Abstract
Kirsten Ras (K-Ras) mutations have been implicated as a key predictive marker of resistance to therapies targeting the epidermal growth factor receptor (EGFR). To determine whether Harvey Ras (H-Ras) mutations also can confer resistance to EGFR-targeted therapy, we expressed a constitutively active H-Ras (Ras G12V) in A431 human vulvar squamous carcinoma cells. Compared with corresponding control cells, A431-Ras cells exhibited marked resistance to the EGFR inhibitors cetuximab and gefitinib, reducing inhibition of Akt and Erk phosphorylation, inhibition of HIF-1α expression and transcriptional activity, and antitumor effects in vitro and in vivo. Our data indicate that constitutively active H-Ras can also confer resistance to anti-EGFR therapy in cancer cells.
Keywords: EGFR, H-Ras, Cetuximab, Gefitinib, Resistance
1. Introduction
The epidermal growth factor receptor (EGFR), a 170-kDa transmembrane glycoprotein with intrinsic tyrosine kinase activity [1], is often highly expressed in a variety of human tumors of epithelial origin, including cancers of the colon, lung, head and neck, esophagus, stomach, prostate, bladder, kidney, pancreas and ovary [2]. Activation of EGFR triggers signal transduction through several well-characterized downstream pathways, including the Ras/Raf/Erk, PI3K/Akt/mTOR, PLCγ, PKC, and JAK/STAT pathways, leading to gene transcription that is responsible for a variety of cellular functions [3]. For example, activation of several of these pathways leads to increased expression of hypoxia-inducible factor-1 alpha (HIF-1α) [4–8], which forms a heterodimer with HIF-1β; together this protein complex regulates more than 100 targeted genes that are critical for both bioenergetic and biosynthetic metabolism in cancer cells and are responsible for many metastatic properties of cancer cells, such as angiogenesis, invasion, drug resistance, increased cell proliferation, and reduced apoptosis [9,10].
Several therapeutic strategies to inhibit EGFR signaling have been devised, and five agents that target EGFR are approved for use in the treatment of patients with several types of cancers. These agents include the monoclonal antibodies cetuximab and panitumumab and the tyrosine kinase inhibitors gefitinib, erlotinib, and lapatinib (a dual inhibitor to EGFR and HER2) [2]. The antitumor mechanisms of these agents have been well explored, resulting in a large body of experimental evidence revealing that they prevent ligand-induced receptor activation and subsequently inhibit downstream signaling, resulting in cell cycle arrest, apoptosis induction, and angiogenesis inhibition in preclinical models; however, despite objective responses in some patients, the clinical benefits of these agents in prolonging patients' overall survival have been modest [2]. Understanding of the mechanisms of tumor resistance to the EGFR-targeting agents has been an active topic of research in the area in recent years.
Resistance to EGFR-targeted therapy has been linked to Ras mutations. The Ras gene subfamily consists of the Harvey, Kirsten, and neuroblastoma Ras genes (H-Ras, K-Ras, and N-Ras), which encode proteins with GTP/GDP binding and GTPase activity [11–14]. Ras proteins alternate between an inactive form bound to GDP (Ras-GDP) and an active form bound to GTP (Ras-GTP); the proteins are activated by a guanine nucleotide-exchange factor (GEF) and inactivated by a GTPase-activating protein (GAP) [15]. Functioning as molecular switches in regulating cell survival, proliferation, and differentiation, Ras proteins are essential mediators that convey extracellular signals from surface receptors to intracellular signaling pathways [16]. Oncogenic mutant Ras proteins are locked into constitutively GTP-bound conformation and thus are independent of EGFR signaling. Recent clinical studies have identified K-Ras mutations as a key predictive marker of resistance to EGFR-targeted therapy [17–25]. Indeed, due to the high percentage of K-Ras mutations in patients with colon cancer (ranging from 30% to 35%) [17–25] and the lack of response by these tumors to cetuximab, patient biopsy specimens are now routinely assessed for K-Ras mutations [26]. Metastatic colon cancer patients with K-Ras mutations are excluded from anti-EGFR therapy because of the likelihood of de novo resistance.
Despite their great biochemical and biological similarities, the Ras proteins may not be fully functionally identical or redundant [27,28]. H-Ras mutations are less common than K-Ras mutations in human cancer; however, rates of H-Ras mutations are not negligible, and genetic analysis of tumor specimens has revealed H-Ras mutations in approximately 10–22% of head and neck cancers [29,30], 11% of bladder cancers, 9% of cervical cancers, and in smaller percentages of several other cancers [31]. It is unknown whether H-Ras mutations, like K-Ras mutations, can confer resistance to EGFR-targeted therapy. In the present study, we examined the impact of the expression of constitutively active H-Ras on the antitumor effects of cetuximab and gefitinib both in vitro and in vivo.
2. Materials and Methods
2.1. Reagents
The EGFR-blocking monoclonal antibody cetuximab and the small molecule EGFR tyrosine kinase inhibitor gefitinib were provided by ImClone Systems (New York, NY, USA) and AstraZeneca (Wilmington, DE, USA), respectively [32]. Antibodies directed against total Akt, ser473-phosphorylated Akt, and the phosphorylated Erk p42/p44 were obtained from Cell Signaling Technology (Beverly, MA, USA). The rabbit anti-MAPK (Erk2) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-HIF-1α antibody was purchased from BD Transduction Laboratories (San Diego, CA, USA). The anti-His G antibody was purchased from Upstate Biotechnology (Charlottesville, VA, USA). The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reagent and the chemotherapeutic agent cisplatin were both purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Cells and cell culture
The human vulvar squamous carcinoma cell line A431, which has been previously described [33], was maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C (normoxic conditions). For hypoxic stimulation, cells were placed in an airtight chamber that was flushed with a gas mixture of 5% CO2 and 95% N2. The O2 concentration inside the chamber was maintained at 1% using the Pro-Ox O2 regulator (Model 110; BioSpherix, Redfield, NY, USA). The hypoxic chamber was incubated at 37°C alongside the chamber containing the cells under normoxic conditions.
For stable expression of the constitutively active H-Ras (H-Ras G12V), A431 cells were transfected with pcDNA3.1 H-Ras G12V vector; control cells were transfected with pcDNA3.1 backbone vector. Both transfections were done using a FuGENE-6 transfection kit (Roche Diagnostics, Indianapolis, IN, USA) and transfectants were subsequently selected with G418 as previously described [34]. The expression of H-Ras G12V in A431-Ras cells was measured by Western blot analysis with anti-His G–tag antibodies in selected pooled cells.
2.3. Western blot analysis
Cells were lysed in a buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 0.5% NP-40, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 μg/mL leupeptin, and 25 μg/mL aprotinin and clarified by centrifugation (14,000 g for 20 min at 4°C). Cell lysates were then separated by sodium dodecyl sulfate polyacrylamide electrophoresis, blotted onto nitrocellulose, and probed with the indicated primary antibodies. The signals were visualized using an ECL chemiluminescence detection kit (GE Life Science/Amersham Biosciences, Piscataway, NJ, USA).
2.4. MTT proliferation assay
Parental A431, A431V, and A431-Ras cells (5 × 103/well) were seeded into 24-well plates in medium containing 10% FBS and allowed to adhere overnight. Following overnight incubation, medium was removed and replaced with DMEM/F12 containing 0.5% FBS and increasing doses of cetuximab or gefitinib for 5 days. For combinational studies, cells were pulsed with 1 μM cisplatin for 3 h, after which cells were cultured for an additional 5 days in medium supplemented with 0.5% FBS containing control vehicle, cetuximab (1 nM), or gefitinib (0.1 μM). The relative number of cells for each group was assayed by adding 50 μL of 10 mg/mL MTT to 500 μL of culture medium and incubating cells for 3 h at 37°C. Following incubation, cells were lysed with 500 μL of lysis buffer (20% sodium dodecyl sulfate in dimethyl formamide/H2O, 1:1 v/v, pH 4.7) at room temperature for at least 6 h. Cell proliferation was then determined by measuring the optimal absorbance of cell lysates at a wavelength of 570 nm and normalizing the value to that for a corresponding control.
2.5. Plasmid transfection and luciferase assay
The pBI-GL-V6L construct, which contains six copies of the VEGF hypoxia response element, has been previously described [35,36]. A431 cells were transiently transfected with the pBI-GL-V6L construct using the FuGENE-6 transfection kit. After a 24-h transfection period, the cells were washed twice with phosphate-buffered saline (PBS) and cultured with vehicle control, cetuximab (20 nM), or gefitinib (0.5 μM) in hypoxic or normoxic conditions as described above for an additional 16 h in serum-free medium. The cells were then harvested and lysed in a lysis buffer (0.2 M Tris HCl, pH 8.0, and 0.1% Triton X-100). The luciferase assay was performed by adding luciferase substrate solution (0.5 mM D-luciferin, 0.25 mM coenzyme A, 20 mM Tris HCl, 4 mM MgSO4, 0.1 mM EDTA, 30 mM DTT, and 0.5 mM ATP) to the samples and immediately measuring for luciferase activity using a multiplate luminometer (Berthold Detection Systems, Oak Ridge, TN, USA). Luciferase activities expressed in arbitrary units were normalized to the amount of protein in each sample. The protein concentration was determined using the Pierce Coomassie Plus colorimetric protein assay method.
2.6. Animal studies
Cells (5 ×106) in 100 μL of serum-free medium were inoculated subcutaneously into both flanks (A431V on the left side and A431-Ras cells on the right side) of 6–8-week-old male NCr-nu/nu athymic mice (The National Cancer Institute at Frederick, MD, USA). Tumor volume in cubic millimeters was determined using the formula (length × width2)/2, where length was the longest axis and width the measurement at a right angle to the length [37]. Xenografts were considered established when the tumor volume reached approximately 60 mm3, at which time mice were treated with 1 mg of cetuximab or PBS control once weekly for 3 weeks. Mice were euthanized 3 days after their last treatment. Data were expressed as mean tumor volume ± standard deviation for each treatment group. This research project was approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
3. Results
3.1. Constitutively active H-Ras blocks cetuximab-mediated inhibition of Akt and Erk phosphorylation
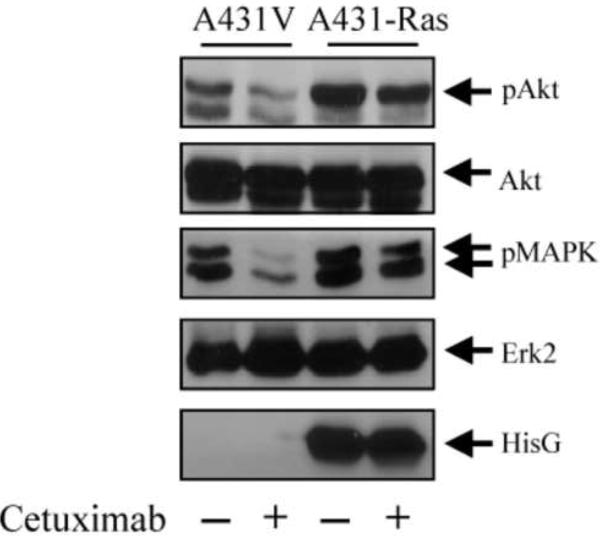
Previous studies have demonstrated that EGFR-targeting agents such as cetuximab and gefitinib inhibit the activation-specific phosphorylation of EGFR downstream targets such as Akt and Erk [38,39]. Therefore, we examined the ability of cetuximab to inhibit the phosphorylation of these EGFR downstream targets after the introduction of constitutively active H-Ras G12V into A431 cells. Fig. 1 shows that A431-Ras cells had higher basal phosphorylation levels of Akt and Erk than did A431V control cells. Furthermore, although cetuximab clearly reduced the levels of phosphorylated Akt and phosphorylated Erk in A431V cells as expected, cetuximab only marginally reduced these levels in A431-Ras cells. No changes in total Akt or Erk were seen in either of the two cell lines, indicating equal protein loading. These results demonstrate that constitutively active H-Ras G12V could activate Akt and Erk independently from the activity of EGFR. Analysis of the same blot with an anti-His G antibody illustrated the presence of His-tagged H-Ras G12V.
Fig. 1.
Resistance to cetuximab-mediated inhibition of Akt and Erk phosphorylation in cells transfected with a constitutively active H-Ras. A431 cells transfected with a control vector (A431V) or an expression vector containing constitutively active H-RasG12V (A431-Ras) were left untreated or were treated with 20 nM cetuximab for 16 h in serum-free medium. Cell lysates were prepared and analyzed for the levels of total and phosphorylated Akt, total and phosphorylated Erk, and His-tagged Ras by Western blot analysis with respective antibodies.
3.2. Constitutively active H-Ras reduces antiproliferative effects of cetuximab and gefitinib
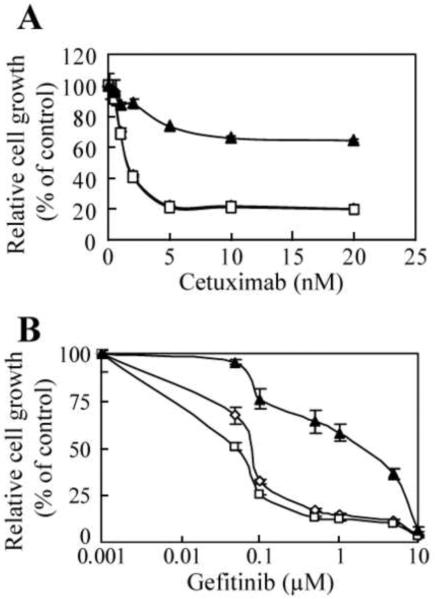
We next determined whether constitutively active H-Ras G12V could confer resistance to the antiproliferative activity of cetuximab and gefitinib. As expected, cetuximab inhibited the proliferation of both parental A431 and control A431V cells, with maximum inhibition reached at ≥ 5 nM (Fig. 2A). In contrast, the proliferation of A431-Ras cells was minimally reduced by cetuximab, with only a 30–35% inhibition seen at the maximum dose of cetuximab used (Fig. 2A). Statistical significance was seen between either parental A431 or A431V and A431-Ras cells in all treatment doses ≥ 1 nM of cetuximab. Like cetuximab, gefitinib inhibited the proliferation of both parental A431 and A431V cells in a dose-dependent manner but had only weak antiproliferative activity against the A431-Ras cells (Fig. 2B). At the maximum dose of gefitinib used (10 μM), which can lead to nonspecific inhibition of kinases other than the EGFR, the drug equally inhibited parental A431, A431V, and A431-Ras cells. Statistical significance was seen between either parental A431 or A431V and A431-Ras cells in all treatment doses ≥ 0.05 μM of gefitinib, except 10 μM. Statistical significance was not observed at any dose of cetuximab or gefitinib between parental A431 and A431V cells.
Fig. 2.
Resistance to cetuximab- and gefitinib-mediated antiproliferative effects in cells transfected with a constitutively active H-Ras. Parental A431 (◇), A431V (□), and A431-Ras (▲) cells were seeded at a density of 5 × 103/well and allowed to adhere overnight. Cells were then treated with increasing doses of (A) cetuximab (0–20 nM) or (B) gefitinib (0–10 μM) in medium containing 0.5% FBS for 5 days. Cell proliferation was assessed by an MTT assay as described in Materials and Methods using the optical density (OD) of cell lysates as relative numbers of cells. The OD value of each treated group was expressed as a percentage of the OD value of the control group.
3.3. Constitutively active H-Ras blocks cetuximab/gefitinib-mediated reduction of HIF-1α
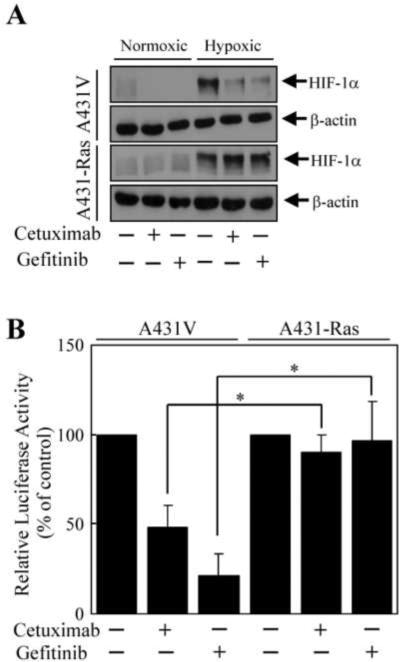
Previous studies have demonstrated that the Ras/PI3K/Akt and Ras/Erk pathways regulate the expression of HIF-1α and VEGF [4–8]. Furthermore, we have previously shown that cetuximab can reduce the expression levels of HIF-1α and VEGF [36,40]. Therefore, we next determined whether the expression of constitutively active H-Ras could influence the effect of cetuximab and gefitinib on HIF-1α expression and subsequent HIF-1α-mediated VEGF transcription under both normoxic and hypoxic conditions. There was no substantial difference in normoxic or hypoxia-induced levels of HIF-1α between the A431V and A431-Ras cells (Fig. 3A). However, both cetuximab and gefitinib reduced the normoxic and hypoxia-induced levels of HIF-1α in A431V cells but failed to reduce the normoxic and hypoxia-induced levels of HIF-1α in A431-Ras cells.
Fig. 3.
Resistance to cetuximab- and gefitinib-mediated downregulation of HIF-α protein and HIF-α transcriptional activity in cells transfected with a constitutively active H-Ras. (A) A431V and A431-Ras cells were left untreated or were treated with cetuximab (20 nM) or gefitinib (0.5 μM) for 16 h in serum-free medium in normoxic or hypoxic conditions. Cell lysates were prepared and analyzed for the levels of HIF-α by Western blot analysis with respective antibodies. The level of β-actin was also measured as a protein-loading control. (B) A431V and A431-Ras cells were transiently transfected with the pBI-GL-V6L vector for 24 h with FuGENE-6 as indicated in Materials and Methods. The cells were left untreated or were treated with cetuximab (20 nM) or gefitinib (0.5 μM) for 16 h in normoxic or hypoxic conditions. Luciferase reporter activity was then measured in each group as described in Materials and Methods. Relative luciferase activity was determined by standardizing the readings of untreated cells to 100% after normalizing values to the total protein concentration of each sample. The data represent the mean of three independent experiments using triplicate wells. The bars indicate standard deviation. * p < 0.05
As HIF-1α regulates the expression of VEGF, we next determined whether the effects of cetuximab and gefitinib on HIF-1α expression described above would correlate to HIF-1α transcriptional activity in cells transfected with the luciferase reporter gene construct pBI-GL-V6L, which contains six tandem repeats of the hypoxia response element from the human VEGF gene [35]. As expected, a substantial increase in luciferase activity was observed in cells cultured in hypoxic conditions compared with that observed in cells cultured under normoxic conditions (data not shown). In hypoxic conditions, cetuximab and gefitinib reduced the luciferase activity in A431V cells by 52% and 79%, respectively, compared with untreated controls (Fig. 3B). In contrast, this reduced luciferase activity was somewhat blocked in the A431-Ras cells, with the luciferase activity reduced by 10% and 4% by cetuximab and gefitinib, respectively, compared with controls. Taken together, these results indicate that constitutively active H-Ras inhibited the effects of cetuximab and gefitinib on both HIF-1α expression and its subsequent transcriptional activity.
3.4. Constitutively active H-Ras reduced antiproliferative effects of combinational treatment with cisplatin plus cetuximab or gefitinib
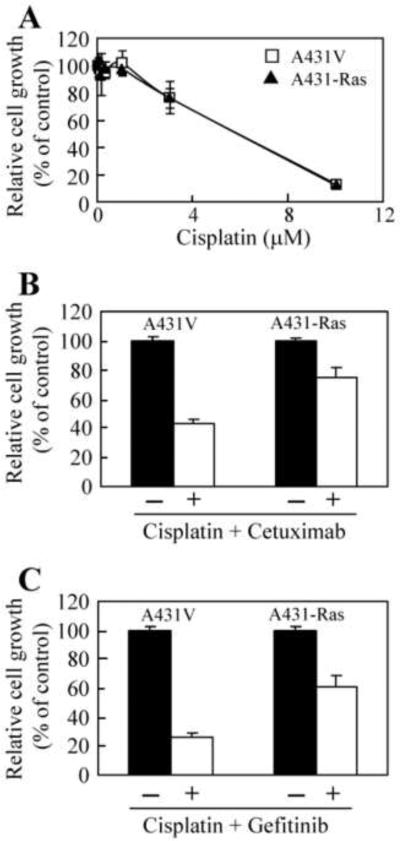
Previous studies have shown that cetuximab and gefitinib can sensitize tumor cells to chemotherapy, leading to additive or synergistic inhibition in both cell culture and animal models [33,41–43]. Therefore, we examined whether constitutively active H-Ras G12V can block the effects of cisplatin as a single agent and the cetuximab- or gefitinib-mediated sensitization to cisplatin. Interestingly, A431V and A431-Ras cells were equally sensitive to cisplatin in a dose-dependent manner (Fig. 4A); however, we observed differences between the antiproliferative effects of combined treatment with cisplatin plus either cetuximab or gefitinib in A431V cells and the antiproliferative effects of the same treatment in A431-Ras cells. The combination of cisplatin and cetuximab inhibited A431V cell proliferation to 43% of the proliferation of untreated cells, while similar treatment inhibited A431-Ras cell proliferation to only 75% of the proliferation of untreated cells (Fig. 4B). Likewise, treatment with cisplatin and gefitinib reduced the A431V cell proliferation to 26% of the proliferation of untreated cells, while similar treatment inhibited A431-Ras cell proliferation to only 62% of the proliferation of untreated cells (Fig. 4C). These results indicate that although no increased resistance to cisplatin was observed when cells expressed constitutively active H-Ras, constitutively active H-Ras could confer increased resistance to combinational treatment with cisplatin plus cetuximab or gefitinib.
Fig. 4.
Reduced antiproliferative effects of cetuximab or gefitinib in combination with cisplatin in cells transfected with a constitutively active H-Ras. A431V and A431-Ras cells (5 × 103) were seeded and allowed to adhere overnight in medium containing 10% FBS. (A) A431V (□) and A431-Ras (▲) cells were then pulsed with increasing doses of cisplatin (0–10 μM) for 3 h, washed in PBS and left for 5 days in medium containing 0.5% FBS. (B) Cells were left untreated (solid column) or were pulsed with 1 μM of cisplatin for 3 h, washed in PBS, and then treated with 1 nM of cetuximab (open column). (C) Cells were untreated (solid column) or were pulsed with 1 μM of cisplatin for 3 h, washed in PBS, and then treated with 0.1 μM of gefitinib (open column) in medium containing 0.5% FBS for 5 days. Cell proliferation was assessed by an MTT assay as described in Materials and Methods using the optical density (OD) of cell lysates as relative numbers of cells. The OD value in the treated groups was expressed as a percentage of the OD value of the control group. The bars indicate standard deviation.
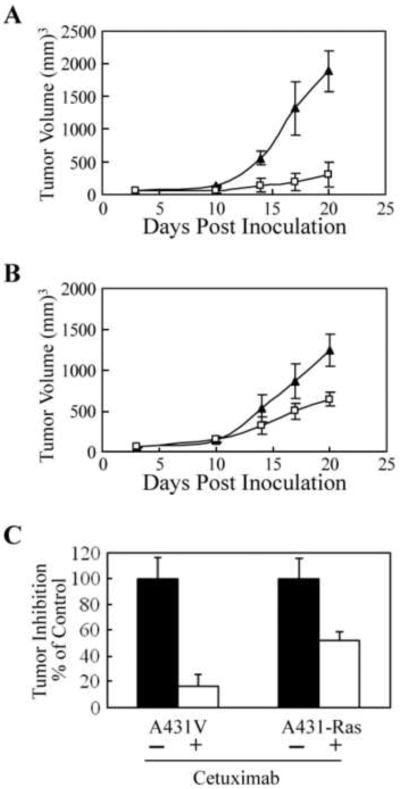
3.5. Constitutively active H-Ras reduced antitumor efficacy of cetuximab in vivo
To determine whether the expression of constitutively active Ras could reduce the antitumor activity of cetuximab in vivo, we inoculated A431V and A431-Ras cells into nude mice, which were then treated with cetuximab or vehicle control as described in Materials and Methods. Interestingly, the A431-Ras xenografts grew slightly slower than the A431-V xenografts, although the mean tumor volumes between groups were not significantly different (p = 0.17). As expected, cetuximab significantly inhibited the growth of A431V xenografts (Fig. 5A). At three days after the last treatment, when the animals were euthanized, the mean A431V xenograft tumor volume was 1,880 ± 310 mm3 for the control group and a significantly smaller 300 ± 180 mm3 for the cetuximab-treated group (p < 0.005). Although cetuximab also significantly inhibited the growth of the A431-Ras xenograft tumors, the level of inhibition was far less then that seen with the A431V xenografts (Fig. 5B). Statistical analysis revealed that cetuximab could significantly inhibit A431V xenograft growth by day 14 but took until day 20 to significantly inhibit A431-Ras xenograft growth (p < 0.05). At the time of euthanasia, the mean A431-Ras xenograft tumor volume was 1,240 ± 200 mm3 for the control group and 640 ± 190 mm3 for the cetuximab-treated group (p < 0.02). Although cetuximab significantly inhibited the growth of both A431V and A431-Ras xenografts, cetuximab inhibited the tumor volume of A431V xenografts to just 16 ± 10% of the tumor volume of the control group while inhibiting the tumor volume of A431-Ras xenografts to 52 ± 7% of the tumor volume of the control group (Fig. 5C, p <0.01). These results indicate that constitutively active H-Ras G12V can negatively affect tumor response to the antitumor activity of cetuximab in vivo.
Fig. 5.
Reduced antitumor effects of cetuximab in xenografts of cells transfected with a constitutively active H-Ras. (A) A431V and (B) A431-Ras cells (5 × 106) were injected subcutaneously into both flanks (A431V, left side; A431-Ras, right side) of 6–8-week-old male NCr nude mice (n = 5; on day 0). Mice were injected intraperitoneally with either 1-mg doses of cetuximab (□) or vehicle control, (▲) starting when tumors had reached a mean tumor volume of 60 mm3. Injections were given on days 3, 10, and 17. Data are expressed as mean tumor volume; bars indicate standard deviation. (C) Percentage inhibition of A431V and A431-Ras xenografts by cetuximab (open columns) compared with vehicle control (solid columns) was calculated on day 20, the final day of the experiment.
4. Discussion
Our in vitro and in vivo preclinical studies confirmed that expression of a constitutively active H-Ras (G12V) can confer resistance to EGFR-targeting agents in a manner similar to that of mutant K-Ras protein, which has been reported to play a key role in mediating resistance to EGFR-blocking antibodies in the treatment of colorectal cancers [17–25]. It is noteworthy that expression of H-Ras mutant did not confer complete resistance in vitro, and particularly not in vivo, in our study, which may be due to that (1) Ras is not the only downstream mediator that EGFR uses to transmit cell signaling, and (2) nude mice have functional macrophages and natural killer cells capable of mediating antibody-dependent cellular cytotoxicity (ADCC) effects [44]; cetuximab can mediate ADCC [45], which should be irrespective to Ras mutation status.
Oncogenic mutations of Ras occur at varying frequencies in different types of tumors in humans [46,47]. For example, H-Ras mutations have been reported in head and neck cancers [29,30], one of only three types of cancers (along with colorectal cancer and lung cancer) for which treatment with EGFR-targeting agents is currently approved by the U.S. Food and Drug Administration. Our results provide laboratory evidence suggesting that screening for H-Ras mutations should also be considered for patients with cancers that may harbor H-Ras mutations, such as head and neck cancers so that these patients may be excluded from treatment with EGFR-targeting agents.
The original discovery that the EGFR plays a critical role in cancer development and progression generated much enthusiasm, as it was hoped that preventing EGFR activation would block cancer growth and dramatically improve clinical outcomes and patient survival durations. It is now known that the genetic profiles of cancer cells with respect to the signaling pathways downstream of EGFR play a pivotal role in determining the efficacy of EGFR-targeted therapies. For example, one study found that only 20–30% of patients with colorectal cancer had disease that responded to EGFR-blocking antibodies [48]. A later study found that among the 70–80% of patients with nonresponsive disease, 30–35% had K-Ras mutations, 20% had B-Raf or PI3K mutations, and the rest had other aberrations [23]. Thus, although EGFR plays important roles in tumorigenesis and tumor progression, cancer cells are genetically unstable and can elude the effect of EGFR-targeted therapy through several well-characterized and some not-yet-known resistance mechanisms.
Much research is now focused on the development of novel combinational therapies targeting EGFR and EGFR downstream signaling pathways in an attempt to overcome various resistance mechanisms. Unfortunately, Ras seems an “undruggable” target, as no direct inhibitor targeting K-Ras or H-Ras has shown meaningful clinical activity to date [49]. Studies of novel agents targeting proteins farther downstream, such as MEK, have shown some encouraging clinical data [50]. HIF-1α—the expression of which is driven by EGFR, other receptor tyrosine kinases, and constitutively active Ras mutants through the activation of the PI3K/Akt/mTOR pathway [4–8]—may represent a promising target for combinational treatment with EGFR-targeting agents [51].
In summary, H-Ras mutations can be a mechanism of resistance to EGFR-targeted therapy. The development of novel agents targeting proteins downstream of the Ras pathways is expected to improve tumor responses to EGFR-targeted therapy.
Acknowledgments
This work was supported in part by a US National Institutes of Health (NIH) R01 award (CA129036 to Z.F.) and by the Cancer Center Support Grant CA016672 from US National Cancer Institute (NCI). We thank Bryan Tutt of the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest None declared
References
- [1].Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- [3].Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3'-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–7355. [PubMed] [Google Scholar]
- [5].Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- [6].Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- [7].Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 2002;277:27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- [8].Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- [9].Semenza GL. HIF-1 inhibitors for cancer therapy: from gene expression to drug discovery. Curr. Pharm. Des. 2009;15:3839–3843. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]
- [10].Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harvey JJ. An unidentified virus which causes the rapid production of tumours in mice. Nature. 1964;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- [12].Kirsten WH, Schauf V, McCoy J. Properties of a murine sarcoma virus. Bibl. Haematol. 1970:246–249. doi: 10.1159/000391714. [DOI] [PubMed] [Google Scholar]
- [13].Cooper GM. Cellular transforming genes. Science. 1982;217:801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- [14].Taparowsky E, Shimizu K, Goldfarb M, Wigler M. Structure and activation of the human N-ras gene. Cell. 1983;34:581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- [15].Buday L, Downward J. Many faces of Ras activation. Biochim. Biophys. Acta. 2008;1786:178–187. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [16].Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- [17].Lievre A, Bachet JB, Le CD, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- [18].Di FF, Blanchard F, Charbonnier F, Le PF, Lamy A, Galais MP, Bastit L, Killian A, Sesboue R, Tuech JJ, Queuniet AM, Paillot B, Sabourin JC, Michot F, Michel P, Frebourg T. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br. J. Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA, III, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J. Clin. Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- [20].Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- [21].Amado RG, Wolf M, Peeters M, Van CE, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- [22].Lievre A, Bachet JB, Boige V, Cayre A, Le CD, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, Diebold MD, Rougier P, Ducreux M, Tomasic G, Emile JF, Penault-Llorca F, Laurent-Puig P. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- [23].Freeman DJ, Juan T, Reiner M, Hecht JR, Meropol NJ, Berlin J, Mitchell E, Sarosi I, Radinsky R, Amado RG. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin. Colorectal Cancer. 2008;7:184–190. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- [24].Van CE, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- [25].Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J. Clin. Oncol. 2009;27:1130–1136. doi: 10.1200/JCO.2008.19.8168. [DOI] [PubMed] [Google Scholar]
- [26].Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- [27].Leon J, Guerrero I, Pellicer A. Differential expression of the ras gene family in mice. Mol. Cell Biol. 1987;7:1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fiorucci G, Hall A. All three human ras genes are expressed in a wide range of tissues. Biochim. Biophys. Acta. 1988;950:81–83. doi: 10.1016/0167-4781(88)90076-0. [DOI] [PubMed] [Google Scholar]
- [29].Lea IA, Jackson MA, Li X, Bailey S, Peddada SD, Dunnick JK. Genetic pathways and mutation profiles of human cancers: site- and exposure-specific patterns. Carcinogenesis. 2007;28:1851–1858. doi: 10.1093/carcin/bgm176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anderson JA, Irish JC, McLachlin CM, Ngan BY. H-ras oncogene mutation and human papillomavirus infection in oral carcinomas. Arch. Otolaryngol. Head Neck Surg. 1994;120:755–760. doi: 10.1001/archotol.1994.01880310059011. [DOI] [PubMed] [Google Scholar]
- [31].Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- [32].Lu Y, Liang K, Li X, Fan Z. Responses of cancer cells with wild-type or tyrosine kinase domain-mutated epidermal growth factor receptor (EGFR) to EGFR-targeted therapy are linked to downregulation of hypoxia-inducible factor-1alpha. Mol. Cancer. 2007;6:63. doi: 10.1186/1476-4598-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- [34].Jin W, Wu L, Liang K, Liu B, Lu Y, Fan Z. Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br. J. Cancer. 2003;89:185–191. doi: 10.1038/sj.bjc.6601048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Post DE, Van Meir EG. Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 2001;8:1801–1807. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- [36].Luwor RB, Lu Y, Li X, Mendelsohn J, Fan Z. The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene. 2005;24:4433–4441. doi: 10.1038/sj.onc.1208625. [DOI] [PubMed] [Google Scholar]
- [37].Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, Scott AM. Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2–7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res. 2001;61:5355–5361. [PubMed] [Google Scholar]
- [38].Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int. J Radiat. Oncol. Biol. Phys. 2003;57:246–254. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- [39].Liu B, Fang M, Lu Y, Mendelsohn J, Fan Z. Fibroblast growth factor and insulin-like growth factor differentially modulate the apoptosis and G1 arrest induced by anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 2001;20:1913–1922. doi: 10.1038/sj.onc.1204277. [DOI] [PubMed] [Google Scholar]
- [40].Li X, Lu Y, Liang K, Pan T, Mendelsohn J, Fan Z. Requirement of hypoxiainducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol. Cancer Ther. 2008;7:1207–1217. doi: 10.1158/1535-7163.MCT-07-2187. [DOI] [PubMed] [Google Scholar]
- [41].Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr., Mendelsohn J. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J. Natl. Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- [42].Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, Bianco AR, Tortora G. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]
- [43].Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin. Cancer Res. 2000;6:4885–4892. [PubMed] [Google Scholar]
- [44].Hasui M, Saikawa Y, Miura M, Takano N, Ueno Y, Yachie A, Miyawaki T, Taniguchi N. Effector and precursor phenotypes of lymphokine-activated killer cells in mice with severe combined immunodeficiency (scid) and athymic (nude) mice. Cell Immunol. 1989;120:230–239. doi: 10.1016/0008-8749(89)90190-1. [DOI] [PubMed] [Google Scholar]
- [45].Bleeker WK, Lammerts van Bueren JJ, van Ojik HH, Gerritsen AF, Pluyter M, Houtkamp M, Halk E, Goldstein J, Schuurman J, van Dijk MA, van de Winkel JG, Parren PW. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol. 2004;173:4699–4707. doi: 10.4049/jimmunol.173.7.4699. [DOI] [PubMed] [Google Scholar]
- [46].Bos JL. The ras gene family and human carcinogenesis. Mutat. Res. 1988;195:255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- [47].Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- [48].Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- [49].Vakiani E, Solit DB. KRAS and BRAF: drug targets and predictive biomarkers. J. Pathol. 2011;223:219–229. doi: 10.1002/path.2796. [DOI] [PubMed] [Google Scholar]
- [50].Zoppoli G, Moran E, Soncini D, Cea M, Garuti A, Rocco I, Cirmena G, Grillo V, Bagnasco L, Icardi G, Ansaldi F, Parodi S, Patrone F, Ballestrero A, Nencioni A. Ras-induced resistance to lapatinib is overcome by MEK inhibition. Curr. Cancer Drug Targets. 2010;10:168–175. doi: 10.2174/156800910791054211. [DOI] [PubMed] [Google Scholar]
- [51].Lu Y, Li X, Lu H, Fan Z. 1, 9-Pyrazoloanthrones downregulate HIF-1alpha and sensitize cancer cells to cetuximab-mediated anti-EGFR therapy. PLoS One. 2010;5:e15823. doi: 10.1371/journal.pone.0015823. [DOI] [PMC free article] [PubMed] [Google Scholar]