER stress in cultured cells correlates with their incorporation into the ER5,6. Therefore, we quantitatively determined all major lipid species and their fatty acid composition in ER samples isolated from lean and obese liver along with the diet consumed by these animals (Supplementary Fig.3, Supplementary Table 3). First, we found that the fatty acid composition of ER lipids in the lean mouse liver was distinct from corresponding dietary lipids, suggesting the contribution of a basal level de novo lipogenesis to the biogenesis of ER membranes in vivo (Supplementary Fig.3a, b, Supplementary Table 3). Almost all ER derived lipids were composed of significantly higher levels of saturated fatty acids (SFA) whereas their polyunsaturated fatty acid (PUFA) content was much lower than those of corresponding dietary lipids, suggesting that de novo synthesized SFAs are preferred over diet derived PUFAs as the substrate for the synthesis of hepatic ER lipids. Second, the liver ER samples of lean and obese mice also had profoundly different composition of fatty acids and lipids as illustrated by the clear separation of lean and obese ER lipidome in cluster analysis (Supplementary Fig.3c). The obese ER was significantly enriched with monounsaturated fatty acids (MUFA, Fig.1d), a bona fide product of de novo lipogenesis, in liver. Third, the obese ER samples contained a higher level of phosphatidylcholine (PC) as compared to phosphatidylethanolamine (PE) (PC/PE=1.97 vs. 1.3, p<0.05, Fig.1e, Supplementary Table 3), two of the most abundant phospholipids on the ER membrane. The rise of PC/PE ratio is likely caused by the up-regulation of two key genes involved in PC synthesis and PE to PC conversion: choline-phosphate cytidylyltransferase A (Pcyt1a) and phosphatidylethanolamine N-methyltransferase (Pemt) (Fig.1c, Supplementary Fig. 3a), and it is consistent with the essential role of PC for lipid packaging in the form of lipid-droplets or lipoproteins, both of which are increased in obesity. In contrast, the PC/PE ratio in the lean hepatic ER was essentially identical as it is in the diet (Supplementary Table 3), indicating that the increase of PC/PE ratio in obesity is not due to food consumption, but the result of increased lipid synthesis in the obese liver.
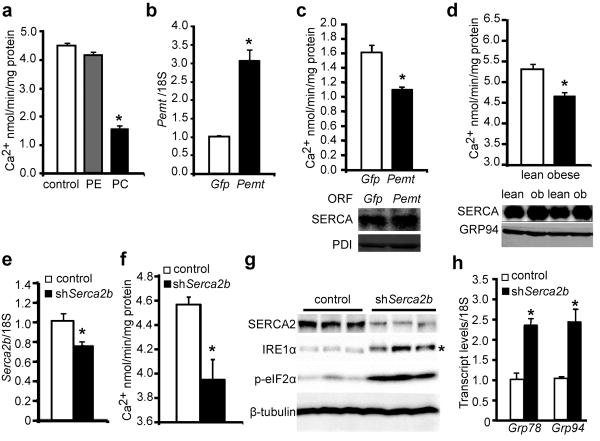
The desaturation of SFA to MUFA in the obese liver likely has a protective role in reducing lipotoxicity, whereas the decrease of PUFA content in the ER may limit its reducing capacity and contribute to ER stress7. However, a potential role of PC/PE ratio in regulating ER homeostasis has not been studied before. Previous biochemical studies have shown that increasing PC content in the membrane inhibits the calcium transport activity of SERCA5,8. Consistently, we found that the addition of PC to liver-derived microsomes in vitro substantially inhibited SERCA activity (Fig.2a). More importantly, over-expression of the PE to PC conversion enzyme, Pemt, in Hepa1-6 cells significantly inhibited microsomal SERCA activity, suggesting changes in the PC/PE balance in a cellular setting can significantly perturb SERCA function (Fig.2b,c). As calcium plays an important role in mediating chaperone function and protein folding in the ER, and given that SERCA is principally responsible in maintaining calcium homeostasis in this organelle, we postulated that the increased PC/PE ratio in the ER of obese liver might impair ER calcium retention and homeostasis in vivo, thereby contributing to protein misfolding and ER stress. In support of this possibility, we found that calcium transport activity of microsomes prepared from obese mice liver were significantly lower than those isolated from lean animals (4.6±0.2 vs. 5.3±0.3, p=0.046, Fig.2d), despite the fact that SERCA protein level was modestly higher in the former, consistent with an inhibitory role of PC/PE ratio on SERCA function.
Figure 2. Elevated PC/PE ratio impairs SERCA activity and ER homeostasis.
a, Calcium transport activity of microsomes loaded with PC and PE in vitro. Transcript levels of Pemt (b) and corresponding microsomal calcium transport activities (c) of Hepa1-6 cells expressing control (Gfp) or mouse Pemt ORF. d, Calcium transport activity (top) and SERCA protein levels (bottom) of microsomes prepared from lean and obese mouse liver. Liver Serca2b transcript levels (e) and microsomal calcium transport activities (f), immunoblot (g) and quantitative RT-PCR (h) measurement of ER stress markers in the livers of lean mice expressing either LacZ (control) or Serca2b shRNAs. “*” in g denotes the phosphorylated IRE1α and “*” in other panels denotes significant difference (p<0.05, n=4) by student's t-test. Values are mean±SEM.
Modest defects in SERCA activity have been implicated in the pathology of Darier's disease9, and we found that a reduction in SERCA expression in vivo (Fig.2e) and a concurrent reduction in its calcium transport activity (Fig.2f) potently activated hepatic ER stress in lean mice as evident by IRE1α and eIF2α phosphorylation and changes in the expression of Grp78 and Grp94 (Fig.2g,h). Therefore, there appears to be little redundancy in the function of SERCA beyond physiological fluctuations to maintain ER homeostasis, and the reduction in calcium transport activity could be a potential mechanism of hepatic ER stress in obesity.
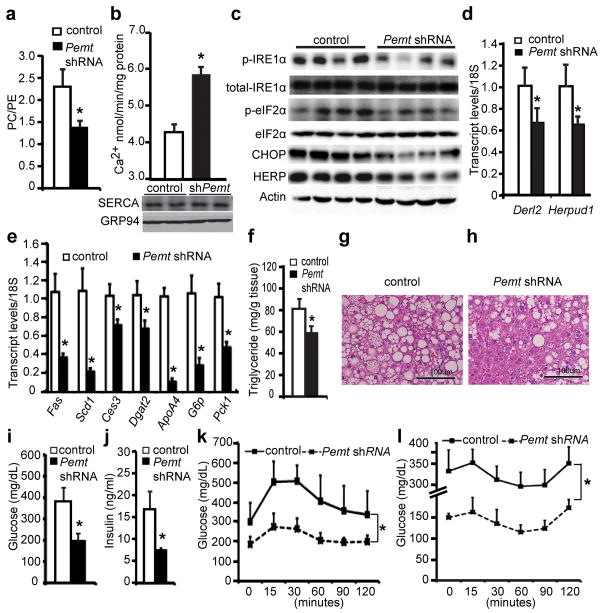
We carried out two different but complimentary approaches to correct aberrant lipid metabolism caused SERCA dysfunction and examined the effects on ER homeostasis in the obese liver. If the alteration in PC/PE ratio seen in obese liver is a significant contributor to ER stress, correction of this ratio to lean levels by reducing Pemt expression should improve calcium transport defects and produce beneficial effects on hepatic ER stress and metabolism. Using an adenovirally-expressed shRNA, we were able to achieve ∼50-70% suppression of the Pemt transcript in obese liver (Supplementary Fig.4a). As postulated, suppression of Pemt led to a decrease of PC content from ∼39% to ∼33%, which was compensated by an ∼7% increase of PE content from ∼17% to 24% (Supplementary Table 4). As a result, the PC/PE ratio is reduced to 1.3 (equivalent to lean ratio), as compared to 2.0 detected in the ER of the obese liver (Fig.3a). The reduction of PC/PE ratio was accompanied by a significant improvement in the calcium transport activity of the ER prepared from the Pemt-knockdown obese mice (Fig.3b). As the improvement of calcium transport function occurred with few and minor changes in the overall fatty acid composition of ER (Supplementary Fig.4b,c Supplementary Table 5), our results confirmed the rise in PC/PE ratio as an inhibitory factor of SERCA activity in obesity. More importantly, hepatic ER stress indicators including the phosphorylation of IRE1α and eIF2α as well as the expression of C/EBP homologous protein (CHOP), homocysteine-inducible, endoplasmic reticulum stress-inducible protein (HERP) and Der1-like domain family member 2 (DERL2) were all reduced upon suppression of Pemt in obese mice (Fig.3c,d, Supplementary Fig.4d). Relief of chronic ER stress in the ob/ob mice has been associated with improvement of hepatic steatosis and glucose homeostasis10,11. Consistently, genes involved in hepatic lipogenesis (Fas, Scd1, Ces3, Dgat2) and lipoprotein synthesis (ApoA4) were significantly down regulated in the obese liver following suppression of Pemt (Fig.3e). As a result, these mice exhibited a significant reduction in hepatic steatosis and liver triglyceride content (Fig.3f-h). Genes involved in glucose production (G6p, Pck1) in the liver were significantly down regulated (Fig.3e), and there were also significant reductions in both hyperglycemia and hyperinsulinemia in obese mice following the suppression of hepatic Pemt expression (Fig.3i,j). Glucose and insulin tolerance tests revealed significantly enhanced glucose disposal following Pemt suppression (Fig.3k,l). A similar phenotype is also observed upon suppression of hepatic Pemt in the high-fat diet induced obesity with reduced ER stress and improved glucose homeostasis (Supplementary Fig.5). These data are consistent with the phenotype seen in Pemt–deficient mice which exhibit protection against diet-induced insulin resistance and atherosclerosis12. Therefore, correcting the PC/PE ratio of ER can significantly improve calcium transport defects, reduce ER stress and improve metabolism, supporting the hypothesis that changes in lipid metabolism contribute to SERCA dysfunction, ER stress and hyperglycemia in both genetic- and diet-induced models of obesity.
Figure 3. Suppression of liver Pemt expression corrects ER PC/PE ratio, relieves ER stress, and improves systemic glucose homeostasis in obesity.
a, PC/PE ratio, and b, calcium transport activity of liver ER from ob/ob mice expressing LacZ (control) or Pemt shRNAs. Immunoblot (c) and quantitative PCR (d) measurement of ER stress markers in the liver. Expression of hepatic lipogenesis and gluconeogenesis genes (e), triglyceride content (f), and Hematoxylin & Eosin staining (g and h) of liver samples. Plasma glucose (i) and insulin (j) levels in control and Pemt shRNA-treated ob/ob mice after 6-hour food withdrawal. k-l, Plasma glucose levels of control and Pemt shRNA-treated ob/ob mice after intraperitoneal administration of either 1g/kg of glucose (k) or 1IU/kg of insulin (l). All data are mean±SEM (n=4 for a-e, n=6 for f-l), “*” denotes p<0.05 (one-way ANOVA for data presented in k and l, and Student's t-test for others).
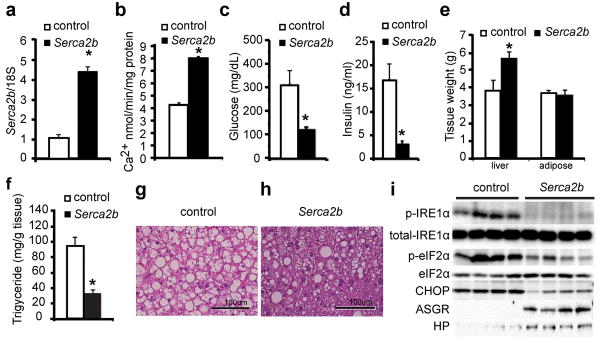
Second, we carried out over-expression of hepatic Serca in vivo to overcome the partial inhibition of SERCA activity by PC (Fig.4a). Indeed, exogenous SERCA expression in the liver of the ob/ob mice improved the calcium import activity of the ER (Fig.4b), restored euglycemia and normoinsulinemia within a few days, and markedly improved glucose tolerance (Fig.4c,d, Supplementary Fig.6). Upon Serca expression, liver showed an increase in size but a marked reduction of lipid infiltration (Fig.4e-h) and suppression of IRE1α and eIF2α phosphorylation, along with significant reduction in CHOP levels (Fig.4i). In these liver samples, there was also a marked increase in two secretory proteins that were otherwise diminished in obesity: asialoglycoprotein receptor (ASGR) and haptoglobin (HP) (Fig.4i). As the folding and maturation of ASGR is most sensitive to perturbations of calcium homeostasis in the ER13, our results support that exogenously increased SERCA expression restored calcium homeostasis and relieved at least some aspects of chronic ER stress in the obese liver. Taken together, these data reinforced the hypothesis that lipid-driven alterations and the ER calcium homeostasis are important contributors to hepatic ER stress in obesity.
Figure 4. Exogenous Serca expression alleviates ER stress and improves systemic glucose homeostasis.
Liver Serca2b transcript levels (a) and microsomal calcium transport activities (b) of control or Serca2b overexpressing obese mice. Plasma glucose (c) Plasma insulin levels (d), tissue weights (e) of ob/ob mice as in panel a. Triglyceride content (f), H&E staining (g, h) and immunoblot analyses (i) of ER stress markers (IRE1α and eIF2α phosphorylation, and CHOP) and secretory proteins (ASGR and HP) in the obese liver expressing Serca2b compared to controls. All values are mean±SEM (n=4 for a-b, n=6 for c-h), “*” denotes p<0.05 (Student's t-test).
The chronic activation of ER stress markers has been observed in a variety of experimental obese models as well as in obese humans14. Furthermore, treatment of obese mice and humans with chemical chaperones result in increased insulin sensitivity10,15. Our systematic, compositional and functional characterization of hepatic ER landscape from lean and obese mice revealed a diametrically opposite regulation of ER functions regarding protein and lipid metabolism and revealed mechanisms giving rise to ER stress. In particular, elevation of the PC/PE ratio in the ER, driven by the up-regulation of de novo lipogenesis in obesity, was linked to SERCA dysfunction and chronic ER stress in vivo. During the review of this manuscript, a study reported down-regulation of SERCA protein level in obese liver16, which was not evident in our analysis and appeared to have resulted from the choice of methodology in ER protein preparations (Supplementary Fig.7). Nevertheless, other mechanisms such as oxidative and inflammatory changes associated with obesity can also perturb ER homeostasis by impacting ER calcium fluxes17-19 and will be important to study in the future.
The identification of a lipid-driven calcium transport dysfunction and ER stress provides a fundamental framework to understand the pathogenesis of hepatic lipid metabolism and chronic ER stress in obesity. First, excessive food intake inevitably stimulates lipogenesis for energy storage, and PC is the preferred phospholipid coat of lipid droplets and lipoproteins20. Therefore, there is a biological need for the synthesis of more PC for packaging and storing the products of hepatic lipogenesis. Second, de novo fatty acid synthesis in the obese liver produces ample amounts of MUFA, which is effectively incorporated into PC but not PE, which further distorts the PC/PE ratio and impairs ER function. The resulting ER stress facilitates the secretion of excessive lipids from liver without ameliorating hyperinsulinemia-induced lipogenesis21, and thus hepatosteosis and ER stress ensue. As a result relieving ER stress in obesity may ultimately depend on breaking this “lipogenesis-ER stress-lipogenesis” vicious cycle and restoring the ER folding capacity. Therefore, we suggest that genetic, chemical or dietary interventions that modulate hepatic phospholipid synthesis and/or ER calcium homeostasis function might represent a new set of therapeutic opportunities for common chronic diseases associated with ER stress such as obesity, insulin resistance, and type 2 diabetes.
Method Summary
Male leptin deficient (ob/ob) and wild type littermates in the C57BL/6J background were either bred in house or purchased from the Jackson Laboratory (strain B6.V-Lepob/J, stock number 000632). Transduction of adenoviruses (serotype 5, Ad5) for the expression of open reading frames (ORF) or shRNAs was carried out between 10-11 weeks after birth, and all mice were sacrificed between 12-13 weeks of age, unless noted otherwise. ER fractionation for proteomic and lipidomic analysis were carried out as described by Cox and Emili (2006)22. Calcium transport experiments were performed according to Moore et al., with some modifications23. Quantitative RT-PCR, Western blot analysis, histology and in vivo animal experiments were carried out as previously described10,24. Oligonucleotide sequences used in this study are listed in Supplementary Table 6. Detailed experimental procedures and protocols are described in the supplementary material.
Supplementary Material
Acknowledgments
We thank Alyssa Porter, Emily Freeman and Ryan Davis for technical assistance. The anti-HERP antibody is a gift of Dr. Yasuhiko Hirabayashi (Tohoku University, Japan). We thank the members of the Hotamisligil lab for scientific discussions and critical reading of the manuscript. This work was supported in part by National Institute of Health (DK52539 and 1RC4-DK090942) and a research grant from Syndexa Pharmaceuticals to G.S.H. S.F. was supported in part by the NIH/NIEHS postdoctoral training grant (T32ES007155).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions S.F. designed, performed experiments, analyzed and interpreted the results and wrote the manuscript; L.Y. and P.L. performed some animal experiments; O.H., L.D., W.H. and X.L. performed statistical and bioinformatic analysis of the proteomic data; S.W.M quantified the lipid composition of ER and analyzed the data; A.I. analyzed the protein composition of ER; G.S.H generated the hypothesis, designed the project, analyzed and interpreted the data and wrote the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints.
The authors declare competing financial interest.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyadomari S, et al. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7(6):520. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erbay E, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15(12):1383. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, et al. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279(35):37030. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 6.Borradaile NM, et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, et al. Omega-3 and omega-6 fatty acids suppress ER- and oxidative stress in cultured neurons and neuronal progenitor cells from mice lacking PPT1. Neurosci Lett. 479(3):292. doi: 10.1016/j.neulet.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng KH, Lepock JR, Hui SW, Yeagle PL. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated phosphatidylethanolamine. J Biol Chem. 1986;261(11):5081. [PubMed] [Google Scholar]
- 9.Miyauchi Y, et al. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+-ATPase mutants associated with Darier disease. J Biol Chem. 2006;281(32):22882. doi: 10.1074/jbc.M601966200. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kammoun HL, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs RL, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010;285(29):22403. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodish HF, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990;265(19):10893. [PubMed] [Google Scholar]
- 14.Gregor MF, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kars M, et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 59(8):1899. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SW, Zhou Y, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107(45):19320. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li SY, et al. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49(6):1434. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 18.Cardozo AK, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54(2):452. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 19.Li G, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186(6):783. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiller J, et al. Lipid analysis of human HDL and LDL by MALDI-TOF mass spectrometry and (31)P-NMR. J Lipid Res. 2001;42(9):1501. [PubMed] [Google Scholar]
- 21.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc. 2006;1(4):1872. doi: 10.1038/nprot.2006.273. [DOI] [PubMed] [Google Scholar]
- 23.Moore L, Chen T, Knapp HR, Jr, Landon EJ. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975;250(12):4562. [PubMed] [Google Scholar]
- 24.Cao H, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.